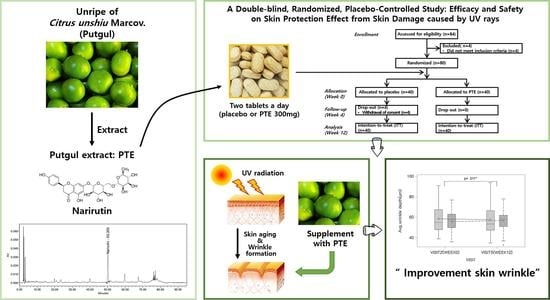
Clinical Evidence of Effects of Green Mandarin (Putgyul) Extract on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Subjects
2.3. Study Design
2.4. Efficacy and Safety Assessment
2.5. Statistical Analysis
3. Results
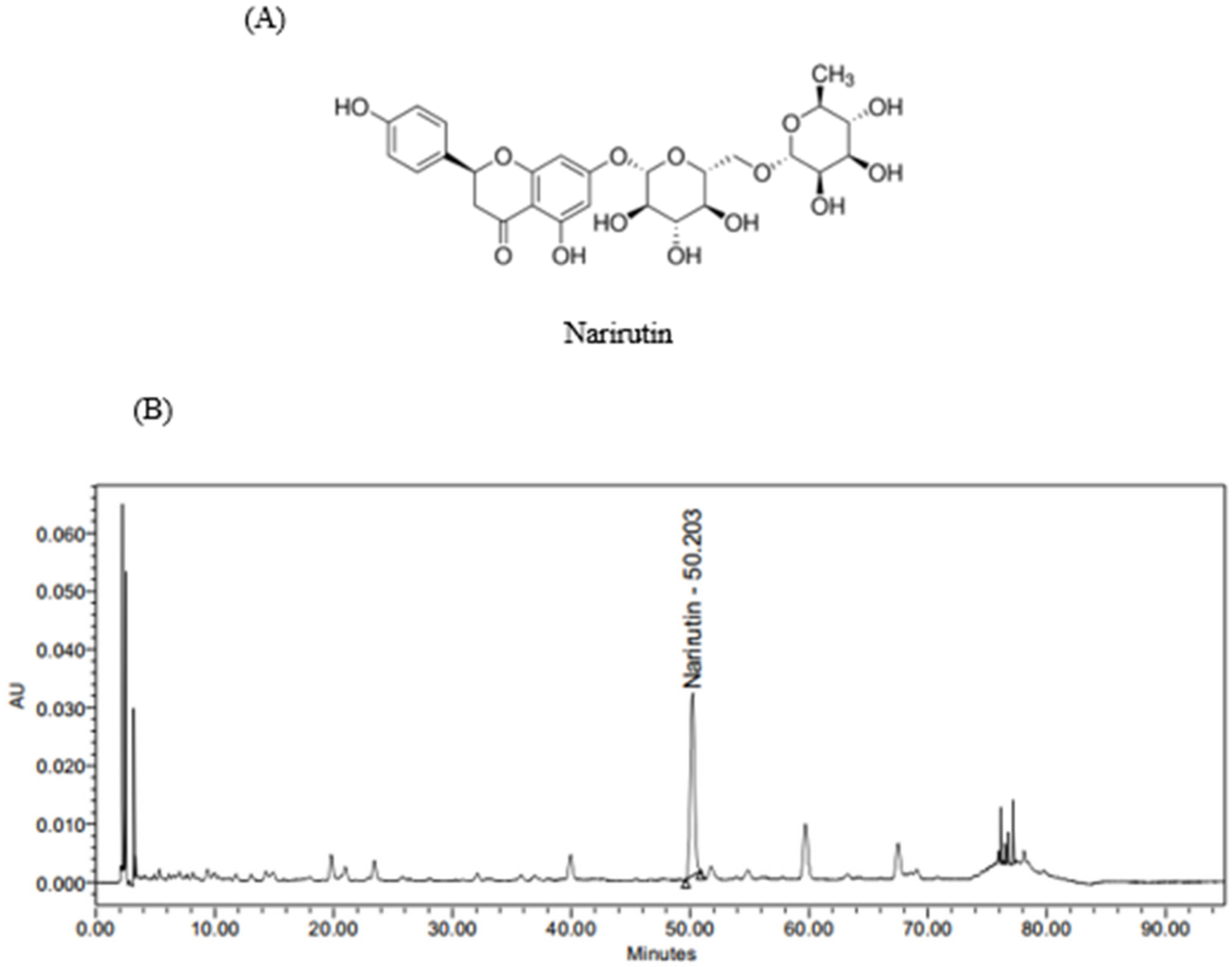
3.1. Analysis of Marker Compound Content in PTE
3.2. Baseline Characteristics
3.3. Primary Efficacy Endpoint: Changes in Eye Wrinkles
3.4. Secondary Efficacy Endpoints
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damiani, E.; Ullrich, S.E. Understanding the connection between platelet-activating factor, a UV-induced lipid mediator of inflammation, immune suppression and skin cancer. Prog. Lipid Res. 2016, 63, 14–27. [Google Scholar]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [PubMed]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of flavonoid constituents in citrus fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [PubMed] [Green Version]
- Azzouz, D.; Khan, M.A.; Sweezey, N.; Palaniyar, N. Two-in-one: UV radiation simultaneously induces apoptosis and NETosis. Cell Death Discov. 2018, 4, 51. [Google Scholar]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine aspects of skin aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar]
- Scharffetter-Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.L.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar] [PubMed]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar]
- Fernández-García, E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014, 5, 1994–2003. [Google Scholar] [PubMed]
- McArdle, F.; Rhodes, L.E.; Parslew, R.; Jack, C.I.A.; Friedmann, P.S.; Jackson, M.J. UVR-induced oxidative stress in human skin in vivo: Effects of oral vitamin C supplementation. Free Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [PubMed]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar]
- Kawada, A.; Konishi, N.; Oiso, N.; Kawara, S.; Date, A. Evaluation of anti-wrinkle effects of a novel cosmetic containing niacinamide. J. Dermatol. 2008, 35, 637–642. [Google Scholar] [PubMed]
- Choi, S.Y.; Hong, J.Y.; Ko, E.J.; Kim, B.J.; Hong, S.W.; Lim, M.H.; Yeon, S.H.; Son, R.H. Protective effects of fermented honeybush (Cyclopia intermedia) extract (HU-018) against skin aging: A randomized, double-blinded, placebo-controlled study. J. Cosmet. Laser Ther. 2018, 20, 313–318. [Google Scholar] [PubMed]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 817. [Google Scholar]
- Fang, Q.; Guo, S.; Zhou, H.; Han, R.; Wu, P.; Han, C. Astaxanthin protects against early burn-wound progression in rats by attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis. Sci. Rep. 2017, 7, 41440. [Google Scholar]
