Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol
Abstract
:1. Introduction
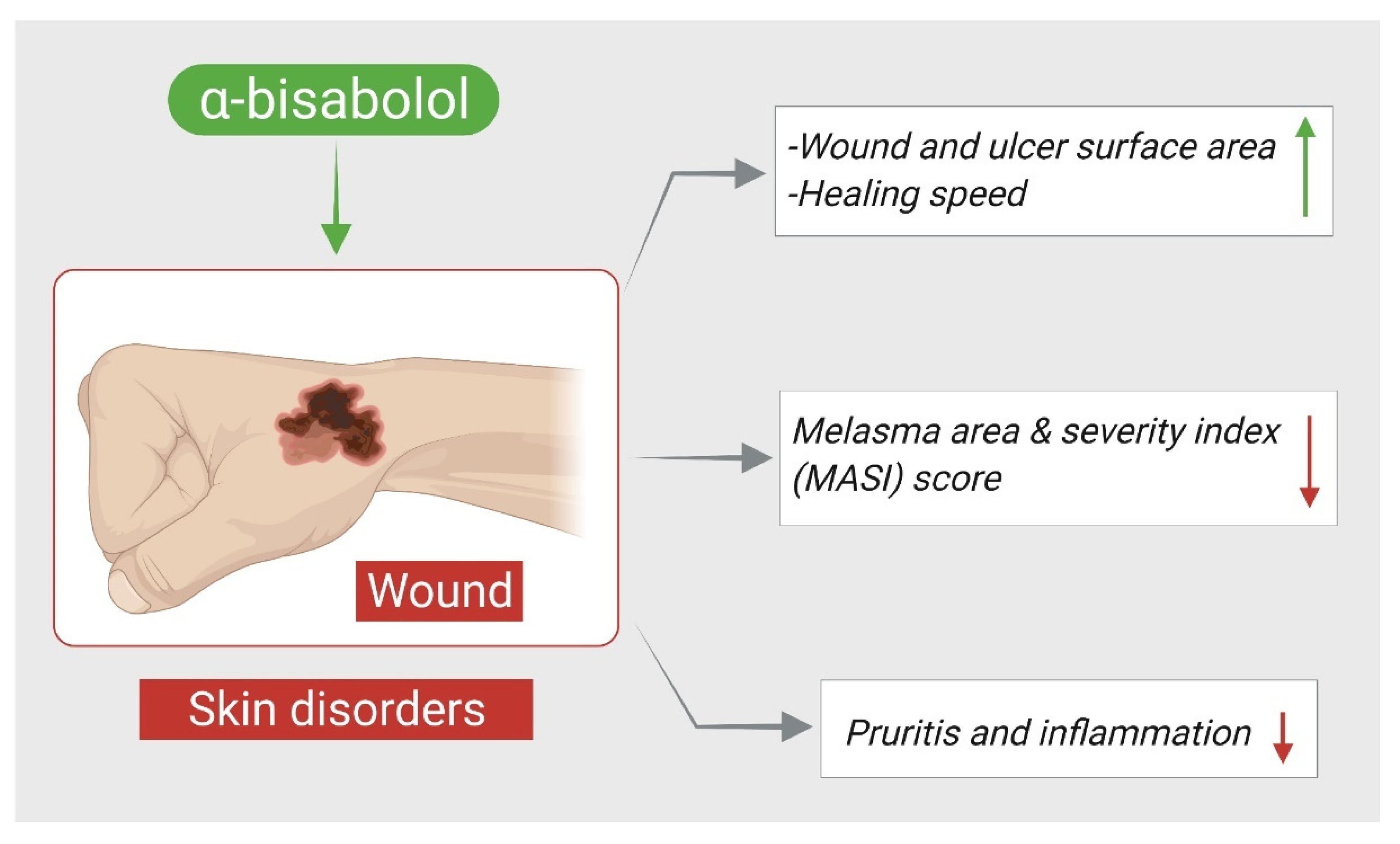
2. α-Bisabolol and Skin Disorders
3. α-Bisabolol and Neuroprotection
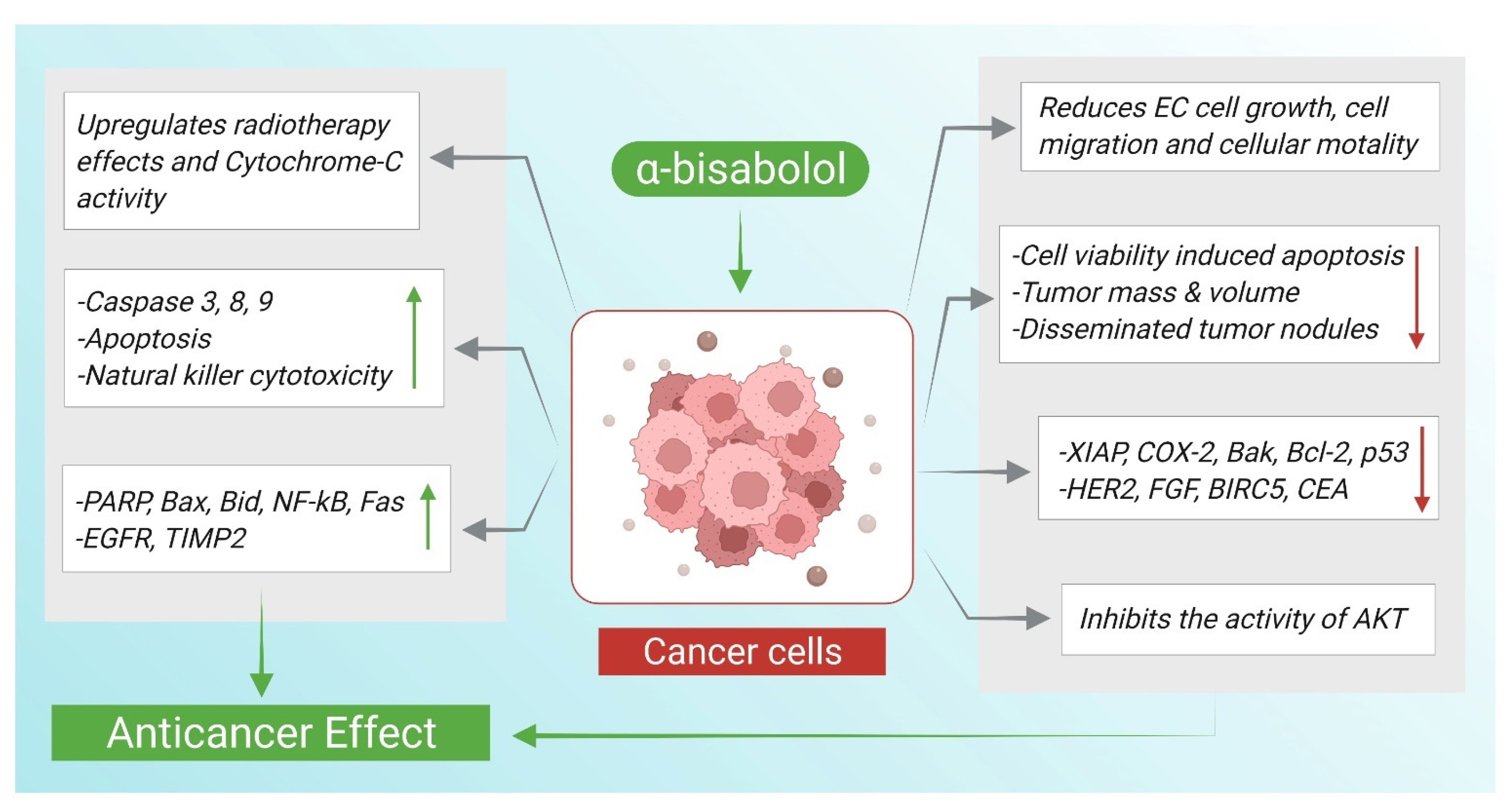
4. α-Bisabolol and Anticancer Effects
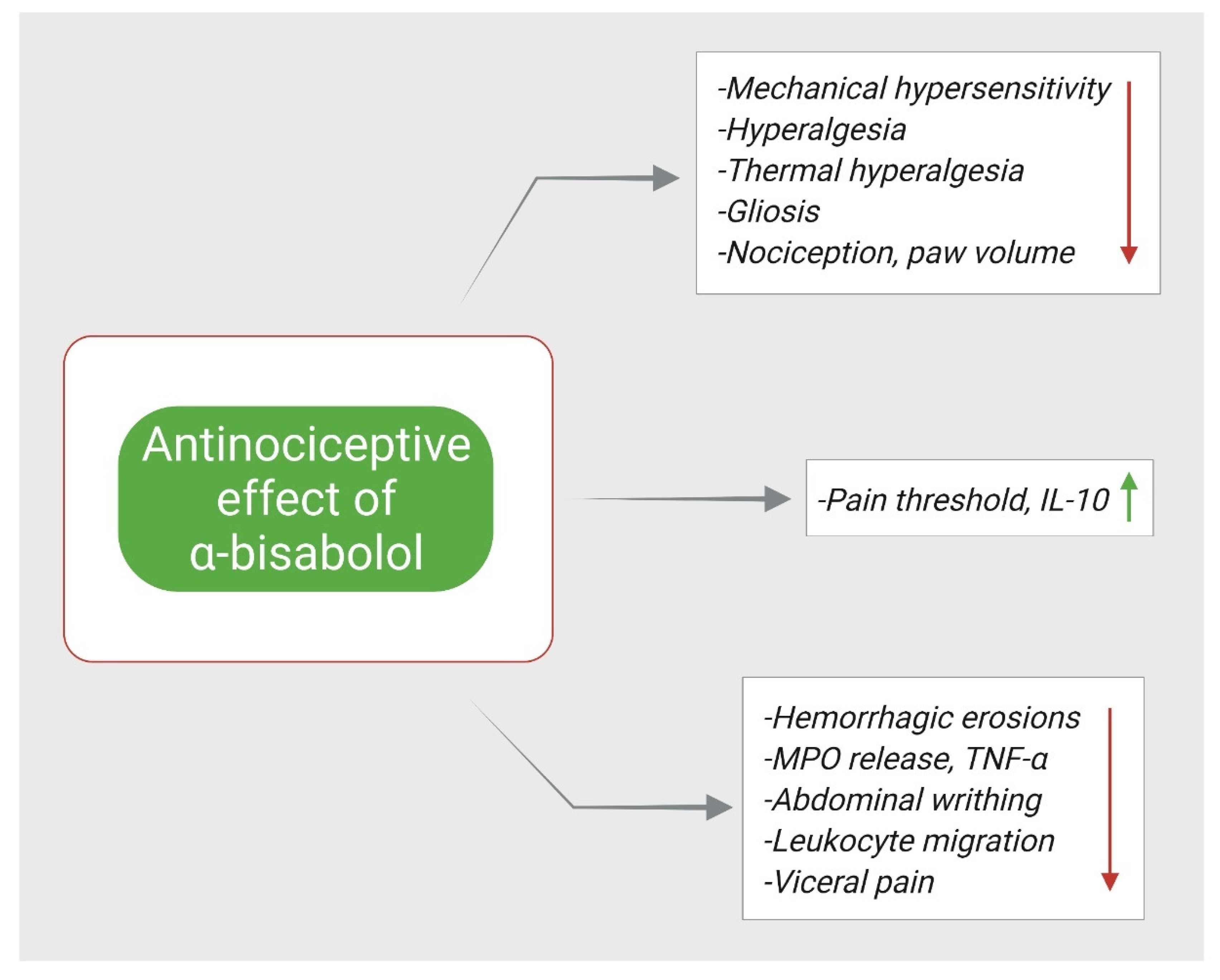
5. α-Bisabolol and Antinociception
6. α-Bisabolol and Cardioprotection
7. α-Bisabolol and Antimicrobial Effects
| Antimicrobial Actions | ||||
|---|---|---|---|---|
| Compound | Dose/Route/ Duration | Model | Major Mechanisms | Reference |
| α-Bisabolol | 4–512 μg/mL (for bacteria) 3–4096 μg/mL (for fungus) | Staphylococcus aureus, Candida albicans, Candida krusei, Candida tropicalis | Inhibited microbial growth | [5] |
| α-Bisabolol | 1024 μg/mL | Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa | Inhibited microbial growth | [97] |
| α-Bisabolol | 1024 µg/mL | Staphylococcus aureus strains: pT181 carrying the TetK efflux pump protein that extrudes tetracycline; and the 1199B that presents resistance to norfloxacin by NorA pump expression | ↓ MIC for tetracycline and norfloxacin | [98] |
| α-Bisabolol | 0.1% | Bacillus Solobacterium moorei | ↓ colonies number ↑ effect of tea tree oil | [99] |
| α-Bisabolol | 0.5–2 mM | Staphylococcus aureus and Escherichia coli | ↑ effect of co-administered antibiotics | [100] |
| α-Bisabolol | 0.281–9 mM for 3 days | Aspergillus fumigatus Af239 | ↓ fungal growth (-) 24-SMT, ↓ erg6 | [102] |
| α-Bisabolol | 5, 10, 20, 50, 100, 200 μg/mL | Microsporum gypseum, Microsporum canis, Trichophyton violaceum, Nannizzia cajetani, Trichophyton mentagrophytes, Epidermophyton floccosum, Arthroderma gypseum, Trichophyton rubrum and Trichophyton tonsurans | ↓ fungal growth (-) spore germination ↑ morphological anomalies | [103] |
| α-Bisabolol | 1 µg/mL | Fusarium oxysporum | ↓ fungal growth ↓ MIC of NaCl | [104] |
| α-Bisabolol | 1000–31.25 μM | Trypanosoma cruzi Y infected LLC-MK2 cells | ↓ cell viability ↑ ROS, ↑ apoptosis | [105] |
| α-Bisabolol | 1000–6.25 μg/mL | Leishmania infantum zymodeme 1 | ↓ parasite growth | [106] |
| α-Bisabolol | IC50 = 9.5, 16.0 | Promastigotes of Leishmania infantum and amazonensis | ↓ parasite growth ↑ apoptosis, ↓ Δψm ↓ ATP, ↑ membrane permeabilization | [107] |
| α-Bisabolol | 1.86–60 μg/mL (IC50 = 8.07 μg/mL) | MHOM/BR/76/Ma-76 Leishmania amazonensis strains | (-) parasite growth ↑ morphological changes | [108] |
| α-Bisabolol | 50, 200, and 1000 mg/kg p.o for 14 days | 107 stationary-phase promastigotes of Leishmania infantum injected in mice | (-) parasite growth | [110] |
| α-Bisabolol | 25 and 100 μM IC50 = 25.2 μM | Leishmania tropica promastigotes | (-) parasite growth ↑ ROS, ↑ apoptosis ↑ ultrastructure changes ↑ PS externalization | [109] |
| α-Bisabolol | 30 mg/kg, p.o, once daily for 28 days | Canine leishmaniosis naturally infected dogs | ↓ parasite load ↓ antibody titers ↑ IFNγ, ↑ Th1/Th2 immunity | [111] |
| α-Bisabolol | 1%, 2.5%, 5% applied ointment, 200 mg/kg p.o. for 21 days | Inoculated 3 × 107 parasites in the left hind footpad of hamsters | ↓ lesion thickness ↓ parasite load | [112] |
8. α-Bisabolol and Gastroprotection
9. α-Bisabolol and Nephroprotection
10. Anti-Inflammatory Effects of α-Bisabolol
11. The Antioxidant Actions of α-Bisabolol
12. Toxicity Assessment of α-Bisabolol
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silveira e Sá Rde, C.; Andrade, L.N.; de Sousa, D.P. Sesquiterpenes from essential oils and anti-inflammatory activity. Nat. Prod. Commun. 2015, 10, 1767–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; Del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Barreto, R.S.S.; Quintans, J.S.S.; Amarante, R.K.L.; Nascimento, T.S.; Amarante, R.S.; Barreto, A.S.; Pereira, E.W.M.; Duarte, M.C.; Coutinho, H.D.M.; Menezes, I.R.A.; et al. Evidence for the involvement of TNF-α and IL-1β in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl. (Lamiaceae) essential oil and (-)-α-Bisabolol, its main compound, in mice. J. Ethnopharmacol. 2016, 191, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.F.G.; Colares, A.V.; Nonato, C.F.A.; Galvão-Rodrigues, F.F.; Mota, M.L.; Moraes Braga, M.F.B.; Costa, J. In vitro antimicrobial activity of the essential oil from Vanillosmopsis arborea Barker (Asteraceae) and its major constituent, α-Bisabolol. Microb. Pathog. 2018, 125, 144–149. [Google Scholar] [CrossRef]
- Farias, K.S.; Kato, N.N.; Boaretto, A.G.; Weber, J.I.; Brust, F.R.; Alves, F.M.; Tasca, T.; Macedo, A.J.; Silva, D.B.; Carollo, C.A. Nectandra as a renewable source for (+)-α-Bisabolol, an antibiofilm and anti-Trichomonas vaginalis compound. Fitoterapia 2019, 136, 104179. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shah, G.C.; Sharma, R.; Dhyani, P. Chemical composition and antibacterial activity of essential oil of Nepeta graciliflora Benth. (Lamiaceae). Nat. Prod. Res. 2016, 30, 1332–1334. [Google Scholar] [CrossRef]
- Hernández, T.; Canales, M.; Avila, J.G.; García, A.M.; Martínez, A.; Caballero, J.; de Vivar, A.R.; Lira, R. Composition and antibacterial activity of essential oil of Lantana achyranthifolia Desf. (Verbenaceae). J. Ethnopharmacol. 2005, 96, 551–554. [Google Scholar] [CrossRef]
- Masoudi, S.; Rustaiyan, A.; Mohebat, R.; Mosslemin, M.H. Composition of the essential oils and antibacterial activities of Hymenocrater yazdianus, Stachys obtusicrena and Nepeta asterotricha three Labiatae herbs growing wild in Iran. Nat. Prod. Commun. 2012, 7, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Ghaderi, A.; Sonboli, A. Chemical composition and antimicrobial activity of the essential oil of Tanacetum walteri (Anthemideae-Asteraceae) from Iran. Nat. Prod. Res. 2019, 33, 1787–1790. [Google Scholar] [CrossRef]
- Xavier, J.; Alves, N.S.F.; Setzer, W.N.; da Silva, J.K.R. Chemical diversity and biological activities of essential oils from Licaria, Nectrandra and Ocotea Species (Lauraceae) with occurrence in Brazilian biomes. Biomolecules 2020, 10, 869. [Google Scholar] [CrossRef]
- Soltanian, S.; Mohamadi, N.; Rajaei, P.; Khodami, M.; Mohammadi, M. Phytochemical composition, and cytotoxic, antioxidant, and antibacterial activity of the essential oil and methanol extract of Semenovia suffruticosa. Avicenna J. Phytomed. 2019, 9, 143–152. [Google Scholar] [PubMed]
- Merghache, D.; Boucherit-Otmani, Z.; Merghache, S.; Chikhi, I.; Selles, C.; Boucherit, K. Chemical composition, antibacterial, antifungal and antioxidant activities of Algerian Eryngium tricuspidatum L. essential oil. Nat. Prod. Res. 2014, 28, 795–807. [Google Scholar] [CrossRef]
