Low Percentage of Vegetable Fat in Red Blood Cells Is Associated with Worse Glucose Metabolism and Incidence of Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Clinical and Laboratory Analyses
2.3. Quantification of Fatty Acids in the Cell Membrane of Erythrocytes
2.4. Definition of Outcomes at Follow-Up
2.5. Statistical Analyses
3. Results
3.1. Subjects Characteristics
3.2. Incidence of Diabetes after Follow-Up
3.3. Association between the Fatty Acid Profile and the Risk of Progression in Glucose Metabolism Category
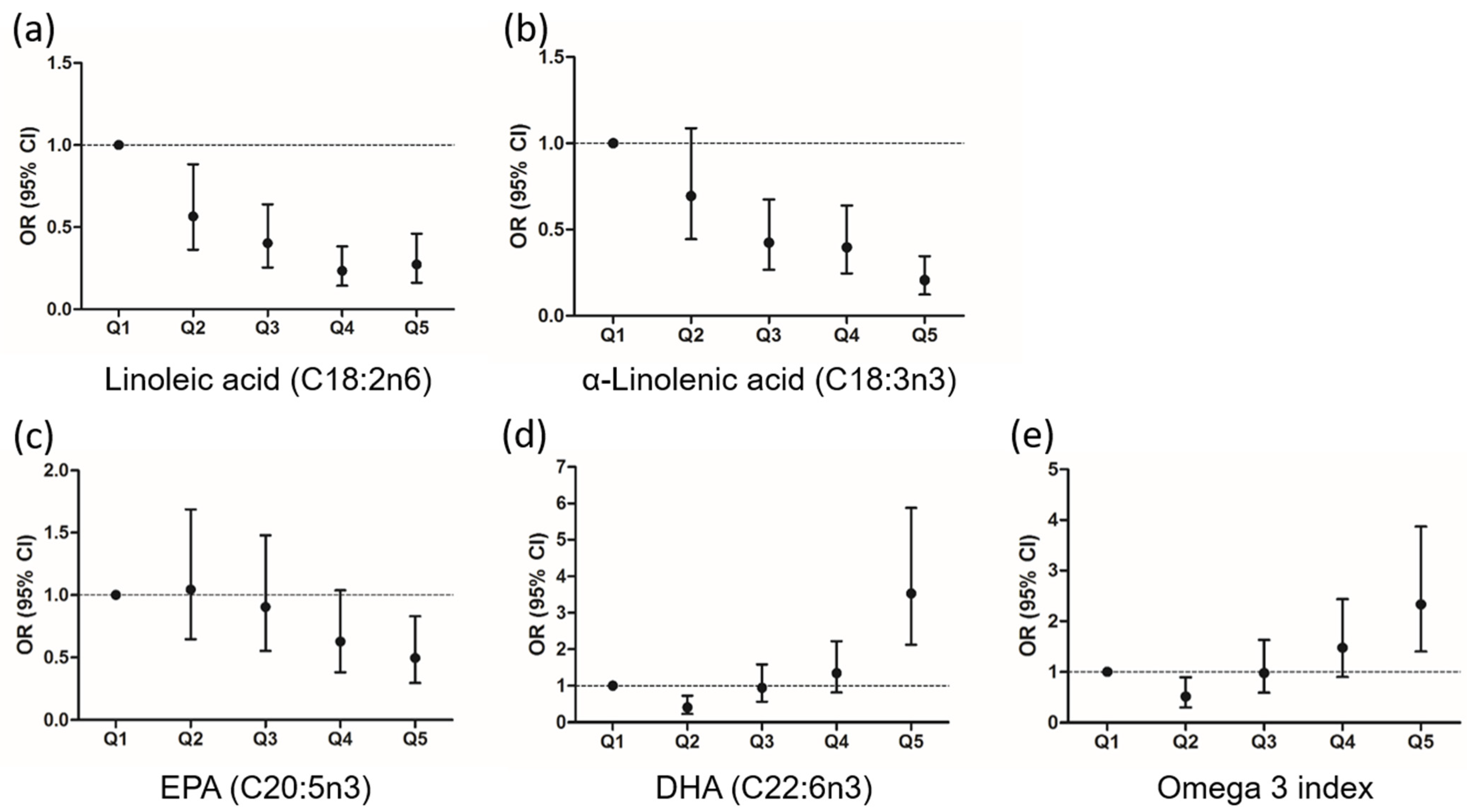
3.4. Association between the Fatty Acid Profile and the Risk of Diabetes
3.5. Red Blood Cells Fatty Acids as Predictive Biomarkers of Progression in Glucose Metabolism Category and Diabetes
3.6. Analysis of Sensitivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Statistics. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/332070/9789240005105-eng.pdf?sequence=1&isAllowed=y (accessed on 19 May 2021).