- Tanizawa, H.; Ohkawa, Y.; Takino, Y.; Miyase, T.; Ueno, A.; Kageyama, T.; Hara, S. Studies on natural antioxidants in citrus species. I. Determination of antioxidative activities of citrus fruits. Chem. Pharm. Bull. 1992, 40, 1940–1942. [Google Scholar]
- Kang, Y.J.; Yang, M.H.; Ko, W.J.; Park, S.R.; Lee, B.G. Studies on the major components and antioxidative properties of whole fruit powder and juice prepared from premature mandarin orange. Korean J. Food Sci. Technol. 2005, 37, 783–788. [Google Scholar]
- Choi, S.H.; Choi, S.I.; Jung, T.D.; Cho, B.Y.; Lee, J.H.; Kim, S.H.; Yoon, S.A.; Ham, Y.M.; Yoon, W.J.; Cho, J.H.; et al. Anti-Photoaging Effect of Jeju Putgyul (Unripe Citrus) Extracts on Human Dermal Fibroblasts and Ultraviolet B-induced Hairless Mouse Skin. Int. J. Mol. Sci. 2017, 18, 2052. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Maibach, H.I. Textbook of Aging Skin, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 15–30. [Google Scholar]
- Kammeyer, A.; Luiten, R. M Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar]
- Choi, S.Y.; Ko, H.C.; Ko, S.Y.; Hwang, J.H.; Park, J.G.; Kang, S.H.; Han, S.H.; Yun, S.H.; Kim, S.J. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol. Pharm. Bull. 2007, 30, 772–778. [Google Scholar]
- Goulas, V.; Manganaris, G.A. Exploring the phytochemical content and the antioxidant potential of Citrus fruits grown in Cyprus. Food Chem. 2012, 131, 39–47. [Google Scholar]
- Gironés-Vilaplana, A.; Moreno, D.A.; García-Viguera, C. Phytochemistry and biological activity of Spanish Citrus fruits. Food Funct. 2014, 5, 764–772. [Google Scholar] [PubMed]
- Han, S.; Kim, H.M.; Lee, S. Simultaneous determination of polymethoxyflavones in Citrus species, Kiyomi tangor and Satsuma mandarin, by high performance liquid chromatography. Food Chem. 2012, 134, 1220–1224. [Google Scholar] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink. Food Chem. 1997, 60, 331–337. [Google Scholar]
- Qurtam, A.A.; Mechchate, H.; Es-safi, I.; Al-zharani, M.; Nasr, F.A.; Noman, O.M.; Aleissa, M.; Imtara, H.; Aleissa, A.M.; Bouhrim, M.; et al. Citrus Flavanone Narirutin, In Vitro and In Silico Mechanistic Antidiabetic Potential. Pharmaceutics 2021, 13, 1818. [Google Scholar]
- Funaguchi, N.; Ohno, Y.; La, B.L.B.; Asai, T.; Yuhgetsu, H.; Sawada, M.; Takemura, G.; Minatoguchi, S.; Fujiwara, T.; Fujiwara, H. Narirutin inhibits airway inflammation in an allergic mouse model. Clin. Exp. Pharmacol. Physiol. 2007, 34, 766–770. [Google Scholar]