- Kurade, N.P.; Jaitak, V.; Kaul, V.K.; Sharma, O.P. Chemical composition and antibacterial activity of essential oils of Lantana camara, Ageratum houstonianum and Eupatorium adenophorum. Pharm. Biol. 2010, 48, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves Gomes Albertti, L.; Delatte, T.L.; Souza de Farias, K.; Galdi Boaretto, A.; Verstappen, F.; van Houwelingen, A.; Cankar, K.; Carollo, C.A.; Bouwmeester, H.J.; Beekwilder, J. Identification of the Bisabolol synthase in the endangered candeia tree (Eremanthus erythropappus (DC) McLeisch). Front Plant Sci. 2018, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Singtothong, C.; Gagnon, M.J.; Legault, J. Chemical composition and biological activity of the essential oil of Amomum biflorum. Nat. Prod. Commun. 2013, 8, 265–267. [Google Scholar] [CrossRef] [Green Version]
- Alva, M.; Popich, S.; Borkosky, S.; Cartagena, E.; Bardón, A. Bioactivity of the essential oil of an argentine collection of Acanthospermum hispidum (Asteraceae). Nat. Prod. Commun. 2012, 7, 245–248. [Google Scholar] [CrossRef] [Green Version]
- De Moura, N.F.; Simionatto, E.; Porto, C.; Hoelzel, S.C.; Dessoy, E.C.; Zanatta, N.; Morel, A.F. Quinoline alkaloids, coumarins and volatile constituents of Helietta longifoliata. Planta Med. 2002, 68, 631–634. [Google Scholar] [CrossRef]
- Yousefi, M.; Gandomkar, S.; Habibi, Z. Essential oil from aerial parts of of Betonica grandiflora Willd. from Iran. Nat. Prod. Res. 2012, 26, 146–151. [Google Scholar] [CrossRef]
- Boussaada, O.; Ammar, S.; Saidana, D.; Chriaa, J.; Chraif, I.; Daami, M.; Helal, A.N.; Mighri, Z. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol. Res. 2008, 163, 87–95. [Google Scholar] [CrossRef]
- Buitrago, A.; Rojas, J.; Rojas, L.; Velasco, J.; Morales, A.; Peñaloza, Y.; Díaz, C. Essential oil composition and antimicrobial activity of Vismia macrophylla leaves and fruits collected in Táchira-Venezuela. Nat. Prod. Commun. 2015, 10, 375–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benomari, F.Z.; Djabou, N.; Medbouhi, A.; Khadir, A.; Bendahou, M.; Selles, C.; Desjobert, J.M.; Costa, J.; Muselli, A. Chemical variability and biological activities of essential oils of micromeria inodora (Desf.) Benth. from Algeria. Chem. Biodivers. 2016, 13, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Campos Ziegenbein, F.; Hanssen, H.P.; König, W.A. Secondary metabolites from Ganoderma lucidum and Spongiporus leucomallellus. Phytochemistry 2006, 67, 202–211. [Google Scholar] [CrossRef]
- Popović, V.; Petrović, S.; Pavlović, M.; Milenković, M.; Couladis, M.; Tzakou, O.; Duraki, S.; Niketić, M. Essential oil from the underground parts of Laserpitium zernyi: Potential source of alpha-Bisabolol and its antimicrobial activity. Nat. Prod. Commun. 2010, 5, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.L.B.; Silva, R.C.; da Silva, J.K.R.; Suemitsu, C.; Mourão, R.H.V.; Maia, J.G.S. Chemical variability in the essential oil of leaves of Araçá (Psidium guineense Sw.), with occurrence in the Amazon. Chem. Cent. J. 2018, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.L.B.; Batista, H.R.F.; Estevam, E.B.B.; Alves, C.C.F.; Forim, M.R.; Nicolella, H.D.; Furtado, R.A.; Tavares, D.C.; Silva, T.S.; Martins, C.H.G.; et al. Chemical composition and in vitro antibacterial and antiproliferative activities of the essential oil from the leaves of Psidium myrtoides O. Berg (Myrtaceae). Nat. Prod. Res. 2019, 33, 2566–2570. [Google Scholar] [CrossRef]
- Al-Ja’fari, A.H.; Vila, R.; Freixa, B.; Tomi, F.; Casanova, J.; Costa, J.; Cañigueral, S. Composition and antifungal activity of the essential oil from the rhizome and roots of Ferula hermonis. Phytochemistry 2011, 72, 1406–1413. [Google Scholar] [CrossRef]
- Vila, R.; Santana, A.I.; Pérez-Rosés, R.; Valderrama, A.; Castelli, M.V.; Mendonca, S.; Zacchino, S.; Gupta, M.P.; Cañigueral, S. Composition and biological activity of the essential oil from leaves of Plinia cerrocampanensis, a new source of alpha-Bisabolol. Bioresour. Technol. 2010, 101, 2510–2514. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Crockett, S.L.; Başer, K.H.; Wedge, D.E. Chemical composition and antifungal activity of Arnica longifolia, Aster hesperius, and Chrysothamnus nauseosus essential oils. J. Agric. Food Chem. 2007, 55, 8430–8435. [Google Scholar] [CrossRef]
- Tolouee, M.; Alinezhad, S.; Saberi, R.; Eslamifar, A.; Zad, S.J.; Jaimand, K.; Taeb, J.; Rezaee, M.B.; Kawachi, M.; Shams-Ghahfarokhi, M.; et al. Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van Tieghem. Int. J. Food Microbiol. 2010, 139, 127–133. [Google Scholar] [CrossRef]
- Sayyad, R.; Farahmandfar, R. Influence of Teucrium polium L. essential oil on the oxidative stability of canola oil during storage. J. Food Sci. Technol. 2017, 54, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Alkan Türkuçar, S.; Aktaş Karaçelik, A.; Karaköse, M. Phenolic compounds, essential oil composition, and antioxidant activity of Angelica purpurascens (Avé-Lall.) Gill. Turk. J. Chem. 2021, 45, 956–966. [Google Scholar] [CrossRef]
- Niazmand, R.; Razavizadeh, B.M. Ferula asafoetida: Chemical composition, thermal behavior, antioxidant and antimicrobial activities of leaf and gum hydroalcoholic extracts. J. Food Sci. Technol. 2021, 58, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Viljoen, A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol -rich oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Invest. Derm. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Mintie, C.A.; Singh, C.K.; Ahmad, N. Whole fruit phytochemicals combating skin damage and carcinogenesis. Transl. Oncol. 2020, 13, 146–156. [Google Scholar] [CrossRef]
- Solovăstru, L.G.; Stîncanu, A.; De Ascentii, A.; Capparé, G.; Mattana, P.; Vâţă, D. Randomized, controlled study of innovative spray formulation containing ozonated oil and α-Bisabolol in the topical treatment of chronic venous leg ulcers. Adv. Ski. Wound Care 2015, 28, 406–409. [Google Scholar] [CrossRef]
- Licari, A.; Ruffinazzi, G.; M, D.E.F.; Castagnoli, R.; Marseglia, A.; Agostinis, F.; Puviani, M.; Milani, M.; Marseglia, G.L. A starch, glycyrretinic, zinc oxide and Bisabolol based cream in the treatment of chronic mild-to-moderate atopic dermatitis in children: A three-center, assessor blinded trial. Minerva Pediatr. 2017, 69, 470–475. [Google Scholar] [CrossRef]
- Arenberger, P.; Arenbergerová, M.; Drozenová, H.; Hladíková, M.; Holcová, S. Effect of topical heparin and levomenol on atopic dermatitis: A randomized four-arm, placebo-controlled, double-blind clinical study. J. Eur. Acad. Derm. Venereol. 2011, 25, 688–694. [Google Scholar] [CrossRef]