- Williams, R.; Colagiuri, S.; Chan, J. IDF DIABETES ATLAS, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- IDF Europe members: SPAIN. Available online: https://idf.org/our-network/regions-members/europe/members/159-spain.html (accessed on 19 May 2021).
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo-Martínez, G.; Valdés, S.; Soriguer, F.; Vendrell, J.; Urrutia, I.; Pérez, V.; Ortega, E.; Ocón, P.; Montanya, E.; Menéndez, E.; et al. Incidence of diabetes mellitus in Spain as results of the nation-wide cohort di@bet.es study. Sci. Rep. 2020, 10, 2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocké, M.C.; de Vries, J.H.M.; Hulshof, P.J.M. Assessment of dietary intake by self-reports and biological markers. In Present Knowledge in Nutrition; Academic Press: Cambridge, MA, USA, 2020; pp. 249–265. [Google Scholar] [CrossRef]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- Yary, T.; Voutilainen, S.; Tuomainen, T.P.; Ruusunen, A.; Nurmi, T.; Virtanen, J.K. Serum n-6 polyunsaturated fatty acids, Δ5- and D6-desaturase activities, and risk of incident type 2 diabetes in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2016, 103, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Qian, F.; Ardisson Korat, A.V.; Imamura, F.; Marklund, M.; Tintle, N.; Virtanen, J.K.; Zhou, X.; Bassett, J.K.; Lai, H.; Hirakawa, Y.; et al. n-3 Fatty Acid Biomarkers and Incident Type 2 Diabetes: An Individual Participant-Level Pooling Project of 20 Prospective Cohort Studies. Diabetes Care 2021, 44, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Imamura, F.; Sharp, S.J.; Koulman, A.; Schulze, M.B.; Zheng, J.; Ye, Z.; Sluijs, I.; Guevara, M.; Huerta, J.M.; et al. Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study. PLoS Med. 2016, 13, e1002094. [Google Scholar] [CrossRef] [Green Version]
- Djoussé, L.; Gaziano, J.M.; Buring, J.E.; Lee, I.M. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am. J. Clin. Nutr. 2011, 93, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Wallin, A.; Di Giuseppe, D.; Orsini, N.; Patel, P.S.; Forouhi, N.G.; Wolk, A. Fish consumption, dietary long-chain n-3 fatty acids, and risk of type 2 diabetes:Systematic review and meta-analysis of prospective studies. Diabetes Care 2012, 35, 918–929. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.H.; Marklund, M.; Imamura, F.; Tintle, N.; Korat, A.V.A.; De Goede, J.; Zhou, X.; Yang, W.S.; de Oliveira Otto, M.C.; Kröger, J.; et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care 2012, 35, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Harris, W.S.; Cofán, M.; Pérez-Heras, A.M.; Pintó, X.; Lamuela-Raventós, R.M.; Covas, M.I.; Estruch, R.; Ros, E. Determinants of the omega-3 index in a Mediterranean population at increased risk for CHD. Br. J. Nutr. 2011, 106, 425–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lázaro, I.; Cofán, M.; Amor, A.J.; Ortega, E.; Freitas-Simoes, T.M.; Llull, L.; Amaro, S.; Mestres, G.; Yugueros, X.; Harris, W.S.; et al. Linoleic Acid Status in Cell Membranes Inversely Relates to the Prevalence of Symptomatic Carotid Artery Disease. Stroke 2021, 52, 703–706. [Google Scholar] [CrossRef]
- Brugnara, L.; Murillo, S.; Novials, A.; Rojo-Martínez, G.; Soriguer, F.; Goday, A.; Calle-Pascual, A.; Castaño, L.; Gaztambide, S.; Valdés, S.; et al. Low Physical Activity and Its Association with Diabetes and Other Cardiovascular Risk Factors: A Nationwide, Population-Based Study. PLoS ONE. 2016, 11, e0160959. [Google Scholar] [CrossRef] [Green Version]