- Niu, L.; Wei, J.; Li, W.; Jin, Y.; Shi, X. Inhibitory activity of narirutin on RBL-2H3 cells degranulation. Immunopharmacol. Immunotoxicol. 2020, 43, 68–76. [Google Scholar]
- Itoh, K.; Hirata, N.; Masuda, M.; Naruto, S.; Murata, K.; Wakabayashi, K.; Matsuda, H. Inhibitory Effects of Citrus hassaku Extract and Its Flavanone Glycosides on Melanogenesis. Biol. Pharm. Bull. 2009, 32, 410–415. [Google Scholar]
- Bae, J.T.; Ko, H.J.; Kim, G.B.; Pyo, H.B.; Lee, G.S. Protective Effects of Fermented Citrus Unshiu Peel Extract against Ultraviolet-A-induced Photoageing in Human Dermal Fibrobolasts. Phytother. Res. 2012, 26, 1851–1856. [Google Scholar]
- Tamaru, E.; Watanabe, M.; Nomura, Y. Dietary immature Citrus unshiu alleviates UVB- induced photoaging by suppressing degradation of basement membrane in hairless mice. Heliyon 2020, 6, e04218. [Google Scholar]
- Kim, Y.D.; Ko, W.J.; Koh, K.S.; Jeon, Y.J.; Kim, S.H. Composition of flavonoids and antioxidative activity from juice of Jeju native citrus fruits during maturation. Korean J. Nutr. 2009, 42, 278–290. [Google Scholar]


| Placebo (n = 40) | PTE (n = 40) | p-Value B | |
|---|---|---|---|
| Gender (N, male/female) | 0/40 | 0/40 | - |
| Age (years) | 46.2 ± 3.9 | 47.1 ± 4.4 | 0.314 |
| BMI (kg/m) | 23.5 ± 2.8 | 22.7 ± 3.0 | 0.244 |
| SBP (mmHg) | 113.1 ± 13.1 | 111.1 ± 13.4 | 0.519 |
| DBP (mmHg) | 78.5 ± 5 | 75.2 ± 11.2 | 0.144 |
| Parameter | Visit | PTE (n = 40) | Placebo (n = 40) | # p | ## p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Min, Max | Mean ± SD | Median | Min, Max | ||||
| Avg.wrinkle depth (μm) | Week 0 | 58.63 ± 14.040 | 55.0 | 39.0, 91.0 | 55.73 ± 9.915 | 57.5 | 36.0, 77.0 | 0.290 | 0.649 |
| Week 12 | 57.68 ± 14.543 | 53.5 | 35.0, 94.0 | 56.89 ± 10.752 | 58.0 | 33.0, 78.0 | 0.790 | 0.594 | |
| Change | −0.95 ± 4.188 | −1.0 | −10.0, 7.0 | 1.51 ± 4.107 | 1.0 | −6.0, 10.0 | 0.011 * | 0.024 * | |
| Wrinkle volume (mm3) | Week 0 | 7.48 ± 2.293 | 7.1 | 3.8, 13.1 | 7.16 ± 1.804 | 7.3 | 3.5, 10.2 | 0.483 | 0.766 |
| Week 12 | 6.87 ± 2.185 | 6.3 | 3.6, 12.5 | 6.78 ± 1.820 | 7.0 | 2.8, 10.0 | 0.838 | 0.768 | |
| Change | −0.61 ± 0.583 | −0.6 | −2.2, 0.5 | −0.28 ± 0.488 | −0.4 | −1.2, 0.9 | 0.009 ** | 0.017 * | |
| Roughness area (μm) | Week 0 | 29.04 ± 6.516 | 27.4 | 18.7, 45.8 | 27.84 ± 4.834 | 28.2 | 19.1, 39.1 | 0.354 | 0.785 |
| Week 12 | 28.07 ± 6.686 | 26.3 | 17.4, 45.9 | 28.14 ± 5.133 | 27.3 | 16.7, 38.4 | 0.956 | 0.506 | |
| Change | −0.97 ± 1.948 | −1.0 | −4.4, 3.7 | 0.43 ± 2.133 | −0.1 | −3.1, 5.0 | 0.004 ** | 0.009 ** | |