- Crocco, E.I.; Veasey, J.V.; Boin, M.F.; Lellis, R.F.; Alves, R.O. A novel cream formulation containing nicotinamide 4%, arbutin 3%, Bisabolol 1%, and retinaldehyde 0.05% for treatment of epidermal melasma. Cutis 2015, 96, 337–342. [Google Scholar]
- Nemelka, O.; Bleidel, D.; Fabrizi, G.; Camplone, G.; Occella, C.; Marzatico, F.; Pecis, L.; Bocchietto, E. Experimental survey of a new topical anti-oxidant based on furfuryl palmitate in the treatment of child’s and baby’s dermatitis with eczema: Results from a multicenter clinical investigation. Minerva Pediatr. 2002, 54, 465–474. [Google Scholar] [PubMed]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Villegas, L.F.; Marçalo, A.; Martin, J.; Fernández, I.D.; Maldonado, H.; Vaisberg, A.J.; Hammond, G.B. (+)-epi-Alpha-Bisabolol [correction of bisbolol] is the wound-healing principle of Peperomia galioides: Investigation of the in vivo wound-healing activity of related terpenoids. J. Nat. Prod. 2001, 64, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Raymick, J.; Imam, S. Neuroprotective and therapeutic strategies against Parkinson’s Disease: Recent perspectives. Int. J. Mol. Sci. 2016, 17, 904. [Google Scholar] [CrossRef] [Green Version]
- Leite, G.O.; Ecker, A.; Seeger, R.L.; Krum, B.N.; Lugokenski, T.H.; Fachinetto, R.; Sudati, J.H.; Barbosa, N.V.; Wagner, C. Protective effect of (-)-α-Bisabolol on rotenone-induced toxicity in Drosophila melanogaster. Can. J. Physiol. Pharm. 2018, 96, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Javed, H.; Meeran, M.F.N.; Azimullah, S.; Bader Eddin, L.; Dwivedi, V.D.; Jha, N.K.; Ojha, S. α-Bisabolol, a dietary bioactive phytochemical attenuates dopaminergic neurodegeneration through modulation of oxidative stress, neuroinflammation and apoptosis in rotenone-induced rat model of Parkinson’s disease. Biomolecules 2020, 10, 1421. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Shanmuganathan, B.; Sathya, S.; Balasubramaniam, B.; Balamurugan, K.; Devi, K.P. Amyloid-β induced neuropathological actions are suppressed by Padina gymnospora (Phaeophyceae) and its active constituent α-Bisabolol in Neuro2a cells and transgenic Caenorhabditis elegans Alzheimer’s model. Nitric. Oxide 2019, 91, 52–66. [Google Scholar] [CrossRef]
- Shanmuganathan, B.; Suryanarayanan, V.; Sathya, S.; Narenkumar, M.; Singh, S.K.; Ruckmani, K.; Pandima Devi, K. Anti-amyloidogenic and anti-apoptotic effect of α-Bisabolol against Aβ induced neurotoxicity in PC12 cells. Eur. J. Med. Chem. 2018, 143, 1196–1207. [Google Scholar] [CrossRef]
- Sathya, S.; Shanmuganathan, B.; Devi, K.P. Deciphering the anti-apoptotic potential of α-Bisabolol loaded solid lipid nanoparticles against Aβ induced neurotoxicity in Neuro-2a cells. Colloids Surf. B Biointerfaces 2020, 190, 110948. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, M.; Sathya, S.; Gandhi, S.; Tharra, P.; Suryanarayanan, V.; Singh, S.K.; Baire, B.; Pandima Devi, K. α-Bisabolol β-D-fucopyranoside as a potential modulator of β-amyloid peptide induced neurotoxicity: An in vitro &in silico study. Bioorg. Chem. 2019, 88, 102935. [Google Scholar] [CrossRef] [PubMed]
- Nour, M.; Scalzo, F.; Liebeskind, D.S. Ischemia-reperfusion injury in stroke. Interv. Neurol. 2012, 1, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, M.Y.D.; Carmo, M.; Fonteles, A.A.; Neves, J.C.S.; Silva, A.; Pereira, J.F.; Ferreira, E.O.; Lima, N.M.R.; Neves, K.R.T.; Andrade, G.M. (-)-α-Bisabolol prevents neuronal damage and memory deficits through reduction of proinflammatory markers induced by permanent focal cerebral ischemia in mice. Eur. J. Pharm. 2019, 842, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Ok, C.; Woda, B.; Kurian, E. The Pathology of Cancer; University of Massachusetts Medical School: Worcester, MA, USA, 2018. [Google Scholar]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Darra, E.; Abdel-Azeim, S.; Manara, A.; Shoji, K.; Maréchal, J.-D.; Mariotto, S.; Cavalieri, E.; Perbellini, L.; Pizza, C.; Perahia, D.; et al. Insight into the apoptosis-inducing action of α-Bisabolol towards malignant tumor cells: Involvement of lipid rafts and Bid. Arch. Biochem. Biophys. 2008, 476, 113–123. [Google Scholar] [CrossRef]
- Passarello, K.; Kurian, S.; Villanueva, V. Endometrial cancer: An overview of pathophysiology, management, and care. Semin. Oncol. Nurs. 2019, 35, 157–165. [Google Scholar] [CrossRef]
- Fang, D.; Wang, H.; Li, M.; Wei, W. α-Bisabolol enhances radiotherapy-induced apoptosis in endometrial cancer cells by reducing the effect of XIAP on inhibiting caspase-3. Biosci. Rep. 2019, 39, BSR20190696. [Google Scholar] [CrossRef] [Green Version]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Mendes, F.B.; Bergamin, L.S.; Dos Santos Stuepp, C.; Braganhol, E.; Terroso, T.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M. Alpha-bisabolol promotes glioma cell death by modulating the adenosinergic system. Anticancer Res. 2017, 37, 1819–1823. [Google Scholar] [CrossRef]
- Cavalieri, E.; Mariotto, S.; Fabrizi, C.; de Prati, A.C.; Gottardo, R.; Leone, S.; Berra, L.V.; Lauro, G.M.; Ciampa, A.R.; Suzuki, H. alpha-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem. Biophys. Res. Commun. 2004, 315, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Aboulthana, W.; Sahu, D.R. Hepatocellular carcinoma: Causes and prevention. UK J. Pharm. Biosci. 2018, 6, 48. [Google Scholar] [CrossRef]
- Chen, W.; Hou, J.; Yin, Y.; Jang, J.; Zheng, Z.; Fan, H.; Zou, G. alpha-Bisabolol induces dose- and time-dependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFkappaB. Biochem. Pharm. 2010, 80, 247–254. [Google Scholar] [CrossRef]
- Akbulut, H.; İncedayı, S.; Atasoy, Ö. Non-small cell lung cancer and its treatment. Demiroglu Sci. Univ. Florence Nightingale Transplant. J. 2020, 4, 23–40. [Google Scholar] [CrossRef]
- Wu, S.; Peng, L.; Sang, H.; Ping Li, Q.; Cheng, S. Anticancer effects of α-Bisabolol in human non-small cell lung carcinoma cells are mediated via apoptosis induction, cell cycle arrest, inhibition of cell migration and invasion and upregulation of P13K/AKT signalling pathway. J. Buon 2018, 23, 1407–1412. [Google Scholar] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; Vecchia, C.; Johnson, C.; Biankin, A.; Neale, R.; Tempero, M.; Tuveson, D.; Hruban, R.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, S.S.; Saito, K.; Williams, S.; Arimura, Y.; Ma, Y.; Ke, Y.; Baron, V.; Mercola, D.; Feng, G.-S.; et al. PTEN regulation by Akt-EGR1-ARF-PTEN axis. EMBO J. 2009, 28, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Seki, T.; Kokuryo, T.; Yokoyama, Y.; Suzuki, H.; Itatsu, K.; Nakagawa, A.; Mizutani, T.; Miyake, T.; Uno, M.; Yamauchi, K.; et al. Antitumor effects of α-Bisabolol against pancreatic cancer. Cancer Sci. 2011, 102, 2199–2205. [Google Scholar] [CrossRef]