- Kröger, J.; Zietemann, V.; Enzenbach, C.; Weikert, C.; Jansen, E.H.; Döring, F.; Joost, H.G.; Boeing, H.; Schulze, M.B. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am. J. Clin. Nutr. 2011, 93, 127–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertiwi, K.; Wanders, A.J.; Harbers, M.C.; Küpers, L.K.; Soedamah-Muthu, S.S.; de Goede, J.; Zock, P.L.; Geleijnse, J.M. Plasma and Dietary Linoleic Acid and 3-Year Risk of Type 2 Diabetes After Myocardial Infarction: A Prospective Analysis in the Alpha Omega Cohort. Diabetes Care 2020, 43, 358–365. [Google Scholar] [CrossRef]
- Mahendran, Y.; Ågren, J.; Uusitupa, M.; Cederberg, H.; Vangipurapu, J.; Stančáková, A.; Schwab, U.; Kuusisto, J.; Laakso, M. Association of erythrocyte membrane fatty acids with changes in glycemia and risk of type 2 diabetes. Am. J. Clin. Nutr. 2014, 99, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.S.; Lin, J.S.; Dong, H.L.; Zeng, F.F.; Li, D.; Song, Y.; Chen, Y.M. Association of erythrocyte n-3 polyunsaturated fatty acids with incident type 2 diabetes in a Chinese population. Clin. Nutr. 2019, 38, 2195–2201. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.; Lin, J.S.; Mao, Y.; Chen, G.D.; Zeng, F.F.; Dong, H.L.; Jiang, Z.; Wang, J.; Xiao, C.; Shuai, M.; et al. Erythrocyte n-6 polyunsaturated fatty acids, gut microbiota, and incident type 2 diabetes: A prospective cohort study. Diabetes Care 2020, 43, 2435–2443. [Google Scholar] [CrossRef] [PubMed]
- Weir, N.L.; Nomura, S.O.; Steffen, B.T.; Guan, W.; Karger, A.B.; Klein, R.; Klein, B.E.; Cotch, M.F.; Tsai, M.Y. Associations between omega-6 polyunsaturated fatty acids, hyperinsulinemia and incident diabetes by race/ethnicity: The Multi-Ethnic Study of Atherosclerosis. Clin. Nutr. 2020, 39, 3031–3041. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Jalilpiran, Y.; Karimi, E.; Aune, D.; Larijani, B.; Mozaffarian, D.; Willett, W.C.; Esmaillzadeh, A. Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Diabetes Care 2021, 44, 2173–2181. [Google Scholar] [CrossRef]
- Coelho, O.G.L.; da Silva, B.P.; Rocha, D.M.U.P.; Lopes, L.L.; Alfenas, R.D.C.G. Polyunsaturated fatty acids and type 2 diabetes: Impact on the glycemic control mechanism. Crit. Rev. Food Sci. Nutr. 2017, 57, 3614–3619. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, M.; Barbaresko, J.; Pischke, C.R.; Iser, N.; Beckhaus, J.; Schwingshackl, L.; Schlesinger, S. Intake of dietary fats and fatty acids and the incidence of type 2 diabetes: A systematic review and dose-response meta-analysis of prospective observational studies. PLoS Med. 2020, 17, e1003347. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients after Myocardial Infarction: A Randomized, Controlled Trial. Circulation 2021, 143, 528–539. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA-J. Am. Med. Assoc. 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Behrouz, V.; Dastkhosh, A.; Sohrab, G. Overview of dietary supplements on patients with type 2 diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 325–334. [Google Scholar] [CrossRef]
- Katan, M.B.; Deslypere, J.P.; Van Birgelen, A.P.; Penders, M.; Zegwaard, M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. J. Lipid Res. 1997, 38, 2012–2022. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Y.; Yu, X.; Hu, S.; Shao, S. Association between omega-3 fatty acids consumption and the risk of type 2 diabetes: A meta-analysis of cohort studies. J. Diabetes Investig. 2017, 8, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.H.; Han, S.H.; Kim, S.H.; Eckel, R.H.; Koh, K.K. Cardiovascular effects of omega-3 fatty acids: Hope or hype? Atherosclerosis 2021, 322, 15–23. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| All (n = 1032) | Normoglycemia (n = 847) | Prediabetes (n = 185) | p | |