| Mean depth biggest wrinkle (μm) | Week 0 | 92.80 ± 38.260 | 89.0 | 33.0, 202.0 | 89.93 ± 38.254 | 82.5 | 38.0, 217.0 | 0.738 | 0.578 |
| Week 12 | 90.53 ± 37.694 | 90.0 | 35.0, 206.0 | 89.43 ± 37.946 | 81.0 | 39.0, 223.0 | 0.900 | 0.867 | |
| Change | −2.28 ± 9.498 | −1.5 | −24.0, 21.0 | −0.76 ± 10.145 | 1.0 | −33.0, 18.0 | 0.500 | 0.259 | |
| Max depth biggest wrinkle (μm) | Week 0 | 352.35 ± 222.127 | 327.0 | 83.0, 1115.0 | 308.05 ± 155.214 | 255.5 | 101.0, 694.0 | 0.390 | 0.490 |
| Week 12 | 333.73 ± 201.506 | 295.5 | 87.0, 963.0 | 308.54 ± 170.860 | 257.0 | 103.0, 870.0 | 0.558 | 0.655 | |
| Change | −18.63 ± 51.793 | −12.5 | −229.0, 86.0 | −2.81 ± 62.583 | −3.0 | −164.0, 215.0 | 0.230 | 0.299 | |
| Wrinkle area (mm2) | Week 0 | 126.62 ± 18.148 | 135.6 | 85.6, 145.0 | 127.24 ± 17.042 | 135.4 | 93.6, 145.1 | 0.875 | 0.927 |
| Week 12 | 118.30 ± 16.146 | 126.5 | 82.5, 139.3 | 117.53 ± 16.524 | 125.2 | 86.8, 136.7 | 0.837 | 0.895 | |
| Change | −8.31 ± 4.059 | −7.0 | −18.0, −2.4 | −8.69 ± 3.335 | −7.8 | −19.0, −3.5 | 0.661 | 0.423 | |
| Length of wrinkle (mm) | Week 0 | 192.28 ± 35.695 | 201.5 | 118.0, 253.0 | 201.68 ± 26.586 | 203.5 | 143.0, 264.0 | 0.186 | 0.336 |
| Week 12 | 176.58 ± 30.836 | 184.5 | 113.0, 229.0 | 183.30 ± 25.500 | 188.0 | 133.0, 234.0 | 0.303 | 0.453 | |
| Change | −15.70 ± 10.920 | −16.0 | −42.0, 11.0 | −16.73 ± 10.090 | −17.0 | −39.0, 10.0 | 0.669 | 0.811 | |
| Variable | Group | Week 0 | Week 4 | Week 8 | Week 12 | Significance | |
|---|---|---|---|---|---|---|---|
| Factor | p | ||||||
| Skin Hydration (A.U.) | PTE | 41.44 ± 5.86 | 42.14 ± 6.20 | 43.18 ± 6.40 | 44.34 ± 6.67 | Group ## | 0.772 |
| Placebo | 41.42 ± 4.56 | 41.42 ± 4.91 | 41.88 ± 5.04 | 42.91 ± 5.35 | Time † | 0.001 *** | |
| p for group # | 0.793 | 0.627 | 0.338 | 0.306 | Group × Time ‡ | 0.057 | |
| Skin Elasticity (%) | PTE | 74.41 ± 5.22 | 74.81 ± 5.15 | 75.40 ± 5.24 | 74.81 ± 5.31 | Group ## | 0.300 |
| Placebo | 75.69 ± 4.33 | 76.12 ± 4.08 | 76.17 ± 4.33 | 76.29 ± 4.57 | Time † | 0.003 *** | |
| P for group # | 0.240 | 0.221 | 0.487 | 0.196 | Group × Time ‡ | 0.170 | |
| Skin glowing (GU) | PTE | 3.37 ± 0.97 | 3.76 ± 0.98 | 3.86 ± 1.01 | 3.90 ± 1.02 | Group ## | 0.094 |
| Placebo | 3.41 ± 0.92 | 3.44 ± 0.80 | 3.46 ± 0.86 | 3.55 ± 0.87 | Time † | 0.001 *** | |
| P for group # | 0.154 | 0.129 | 0.066 | 0.111 | Group × Time ‡ | 0.486 | |
| Transepidermal water loss (g/h/m2) | PTE | 15.09 ± 4.14 | 14.78 ± 3.77 | 15.10 ± 4.83 | 14.64 ± 3.61 | Group ## | 0.0.211 |
| Placebo | 16.25 ± 4.48 | 16.19 ± 3.64 | 15.44 ± 3.10 | 15.34 ± 2.69 | Time † | 0.114 | |