- Uno, M.; Kokuryo, T.; Yokoyama, Y.; Senga, T.; Nagino, M. α-bisabolol inhibits invasiveness and motility in pancreatic cancer through KISS1R activation. Anticancer Res. 2016, 36, 583–589. [Google Scholar]
- Blundell, R. Acute Leukaemia. Int. J. Mol. Med. Adv. Sci. 2007, 3, 1380144. [Google Scholar]
- Cavalieri, E.; Rigo, A.; Bonifacio, M.; Carcereri de Prati, A.; Guardalben, E.; Bergamini, C.; Fato, R.; Pizzolo, G.; Suzuki, H.; Vinante, F. Pro-apoptotic activity of α-Bisabolol in preclinical models of primary human acute leukemia cells. J. Transl. Med. 2011, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costarelli, L.; Malavolta, M.; Giacconi, R.; Cipriano, C.; Gasparini, N.; Tesei, S.; Pierpaoli, S.; Orlando, F.; Suzuki, H.; Perbellini, L.; et al. In vivo effect of alpha-Bisabolol, a nontoxic sesquiterpene alcohol, on the induction of spontaneous mammary tumors in HER-2/neu transgenic mice. Oncol. Res. 2010, 18, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Kokuryo, T.; Yokoyama, Y.; Yamaguchi, J.; Miwa, T.; Shibuya, M.; Yamamoto, Y.; Nagino, M. The anticancer effects of novel α-bisabolol derivatives against pancreatic cancer. Anticancer Res. 2017, 37, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Piochon, M.; Legault, J.; Gauthier, C.; Pichette, A. Synthesis and cytotoxicity evaluation of natural alpha-Bisabolol beta-D-fucopyranoside and analogues. Phytochemistry 2009, 70, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.; Martini, M.V.; de Oliveira, C.M.; Cunha, S.; de Carvalho, J.E.; Ruiz, A.L.; da Silva, C.C. Antitumor activity of (-)-alpha-Bisabolol-based thiosemicarbazones against human tumor cell lines. Eur. J. Med. Chem. 2010, 45, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Smith, E. Advances in understanding nociception and neuropathic pain. J. Neurol. 2018, 265, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, L.T.; Panchalingam, V.; Cherkas, P.; Campos, A.R.; Avivi-Arber, L.; Sessle, B.J. (-)-α-Bisabolol reduces nociception and trigeminal central sensitisation in acute orofacial neuropathic pain induced by infraorbital nerve injury. Life Sci. 2019, 227, 122–128. [Google Scholar] [CrossRef]
- Fontinele, L.L.; Heimfarth, L.; Pereira, E.W.M.; Rezende, M.M.; Lima, N.T.; Barbosa Gomes de Carvalho, Y.M.; Afonso de Moura Pires, E.; Guimarães, A.G.; Bezerra Carvalho, M.T.; de Souza Siqueira Barreto, R.; et al. Anti-hyperalgesic effect of (-)-α-Bisabolol and (-)-α-Bisabolol /β-Cyclodextrin complex in a chronic inflammatory pain model is associated with reduced reactive gliosis and cytokine modulation. Neurochem. Int. 2019, 131, 104530. [Google Scholar] [CrossRef]
- Melo, L.T.; Duailibe, M.A.; Pessoa, L.M.; da Costa, F.N.; Vieira-Neto, A.E.; de Vasconcellos Abdon, A.P.; Campos, A.R. (-)-α-Bisabolol reduces orofacial nociceptive behavior in rodents. Naunyn. Schmiedebergs Arch. Pharm. 2017, 390, 187–195. [Google Scholar] [CrossRef]
- Leite Gde, O.; Fernandes, C.N.; de Menezes, I.R.; da Costa, J.G.; Campos, A.R. Attenuation of visceral nociception by α-Bisabolol in mice: Investigation of mechanisms. Org. Med. Chem. Lett. 2012, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Alves Ade, M.; Gonçalves, J.C.; Cruz, J.S.; Araújo, D.A. Evaluation of the sesquiterpene (-)-alpha-Bisabolol as a novel peripheral nervous blocker. Neurosci. Lett. 2010, 472, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.F.; Rios, E.R.; Carvalho, A.M.; Cerqueira, G.S.; Lopes Ade, A.; Leal, L.K.; Dias, M.L.; de Sousa, D.P.; de Sousa, F.C. Anti-nociceptive and anti-inflammatory activities of (-)-α-Bisabolol in rodents. Naunyn. Schmiedebergs Arch. Pharm. 2011, 384, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Leite Gde, O.; Leite, L.H.; Sampaio Rde, S.; Araruna, M.K.; de Menezes, I.R.; da Costa, J.G.; Campos, A.R. (-)-α-Bisabolol attenuates visceral nociception and inflammation in mice. Fitoterapia 2011, 82, 208–211. [Google Scholar] [CrossRef]
- Teixeira, G.F.; Costa, F.N.; Campos, A.R. Corneal antinociceptive effect of (-)-α-Bisabolol. Pharm. Biol. 2017, 55, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I.; Cariño-Cortés, R.; Ponce-Monter, H.A.; Castañeda-Hernández, G.; Chávez-Piña, A.E. Pharmacological interaction of α-Bisabolol and diclofenac on nociception, inflammation, and gastric integrity in rats. Drug Dev. Res. 2018, 79, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.F.D.; Vieira-Neto, A.E.; da Costa, F.N.; ARA, E.S.; Campos, A.R. Antinociceptive effect of (-)-α-Bisabolol in nanocapsules. Biomed. Pharm. 2017, 91, 946–950. [Google Scholar] [CrossRef]
- Amora-Silva, B.F.; Ribeiro, S.C.; Vieira, C.L.; Mendes, F.R.; Vieira-Neto, A.E.; Abdon, A.P.V.; Costa, F.N.; Campos, A.R. Clinical efficacy of new α-Bisabolol mouthwashes in postoperative complications of maxillofacial surgeries: A randomized, controlled, triple-blind clinical trial. Clin. Oral Investig. 2019, 23, 577–584. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef]
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial infarction: Symptoms and treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Azimullah, S.; Laham, F.; Tariq, S.; Goyal, S.N.; Adeghate, E.; Ojha, S. α-Bisabolol protects against β-adrenergic agonist-induced myocardial infarction in rats by attenuating inflammation, lysosomal dysfunction, NLRP3 inflammasome activation and modulating autophagic flux. Food Funct. 2020, 11, 965–976. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Laham, F.; Azimullah, S.; Tariq, S.; Ojha, S. α-Bisabolol abrogates isoproterenol-induced myocardial infarction by inhibiting mitochondrial dysfunction and intrinsic pathway of apoptosis in rats. Mol. Cell Biochem. 2019, 453, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Laham, F.; Al-Taee, H.; Azimullah, S.; Ojha, S. Protective effects of α-Bisabolol on altered hemodynamics, lipid peroxidation, and nonenzymatic antioxidants in isoproterenol-induced myocardial infarction: In vivo and in vitro evidences. J. Biochem. Mol. Toxicol. 2018, 32, e22200. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, A.; Sakhteman, A.; Moheimani, S.M. An in silico approach towards investigation of possible effects of essential oils constituents on receptors involved in cardiovascular diseases (CVD) and associated risk factors (Diabetes Mellitus and Hyperlipidemia). Cardiovasc. Hematol. Agents Med. Chem. 2021, 19, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Amerikova, M.; Pencheva, I.; Maslarska, V.; Bozhanov, S.; Tachkov, K. Antimicrobial activity, mechanism of action, and methods for stabilisation of defensins as new therapeutic agents. Biotechnol. Biotechnol. Equip. 2019, 33, 671–682. [Google Scholar] [CrossRef]