|---|---|---|---|---|
| Female sex | 596 (57.8) | 488 (57.6) | 108 (58.4) | 0.849 |
| Age, years | 49.41 ± 14.94 | 48.01 ± 14.94 | 55.79 ± 13.25 | <0.0001 |
| Hypertension | 219 (21.2) | 153 (18.1) | 66 (35.7) | <0.0001 |
| Family history of diabetes | 389 (37.7) | 312 (36.8) | 77 (41.6) | 0.004 |
| Dyslipidemia | 340 (32.9) | 265 (31.3) | 75 (40.5) | 0.007 |
| Sedentarism | 75 (7.5) | 52 (6.1) | 23 (12.4) | 0.002 |
| Glucose metabolism category | <0.0001 | |||
| Normoglycemia | 847 (82.1) | 847 (100) | - | |
| Impaired fasting glucose (IFG) | 61 (5.9) | - | 61 (33.0) | |
| Impaired glucose tolerance (IGT) | 98 (9.5) | - | 98 (53.0) | |
| IFG and IGT | 26 (2.5) | - | 26 (14.0) | |
| Glucose, mmol/L | 5.22 ± 0.67 | 5.09 ± 0.58 | 5.81 ± 0.71 | <0.0001 |
| Insulin, pmol/L | 66.60 ± 36.89 | 61.80 ± 31.74 | 88.77 ± 49.04 | <0.0001 |
| HOMA-IR | 2.28 ± 1.44 | 2.05 ± 1.16 | 3.34 ± 2.02 | <0.0001 |
| Waist-to-hip ratio | 0.89 ± 0.09 | 0.88 ± 0.09 | 0.93 ± 0.08 | <0.0001 |
| Body fat, % | 33.09 ± 9.36 | 32.22 ± 9.41 | 37.09 ± 8.03 | <0.0001 |
| BMI, Kg/m2 | 27.94 ± 4.75 | 27.33 ± 4.43 | 30.79 ± 5.12 | <0.0001 |
| BMI > 30 Kg/m2 | 306 (29.7) | 210 (24.8) | 96 (51.9) | <0.0001 |
| Total cholesterol, mg/dL | 199.99 ± 40.52 | 198.52 ± 40.14 | 206.74 ± 41.67 | 0.014 |
| cHDL, mg/dL | 52.45 ± 13.15 | 52.80 ± 13.25 | 50.85 ± 12.59 | 0.073 |
| cLDL, mg/dL | 108.40 ± 30.49 | 107.15 ± 29.99 | 114.09 ± 32.15 | 0.006 |
| Triglycerides, mg/dL | 122.90 ± 104.06 | 118.95 ± 109.38 | 140.98 ± 72.63 | 0.010 |
| n = 1032 | Baseline | End of the Follow-Up |
|---|---|---|
| Diabetes Mellitus (DM) | 0 (0) | 131 (12.7) |
| Known DM | 0 (0) | 70 (6.8) |
| Unknown DM | 0 (0) | 61 (5.9) |
| Glucose metabolism category | ||
| Impaired fasting glucose (IFG) | 61 (5.9) | 72 (7.0) |
| Impaired glucose tolerance (IGT) | 98 (9.5) | 104 (10.1) |
| IFG and IGT | 26 (2.5) | 51 (4.9) |
| Prediabetes | 185 (17.9) | 227 (22.0) |
| Progressors | - | 324 (31.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiva-Blanch, G.; Giró, O.; Cofán, M.; Calle-Pascual, A.L.; Delgado, E.; Gomis, R.; Jiménez, A.; Franch-Nadal, J.; Rojo Martínez, G.; Ortega, E. Low Percentage of Vegetable Fat in Red Blood Cells Is Associated with Worse Glucose Metabolism and Incidence of Type 2 Diabetes. Nutrients 2022, 14, 1368. https://doi.org/10.3390/nu14071368
Chiva-Blanch G, Giró O, Cofán M, Calle-Pascual AL, Delgado E, Gomis R, Jiménez A, Franch-Nadal J, Rojo Martínez G, Ortega E. Low Percentage of Vegetable Fat in Red Blood Cells Is Associated with Worse Glucose Metabolism and Incidence of Type 2 Diabetes. Nutrients. 2022; 14(7):1368. https://doi.org/10.3390/nu14071368
Chicago/Turabian StyleChiva-Blanch, Gemma, Oriol Giró, Montserrat Cofán, Alfonso L. Calle-Pascual, Elías Delgado, Ramon Gomis, Amanda Jiménez, Josep Franch-Nadal, Gemma Rojo Martínez, and Emilio Ortega. 2022. "Low Percentage of Vegetable Fat in Red Blood Cells Is Associated with Worse Glucose Metabolism and Incidence of Type 2 Diabetes" Nutrients 14, no. 7: 1368. https://doi.org/10.3390/nu14071368
APA StyleChiva-Blanch, G., Giró, O., Cofán, M., Calle-Pascual, A. L., Delgado, E., Gomis, R., Jiménez, A., Franch-Nadal, J., Rojo Martínez, G., & Ortega, E. (2022). Low Percentage of Vegetable Fat in Red Blood Cells Is Associated with Worse Glucose Metabolism and Incidence of Type 2 Diabetes. Nutrients, 14(7), 1368. https://doi.org/10.3390/nu14071368