| P for group # | 0.233 | 0.101 | 0.710 | 0.346 | Group × Time ‡ | 0.227 | |
| Variable | Group | Week 4 | Week 8 | Week 12 | Significance | |
|---|---|---|---|---|---|---|
| Factor | p | |||||
| Increase skin moisture | PTE | 3.33 ± 0.57 | 3.63 ± 0.62 | 3.63 ± 0.58 | Group ## | 0.599 |
| Placebo | 3.22 ± 0.63 | 3.65 ± 0.48 | 3.54 ± 0.65 | Time † | 0.001 *** | |
| p for group # | 0.430 | 0.855 | 0.550 | Group × Time ‡ | 0.620 | |
| Increase skin gloss | PTE | 3.20 ± 0.56 | 3.63 ± 0.58 | 3.60 ± 0.67 | Group ## | 0.182 |
| Placebo | 3.14 ± 0.48 | 3.41 ± 0.49 | 3.46 ± 0.69 | Time † | 0.001 *** | |
| P for group # | 0.590 | 0.082 | 0.369 | Group × Time ‡ | 0.563 | |
| Increase skin elacsticity | PTE | 3.25 ± 0.58 | 3.55 ± 0.55 | 3.65 ± 0.70 | Group ## | 0.066 |
| Placebo | 3.22 ± 0.58 | 3.27 ± 0.45 | 3.38 ± 0.59 | Time † | 0.001 *** | |
| P for group # | 0.801 | 0.018 * | 0.071 | Group × Time ‡ | 0.151 | |
| Improvement crow’s feet | PTE | 3.05 ± 0.50 | 3.25 ± 0.49 | 3.65 ± 0.58 | Group ## | 0.337 |
| Placebo | 3.11 ± 0.39 | 3.22 ± 0.53 | 3.35 ± 0.67 | Time† | 0.001 *** | |
| P for group # | 0.5770. | 0.744 | 0.040 * | Group × Time ‡ | 0.025 * | |
| Improvement total skin condition | PTE | 3.40 ± 0.54 | 3.68 ± 0.52 | 3.90 ± 0.59 | Group ## | 0.138 |
| Placebo | 3.32 ± 0.62 | 3.62 ± 0.54 | 3.57 ± 0.64 | Time † | 0.001 *** | |
| P for group # | 0.573 | 0.663 | 0.021 * | Group × Time ‡ | 0.106 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ham, Y.-M.; Yoon, S.-A.; Hyeon, H.; Hyun, H.-B.; Kim, S.-C.; Go, B.; Jung, Y.-H.; Yoon, W.-J. Clinical Evidence of Effects of Green Mandarin (Putgyul) Extract on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. Nutrients 2022, 14, 1352. https://doi.org/10.3390/nu14071352
Ham Y-M, Yoon S-A, Hyeon H, Hyun H-B, Kim S-C, Go B, Jung Y-H, Yoon W-J. Clinical Evidence of Effects of Green Mandarin (Putgyul) Extract on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. Nutrients. 2022; 14(7):1352. https://doi.org/10.3390/nu14071352
Chicago/Turabian StyleHam, Young-Min, Seon-A Yoon, Hyejin Hyeon, Ho-Bong Hyun, Sung-Chun Kim, Boram Go, Yong-Hwan Jung, and Weon-Jong Yoon. 2022. "Clinical Evidence of Effects of Green Mandarin (Putgyul) Extract on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study" Nutrients 14, no. 7: 1352. https://doi.org/10.3390/nu14071352
APA StyleHam, Y. -M., Yoon, S. -A., Hyeon, H., Hyun, H. -B., Kim, S. -C., Go, B., Jung, Y. -H., & Yoon, W. -J. (2022). Clinical Evidence of Effects of Green Mandarin (Putgyul) Extract on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. Nutrients, 14(7), 1352. https://doi.org/10.3390/nu14071352