- Amenu, D. Antimicrobial activity of medicinal plant extracts and their synergistic effect on some selected pathogens. Am. J. Ethnomed. 2014, 1, 18–29. [Google Scholar]
- Oliveira, F.S.; Freitas, T.S.; Cruz, R.P.D.; Costa, M.D.S.; Pereira, R.L.S.; Quintans-Júnior, L.J.; Andrade, T.A.; Menezes, P.D.P.; Sousa, B.M.H.; Nunes, P.S.; et al. Evaluation of the antibacterial and modulatory potential of α-Bisabolol, β-cyclodextrin and α-Bisabolol /β-cyclodextrin complex. Biomed. Pharm. 2017, 92, 1111–1118. [Google Scholar] [CrossRef]
- Pereira da Cruz, R.; Sampaio de Freitas, T.; Socorro Costa, M.d.; Lucas Dos Santos, A.T.; Ferreira Campina, F.; Pereira, R.L.S.; Bezerra, J.W.A.; Quintans-Júnior, L.J.; De Souza Araújo, A.A.; Júnior, J.P.D.S.; et al. Effect of α-Bisabolol and its β-cyclodextrin complex as TetK and NorA efflux pump inhibitors in staphylococcus aureus strains. Antibiotics 2020, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Forrer, M.; Kulik, E.M.; Filippi, A.; Waltimo, T. The antimicrobial activity of alpha-Bisabolol and tea tree oil against Solobacterium moorei, a Gram-positive bacterium associated with halitosis. Arch. Oral. Biol. 2013, 58, 10–16. [Google Scholar] [CrossRef]
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, Bisabolol, and apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef] [Green Version]
- Elgharbawy, A.; Samsudin, N.; Hashim, Y.; Salleh, H.; Santhanam, J.; Ben belgacem, F. Phytochemicals with antifungal properties: Cure from nature. Int. J. Eng. Computat. 2020, 16, 323–345. [Google Scholar] [CrossRef]
- Jahanshiri, Z.; Shams-Ghahfarokhi, M.; Asghari-Paskiabi, F.; Saghiri, R.; Razzaghi-Abyaneh, M. α-Bisabolol inhibits Aspergillus fumigatus Af239 growth via affecting microsomal ∆(24)-sterol methyltransferase as a crucial enzyme in ergosterol biosynthesis pathway. World J. Microbiol. Biotechnol. 2017, 33, 55. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Baldisserotto, A.; Malisardi, G.; Vicentini, C.B.; Mares, D.; Andreotti, E.; Vertuani, S.; Manfredini, S. A multi-target approach toward the development of novel candidates for antidermatophytic activity: Ultrastructural evidence on α-bisabolol -treated microsporum gypseum. Molecules 2015, 20, 11765–11776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Medeiros, C.A.C.; Pinto, Â.V.; de Oliveira, J.C.; Silva, G.S.; Arrua, J.M.M.; Lima, I.O.; Pereira, F.O. Evaluating the antifungal activity of α-bisabolol in association with NaCl on Fusarium oxysporum in maize grains. Curr. Microbiol. 2021, 78, 604–610. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, R.; Sampaio, T.L.; Lima, D.B.; Sousa, P.L.; de Azevedo, I.E.P.; Magalhães, E.P.; Tessarolo, L.D.; Marinho, M.M.; Dos Santos, R.P.; Martins, A.M.C. Antiparasitic effect of (-)-α-Bisabolol against Trypanosoma cruzi Y strain forms. Diagn. Microbiol. Infect. Dis. 2019, 95, 114860. [Google Scholar] [CrossRef] [PubMed]
- Morales-Yuste, M.; Morillas-Márquez, F.; Martín-Sánchez, J.; Valero-López, A.; Navarro-Moll, M.C. Activity of (-)alpha-Bisabolol against Leishmania infantum promastigotes. Phytomedicine 2010, 17, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Hajaji, S.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Jiménez, I.A.; Bazzocchi, I.L.; Valladares, B.; Akkari, H.; Lorenzo-Morales, J.; Piñero, J.E. Leishmanicidal activity of α-Bisabolol from Tunisian chamomile essential oil. Parasitol. Res. 2018, 117, 2855–2867. [Google Scholar] [CrossRef]
- Rottini, M.M.; Amaral, A.C.; Ferreira, J.L.; Silva, J.R.; Taniwaki, N.N.; Souza Cda, S.; d’Escoffier, L.N.; Almeida-Souza, F.; Hardoim Dde, J.; Gonçalves da Costa, S.C.; et al. In vitro evaluation of (-)α-Bisabolol as a promising agent against Leishmania amazonensis. Exp. Parasitol. 2015, 148, 66–72. [Google Scholar] [CrossRef]
- Corpas-López, V.; Merino-Espinosa, G.; Díaz-Sáez, V.; Morillas-Márquez, F.; Navarro-Moll, M.C.; Martín-Sánchez, J. The sesquiterpene (-)-α-Bisabolol is active against the causative agents of Old World cutaneous leishmaniasis through the induction of mitochondrial-dependent apoptosis. Apoptosis 2016, 21, 1071–1081. [Google Scholar] [CrossRef]
- Corpas-López, V.; Morillas-Márquez, F.; Navarro-Moll, M.C.; Merino-Espinosa, G.; Díaz-Sáez, V.; Martín-Sánchez, J. (-)-α-Bisabolol, a Promising oral compound for the treatment of visceral leishmaniasis. J. Nat. Prod. 2015, 78, 1202–1207. [Google Scholar] [CrossRef]
- Corpas-López, V.; Merino-Espinosa, G.; Acedo-Sánchez, C.; Díaz-Sáez, V.; Navarro-Moll, M.C.; Morillas-Márquez, F.; Martín-Sánchez, J. Effectiveness of the sesquiterpene (-)-α-Bisabolol in dogs with naturally acquired canine leishmaniosis: An exploratory clinical trial. Vet. Res. Commun. 2018, 42, 121–130. [Google Scholar] [CrossRef]
- Corpas-López, V.; Merino-Espinosa, G.; López-Viota, M.; Gijón-Robles, P.; Morillas-Mancilla, M.J.; López-Viota, J.; Díaz-Sáez, V.; Morillas-Márquez, F.; Navarro Moll, M.C.; Martín-Sánchez, J. Topical treatment of Leishmania tropica Infection using (-)-α-Bisabolol ointment in a hamster model: Effectiveness and safety assessment. J. Nat. Prod. 2016, 79, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- Fornai, M.; Antonioli, L.; Colucci, R.; Tuccori, M.; Blandizzi, C. Pathophysiology of gastric ulcer development and healing: Molecular mechanisms and novel therapeutic options. In Peptic Ulcer Disease; IntechOpen: London, UK, 2011. [Google Scholar]
- Bezerra, S.B.; Leal, L.K.; Nogueira, N.A.; Campos, A.R. Bisabolol-induced gastroprotection against acute gastric lesions: Role of prostaglandins, nitric oxide, and KATP+ channels. J. Med. Food 2009, 12, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Moura Rocha, N.F.; Venâncio, E.T.; Moura, B.A.; Gomes Silva, M.I.; Aquino Neto, M.R.; Vasconcelos Rios, E.R.; de Sousa, D.P.; Mendes Vasconcelos, S.M.; de França Fonteles, M.M.; de Sousa, F.C. Gastroprotection of (-)-alpha-Bisabolol on acute gastric mucosal lesions in mice: The possible involved pharmacological mechanisms. Fundam. Clin. Pharm. 2010, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.F.; Oliveira, G.V.; Araújo, F.Y.; Rios, E.R.; Carvalho, A.M.; Vasconcelos, L.F.; Macêdo, D.S.; Soares, P.M.; Sousa, D.P.; Sousa, F.C. (-)-α-Bisabolol -induced gastroprotection is associated with reduction in lipid peroxidation, superoxide dismutase activity and neutrophil migration. Eur. J. Pharm. Sci. 2011, 44, 455–461. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-e-Silva, O. Ischemia/Reperfusion injury revisited: An overview of the latest pharmacological strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, T.L.; Menezes, R.R.; da Costa, M.F.; Meneses, G.C.; Arrieta, M.C.; Chaves Filho, A.J.; de Morais, G.B.; Libório, A.B.; Alves, R.S.; Evangelista, J.S.; et al. Nephroprotective effects of (-)-α-Bisabolol against ischemic-reperfusion acute kidney injury. Phytomedicine 2016, 23, 1843–1852. [Google Scholar] [CrossRef]
- Sampaio, T.L.; Menezes, R.; Lima, D.B.; Costa Silva, R.A.; de Azevedo, I.E.P.; Magalhães, E.P.; Marinho, M.M.; Dos Santos, R.P.; Martins, A.M.C. Involvement of NADPH-oxidase enzyme in the nephroprotective effect of (-)-α-Bisabolol on HK2 cells exposed to ischemia - reoxygenation. Eur. J. Pharmacol. 2019, 855, 1–9. [Google Scholar] [CrossRef]
- Roe, K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef]
- Xu, C.; Sheng, S.; Dou, H.; Chen, J.; Zhou, K.; Lin, Y.; Yang, H. α-Bisabolol suppresses the inflammatory response and ECM catabolism in advanced glycation end products-treated chondrocytes and attenuates murine osteoarthritis. Int. Immunopharmacol. 2020, 84, 106530. [Google Scholar] [CrossRef]
- D’Almeida, A.P.L.; Pacheco de Oliveira, M.T.; de Souza, É.T.; de Sá Coutinho, D.; Ciambarella, B.T.; Gomes, C.R.; Terroso, T.; Guterres, S.S.; Pohlmann, A.R.; Silva, P.M.; et al. α-Bisabolol-loaded lipid-core nanocapsules reduce lipopolysaccharide-induced pulmonary inflammation in mice. Int. J. Nanomed. 2017, 12, 4479–4491. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Pérez, V.M.; Ortiz, M.I.; Ponce-Monter, H.A.; Monter-Pérez, V.; Barragán-Ramírez, G. Anti-inflammatory and utero-relaxant effect of α-Bisabolol on the pregnant human uterus. Korean J. Physiol. Pharm. 2018, 22, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, H.A.O.; Silva-Filho, S.E.; Wiirzler, L.A.M.; Cardia, G.F.E.; Uchida, N.S.; Silva-Comar, F.M.S.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of (-)-α-Bisabolol on the inflammatory response in systemic infection experimental model in C57BL/6 mice. Inflammation 2020, 43, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, E.; Kim, J.H.; Park, Y.H.; Lee, J.; Park, D. Inhibitory effects of (-)-α-Bisabolol on LPS-induced inflammatory response in RAW264.7 macrophages. Food Chem. Toxicol. 2011, 49, 2580–2585. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Singh, M.; Dubey, V.; Srivastava, S.; Luqman, S.; Bawankule, D.U. α-(-)-Bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr. Pharm. Biotechnol. 2014, 15, 173–181. [Google Scholar] [CrossRef]
- Jat, D.; Nahar, M. Oxidative stress and antioxidants: An overview. IJARR Int. J. Adv. Res. Rev. 2017, 2, 110–119. [Google Scholar]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Dal Sasso, M.; Fonti, E.; Culici, M. Antioxidant activity of Bisabolol: Inhibitory effects on chemiluminescence of human neutrophil bursts and cell-free systems. Pharmacology 2009, 83, 110–115. [Google Scholar] [CrossRef]
- Ren, G.; Xue, P.; Sun, X.; Zhao, G. Determination of the volatile and polyphenol constituents and the antimicrobial, antioxidant, and tyrosinase inhibitory activities of the bioactive compounds from the by-product of Rosa rugosa Thunb. var. plena Regal tea. BMC Complement Altern. Med. 2018, 18, 307. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.; Singh, K.; Khedkar, R. 11—Phytochemicals: Extraction process, safety assessment, toxicological evaluations, and regulatory issues. In Functional and Preservative Properties of Phytochemicals; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 341–361. [Google Scholar]
- Api, A.M.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; et al. RIFM fragrance ingredient safety assessment, α-Bisabolol, CAS registry number 515-69-5. Food Chem. Toxicol. 2020, 141, 111238. [Google Scholar] [CrossRef]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on alpha-Bisabolol. Food Chem. Toxicol. 2008, 46, S72–S76. [Google Scholar] [CrossRef]
- Gomes-Carneiro, M.R.; Dias, D.M.; De-Oliveira, A.C.; Paumgartten, F.J. Evaluation of mutagenic and antimutagenic activities of alpha-Bisabolol in the Salmonella/microsome assay. Mutat. Res. 2005, 585, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ceruelos, A.; Sánchez-Gutiérrez, M.; Mojica-Villegas, M.; Chamorro, G. Chemoprotection of fertility by chamomile essential oil over the toxic effect of. Toxicol. Lett. 2007, 172, S185–S186. [Google Scholar] [CrossRef]


| Plants | Percentage Occurrence | Reference |
|---|---|---|
| Stachys lavandulifolia Vahl. (Lamiaceae) | 56.4 | [4] |
| Vanillosmopsis arborea Barker (Asteraceae) | 91.02 | [5] |
| Nectandra megapotamicav (Spreng.) Mez. (Lauraceae) | 93.7 | [6] |
| Nepeta graciliflora Benth. (Lamiaceae) | 8.97 | [7] |
| Lantana achyranthifolia Desf. (Verbenaceae) | 11.23 | [8] |
| Hymenocrater yazdianus, Stachys obtusicrena Boiss, and Nepeta asterotricha | 23.5% | [9] |
| Tanacetum walteri (Anthemideae-Asteraceae) | 6.3 | [10] |
| Licaria, Nectrandra and Ocotea Species (Lauraceae) | 59.7–93.7 | [11] |
| Semenovia suffruticosa | 13.3 | [12] |
| Genus Matricaria | 29–81 | [2] |
| Algerian Eryngium tricuspidatum L. | 32.6 | [13] |
| Eupatorium adenophorum | 9.53 | [14] |
| Candeia Tree (Eremanthus erythropappus (DC) McLeisch) | 66–91 | [15] |
| Amomum biflorum | 16.0 | [16] |
| Acanthospermum hispidum (Asteraceae) | 11.4 | [17] |
| Helietta longifoliata | 7.24 | [18] |
| Betonica grandiflora Willd. | 4.9 | [19] |
| Rhaponticum acaule DC | 4.8 | [20] |
| Vismia macrophylla | 14.9 | [21] |
| Micromeria inodora (Desf.) Benth. | 2.9 | [22] |
| Ganoderma lucidum and Spongiporus leucomallellus | 2 | [23] |
| Laserpitium zernyi | 30.9 | [24] |
| Araçá (Psidium guineense Sw.) | 6.5–18.1 | [25] |
| Psidium myrtoides O. Berg (Myrtaceae) | 5.3 | [26] |
| Ferula hermonis Boiss | 11.1 | [27] |
| Plinia cerrocampanensis | 42.8 | [28] |
| Arnica longifolia, Aster hesperius, and Chrysothamnus nauseosus | 8.2 | [29] |
| Matricaria chamomilla L. | 56.86 | [30] |
| Teucrium polium L. | 24.6 | [31] |
| Angelica purpurascens (Avé-Lall.) Gill | 22.93 | [32] |
| Ferula asafoetida | 9.75 | [33] |
| PubChem CID | 1549992 |
| Molecular Formula | C15H26O |
| Synonyms | (+)-α-Bisabolol, D-α-Bisabolol, (2R)-6-Methyl-2-(4-methyl-3-cyclohexenyl)-5-heptene-2-ol, Dragosantol, Camilol, Hydagen B, (+)-6R,7R-α-Bisabolol, |
| Molecular Weight | 222.37 |
| XLogP3-AA | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | Rotatable Bond Count |
| Exact Mass | 222.198365449 |
| Monoisotopic Mass | 222.198365449 |
| Topological Polar Surface Area | 20.2 Å2 |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 284 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Solubility | 1.688 mg/L @ 25 °C (est), Practically insoluble or insoluble in water, slightly soluble in ethanol |
| Density | 0.922–0.931 |
| LogP | 5.070 (est) |
| Refractive Index | 1.491–1.500 |
| Food additive class | Flavoring agent |
| Skin Disorders | ||||
|---|---|---|---|---|
| Compound | Dose/Route/ Duration | Model | Major Mechanisms | Reference |
| α-Bisabolol | 1% topical spray | Randomized controlled trial on chronic venous leg ulcer patients | ↓ wound and ulcer surface area ↑ healing speed | [37] |
| α-Bisabolol | 0.3 g/100 g cream twice daily for 8 weeks | Prospective, randomized, reference-controlled, double-blind, two-center and four-armed parallel group study on patients with atopic dermatitis | ↓ pruritis, inflammation ↑ healing | [39] |
| α-Bisabolol | 1% cream once-daily for 30 days | Single-center, single-arm, prospective, open-label study on patients with melasma | ↓ melasma area and severity index (MASI) score ↑ patient satisfaction | [40] |
| Neuroprotective | ||||
|---|---|---|---|---|
| Compound | Dose/Route/ Duration | Model | Major Mechanisms | Reference |
| α-Bisabolol | 5, 25, and 250 μmol/L for 7 days | Rotenone (500 μmol/L) induced neurotoxicity in Drosophila | ↓ mortality and motor deficits, ↓ thiol level ↑ SOD, CAT and Keap1 | [46] |
| α-Bisabolol | 50 mg/kg i.p, 30 min before rotenone for 4 weeks | Rotenone (2.5 mg/kg) induced Parkinson’s disease | ⇥ neuronal loss, ↓ MDA, ↑ GSH, SOD and CAT, ↓ glial activation, ↓ IL-1β, IL-6, TNF-α, iNOS and COX-2, ↑ Bcl-2, ↓ Bax, caspases-3, 9 and cytochrome-C, restored ATP and MC-I activity | [47] |
| α-Bisabolol | 5, 10 μg/mL for 2 h in N2a cells and 25, 50 and 100 μg/mL in elegans | Aβ25–35 peptide (50 μM for 24 h) induced toxicity in N2a cells and Caenorhabditis elegans CL4176 and CL2006 | ⇥ cholinesterase and β-secretase, ↓ ROS and RNS ↓ Bax and caspase-3 ↓ ace-1, hsp-4 and Aβ genes | [49] |
| α-Bisabolol | 5 mg/mL for 2 h | Aβ25–35 peptide (50 μM for 24 h) induced toxicity in PC12 cells | ↓ Aβ aggregation ↑ cell survival | [50] |
| α-Bisabolol | 5 and 10 μg/mL for 2 h | Aβ25–35 peptide (50 μM for 24 h) induced toxicity in Neuro-2a cells | ↓ ROS and RNS, ↓ β-secretase and AchE activities, ↓ Bax, caspase3, and ↑ Bcl-2 | [51] |
| α-Bisabolol β-D-fucopyranoside | 10–50 μg/mL | Aβ25–35 (100 μM for 24 h, 48 h, 96 h, 9 d) induced toxicity in Neuro 2a cells | Inhibited AChE, ↓ H2O2 and OH•, ↓ Aβ aggregation ↑ cell survival | [52] |
| α-Bisabolol | 50, 100 and 200 mg/kg/day, p.o | Permanent occlusion of the middle cerebral artery induced cerebral ischemia in mice | ↓ infarct size, ↑ motor performance, ↑ crossings and rearings | [54] |
| Anticancer Effects | ||||
|---|---|---|---|---|
| Compound | Dose/Route/Duration | Model | Major Mechanisms | Reference |
| α-Bisabolol | 0 to 32 μmol/L for 24 h | EC cell lines including RL95-2, ECC001 and ECC003 cells | (-) EC cells growth ↑ caspase-3, ↑ PARP ↓ XIAP, COX-2 ↑ radiotherapy effect | [59] |
| α-Bisabolol | 35, 45 or 55 μM for C6 glioma cells and 55, 65 or 75 μM for U138-MG | U138-MG human and C6 rat glioma cell lines | ↓ cell viability ↑ ecto5′-NT/CD73 | [61] |
| α-Bisabolol | 100 and 250 μM | Human and rat glioma cell lines | ↓ cell viability ↑ Cytochrome-C | [62] |
| α-Bisabolol | 1 mM | Human prostate cancer cell line PC-3, human cervical carcinoma cell line Hela, human esophageal ECA-109, and human liver carcinoma cell line HepG2 | ↑ caspases 3, 8 and 9 ↑ cytochrome-C ↑ Bax, Bid, ↓ Bak and Bcl-2, p53, ↑ NF-κB and Fas | [64] |
| α-Bisabolol | 0 t0 100 μM for 24 h (IC50 = 15 µM) | NSCLC cell line A549 | (-) migration of A549 cells, (-) PI3K/AKT, ↑ apoptotic cells ↑ Bax, ↓ Bcl-2, triggers G2/M cell cycle arrest | [66] |
| α-Bisabolol | 0–250 μM 1000 mg/kg, (21–27 mg/mouse) once a week for 3 weeks | KLM1, Panc1, MIA Paca2 and KP4 human pancreatic cancer cell lines, BALB ⁄ c nude mice xenograft model inoculated with KLM1 and KP4 cells (1 × 107 s.c.) in femoral area | ↓ cells viability, ↑ apoptosis, (-) AKT, ↑ EGR1, ↓ tumor volume and weight | [69] |
| α-Bisabolol | 1.56 μM | KLM1, KP4 and Panc1 human pancreatic cancer cell lines | (-) motility of cells ↑ KISS1R, MTSS1 and TIMP2 | [70] |
| α-Bisabolol | 0, 3, 15, 30, 60, 125, 250 μM | CML-T1primary human acute leukemia cell line | ↓ cells viability, induced apoptosis | [72] |
| α-Bisabolol | 300 µL intra-mammary injection (3.6 mg and 10 mg per mouse) | HER2/neu transgenic mice | ↓ tumor mass ↓ HER2/neu, Fgf and Birc5 ↑ natural killer cytotoxicity | [73] |
| α-Bisabolol and its derivative | 62.5 μM and 125 μM, 1000 mg/kg | KLM1 and Panc1 human pancreatic cancer cell lines, BALB/c nude mice implanted with KLM1; cells (1 × 107 cells/100 μL, s.c.) into femoral area | ↑ cell death ↓ volume of tumor ↓ CEA and CA19-9, ↓ disseminated tumorous nodules ⇥ AKT | [74] |
| α-Bisabolol β-D-fucopyranoside | IC50 > 100 μM | human lung carcinoma (A549), colon adeno-carcinoma (DLD-1), breast adeno-carcinoma (MCF-7), melanoma (SK-MEL-2), ovary teratocarcinoma (PA-1), prostate adeno-carcinoma (PC-3), pancreas adeno-carcinoma (PANC 05.04), glioma (U-251), glioblastoma (U-87) and murine glioma (GL-261) | ↑ α-Bisabolol cytotoxicity ↑ BBB penetration ↑ α-Bisabolol lipophilicity | [75] |
| α-Bisabolol-based thiosemicarbazones compounds | 0.25 to 250 mg/mL | Melanoma UACC-62, breast MCF-7, breast resistant NCI-ADR, lung NCI-460, leukemia K-562, ovarian OVCAR, prostate PCO-3, and colon HT29 cell lines | (-) cell growth | [76] |
| Antinociceptive Effects | ||||
|---|---|---|---|---|
| Compound | Dose/Route/ Duration | Model | Major Mechanisms | Reference |
| α-Bisabolol | 200 mg/kg p.o | IONX-induced acute orofacial neuropathic pain in rats | ↓ mechanical hypersensitivity ↑ pain threshold | [78] |
| α-Bisabolol | 50 mg/kg, p.o | FCA (25 μL, i.p.) and PLSN induced pain in mice | ↓ mechanical and thermal hyperalgesia ↓ gliosis, ↑ IL-10, ↓ TNF-α | [79] |
| α-Bisabolol | 25 or 50 mg/kg, p.o 1 h before the local injection of inducing agents | Formalin (20 μL of 2% s.c.), capsaicin (20 μL of 2.5 µg, s.c.) or glutamate (40 μL of 25 mM, s.c.) induced orofacial nociception Carrageenan (100 µL of 1%w/v intrapleural) induced pleurisy in mice | ↓ orofacial pain ↓ TNF-α | [4] |
| α-Bisabolol | 30, 56, 100, and 180 mg/kg p.o. of α-Bisabolol alone α-Bisabolol -diclofenac (5.1, 10.3, 20.6, and 41.2 mg/kg) | Formalin (50 µL of 1%, s.c.) induced nociception Carrageenan (100 µL of a 1%, s.c.)-induced inflammation in rats | ↓ nociception ↓ paw volume ↓ hemorrhagic erosion | [86] |
| α-Bisabolol | 25, 50, 100 and 200 mg/kg p.o | Carrageenan (20 μL 1%w/v, intraplantar injection), dextran (20 μL of 0.15%, w/v), histamine (200 μg/paw) or serotonin (200 μg/paw) induced inflammation, formalin (20 μL of 1%) induced nociception, acetic acid (0.1 mL/10 g of 0.6% solution)-induced abdominal writhing in rats | ↓ paw licking ↓ edema volume ↓ abdominal writhing ↓ leukocytes migration ↓ MPO release ↓ TNF-α | [83] |
| α-Bisabolol | 100, 200, or 400 mg/kg p.o., or 50, 100, or 200 mg/mL topical 60 min before injection | Formalin (20 μL of 1.5% s.c.), cinnamaldehyde (13.2 μg/lip) induced nociception in rodents | ↓ face rubbing ↓ head flinching | [80] |
| α-Bisabolol | 50, 100 or 200 mg/kg, p.o | Cyclophosphamide (400 mg/kg, i.p.), mustard oil (50 μL/animal intracolonic) induced visceral nociception in mice | ↓ visceral pain | [84] |
| α-Bisabolol | 50–200 mg/mL ointment | Hypertonic saline (20 μL of 5 M NaCl)-induced corneal nociception in mice | ↓ eye wiping | [85] |
| α-Bisabolol nanocapsules | 100 or 200 mg/mL | Hypertonic saline (20 μL of 5M NaCl)-induced corneal nociception in mice | ↓ eye wiping | [87] |
| α-Bisabolol | 50, 100 or 200 mg/kg, p.o | Acetic acid (0.6%, i.p.), Capsaicin (50 μL/animal, intracolonic), Formalin (10%, 10 μL/animal, intracolonic), (0.75%, 50 μL/animal, intracolonic) induced visceral nociception in mice | ↓ abdominal constrictions ↓ pain-related behavior | [81] |
| α-Bisabolol | 0.5, 1, 5 and 10 mM | Supramaximal stimulation consisted of 50–100 μs isolated rectangular voltage pulses applied on mice sciatic nerves | ↓ nerve excitability | [82] |
| α-Bisabolol | 1–0.5% mouthwash | postoperative complications of maxillofacial surgeries, a randomized, controlled, triple-blind clinical trial | ↓ pain during brushing ↓ lesion wiping | [88] |
| Cardioprotective Effects | ||||
|---|---|---|---|---|
| Compound | Dose/Route/ Duration | Model | Major Mechanisms | Reference |
| α-Bisabolol | 25 mg/kg, i.p for 10 days | Isoproterenol (85 mg/kg, s.c. for 2 days) induced myocardial infarction in rats | ↓ LDH, ↓ infarct size ↓ TBARS, ↑ SOD, CAT, ↓ β-glucuronidase, β-galactosidase, cathepsin-B &D, ↓ TNF-α, IL-6, IL-1β, iNOS and COX-2, ↑ IL-10, ↓ NLRP3, (-) NFκB/MAPK, ↑ Beclin-1, LC3BI/II, ↓ p-mTOR | [91] |
| α-Bisabolol | 25 mg/kg, i.p daily for 10 days | Isoproterenol (85 mg/kg, s.c. for 2 days) induced myocardial infarction in rats | ↑ CK and LDH, ↓ LOOH, TBARS, ↑ SOD, catalase and GSH, ↓ Ca2+ overload ↓ mitochondrial swelling, ↑ ATP, ↑ ICDH, SDH, MDH, α-KGDH, and complexes I-IV, ↓ Bax, P53, APAF-1, active caspase-3 and 9, ↑ Bcl-2 | [92] |
| α-Bisabolol | 25 mg/kg, i.p daily for 10 days | Isoproterenol (85 mg/kg, s.c. for 2 days) induced myocardial infarction in rats | ↓ CK ↓ TBARS and LOOH ↑ GSH and vitamin-C ↓ HR, SBP and DBP | [93] |
| Dose/Route/Duration | Model | Major Mechanisms | Reference |
|---|---|---|---|
| 100 mg/kg p.o. | Ethanol (96%, 1 mL per animal) induced gastric damage in rats | ↓ gastric damage | [114] |
| 100 or 200 mg/kg p.o. | Ethanol (0.2 mL/animal p.o.) and Indomethacin (20 mg/kg p.o.) induced ulcer model in mice | ↓ gastric lesions ↑ GSH | [115] |
| 100 and 200 mg/kg, p.o. | Ethanol (0.2 mL) induced gastric lesion in mice | ↓ MDA, MPO, ↑ SOD, ↓ neutrophils influx | [116] |
| 100 mg/kg p.o. and 500, 250, 125, 62.5 and 31.25 μM | Clamping of the renal artery in the left kidney for 60 min. in rats and Ischemia/reperfusion model on tubular epithelial cells (LLC-MK2) by anaerobic chamber method | ↓ creatinine, urea, uric acid ↓ urinary osmolality ↓ FeNa+, FeK+, FeCl− ↓ microalbuminuria ↓ KIM-1, ↓ TBARS, ↑ GSH ↑ cell viability | [118] |
| 1000, 500, 250, 125, 62.5 and 31.25 μM | Ischemia/reperfusion model on human tubular kidney cells (HK2) by anaerobic chamber method | ↑ cell viability, ↓ apoptosis, ↓ TBARS, (-) NADPH oxidase ↑ GSH, ↓ NOX4, ↑ ΔΨm, ↓ KIM-1 | [119] |
| Dose/Route/ Duration | Model | Major Mechanisms | Reference |
|---|---|---|---|
| Cells treated with: 2.5, 5, 10 μM for 24 h Mice treated with 30 mg/kg/day p.o daily for 8 weeks | AGEs (50μg/mL for 2 h) induced OA in chondrocytes and Destabilization of the medial meniscus in mice | ↓ iNOS, COX-2, TNF-α, p65 PGE2, nitrite, IL-6, ↓ MMP13 ↑ collagen II, aggrecan ADAMT-S5, ↓ NF-κB, IκBα, ↓ pJNK, ↓ p-p38, ↑ chondrocytes and proteoglycans | [121] |
| 30, 50, or 100 mg/kg, p.o, 4 h before LPS | LPS (25 µg/25 µL intranasal) induced acute lung inflammation in mice | ↓ neutrophils, ↓ MPO, ↓ AHR, ↓ elastance, ↓ MIP-2 and KC ↓ alveolar wall thickening, inflammatory cell infiltration, alveolar hemorrhage, and lung tissue damage | [122] |
| 560, 860 and 1200 μM | LPS (10 μg/mL for 24 h) induced inflammation in human myometria biopsies | ↓ TNF-α, IL-1β | [123] |
| 50 mg/kg, p.o | Carrageenan (100 μL of 1% (w/v)) induced pleurisy in mice | ↓ TNF-α | [4] |
| In vitro: 0.5, 1, 3, 10, 30, or 90 μg/mL and in vivo: 50, 100, or 200 mg/kg p.o 1 h before surgery | Zymosan (1 mg/cavity, i.p.) induced neutrophils in peritoneal cavity of mice and Cecal ligation and puncture induced systemic infection | ↑ phagocytosis of neutrophils, ↓ leukocytes, ↓ NO, ↓ mortality ↓ colony forming units | [124] |
| 25 and 100 μM for 2h | LPS (500 ng/mL) induced inflammatory response in RAW264.7 macrophages cells | ↓ NO, PGE2, ↓ iNOS, COX-2, ↓ NF-jB, AP-1, ↓ pERK, p-p38 | [125] |
| Dose/Route/ Duration | Model | Major Mechanisms | Reference |
|---|---|---|---|
| 1.9 to 31 g/m | Candida albicans and fMLP induced Human polymorphonuclear neutrophils respiratory, Bursts and ROS production | ↑ LACL inhibition | [129] |
| 1000 μg/mL to 62.5 μg/mL | In vitro tests (DPPH and ABTS) | ↓ concentration of free radicals | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. https://doi.org/10.3390/nu14071370
Eddin LB, Jha NK, Goyal SN, Agrawal YO, Subramanya SB, Bastaki SMA, Ojha S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients. 2022; 14(7):1370. https://doi.org/10.3390/nu14071370
Chicago/Turabian StyleEddin, Lujain Bader, Niraj Kumar Jha, Sameer N. Goyal, Yogeeta O. Agrawal, Sandeep B. Subramanya, Salim M. A. Bastaki, and Shreesh Ojha. 2022. "Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol" Nutrients 14, no. 7: 1370. https://doi.org/10.3390/nu14071370
APA StyleEddin, L. B., Jha, N. K., Goyal, S. N., Agrawal, Y. O., Subramanya, S. B., Bastaki, S. M. A., & Ojha, S. (2022). Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients, 14(7), 1370. https://doi.org/10.3390/nu14071370








