Prolonged or Transition to Metabolically Unhealthy Status, Regardless of Obesity Status, Is Associated with Higher Risk of Cardiovascular Disease Incidence and Mortality in Koreans
Abstract
:1. Introduction
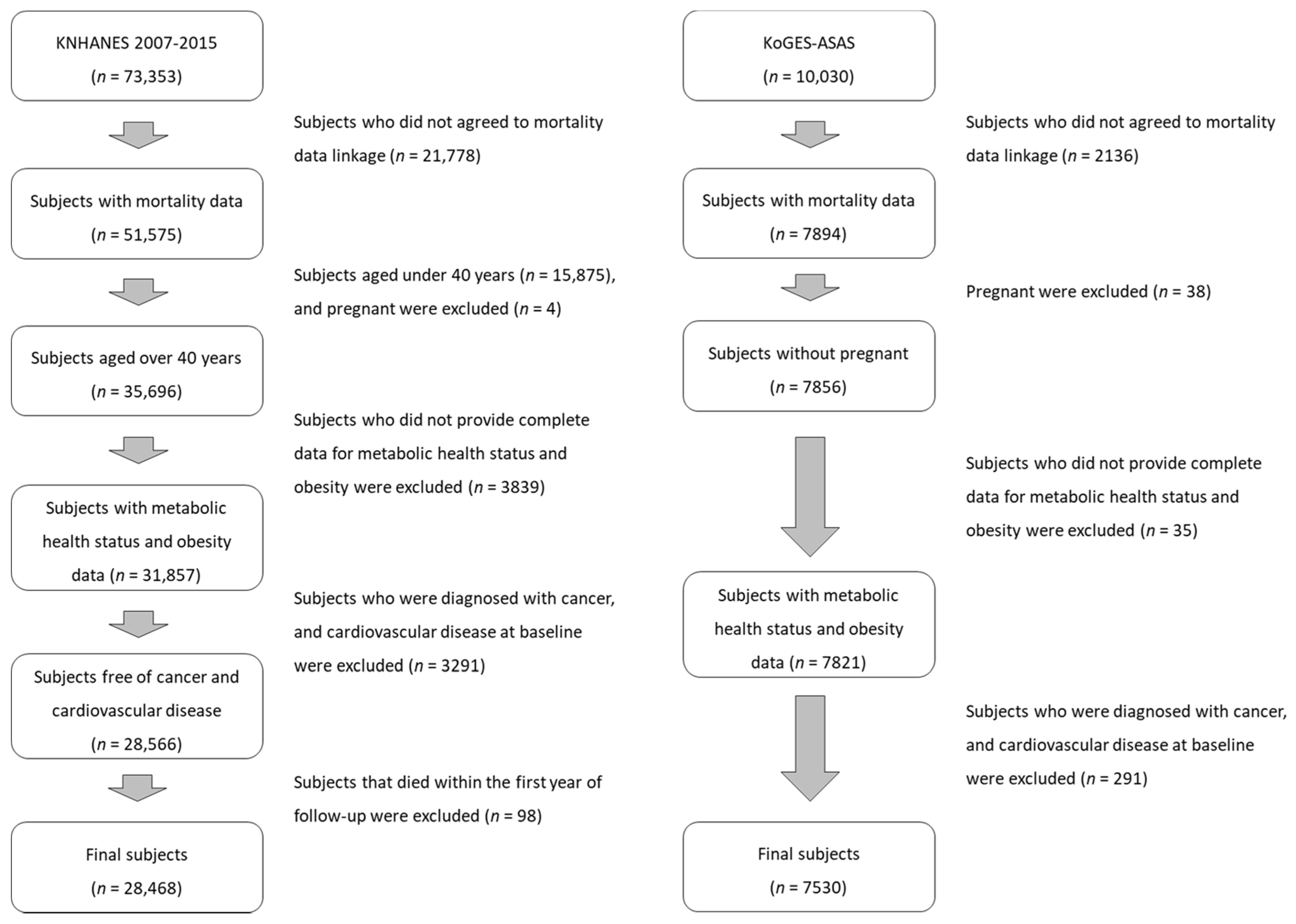
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Assessment of Metabolic Health Status and Obesity
2.4. CVD and Cancer Incidence and Mortality Ascertainment
2.5. Covariate Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of KNHANES Study Participants
3.2. Risk of Cardiovascular, Cancer, and All-Cause Mortality According to Obesity and Metabolic Health Risk in KNHANES
3.3. Baseline Characteristics of KoGES Study Participants
3.4. Risk of CVD and Cancer Incidence and Mortality and All-Cause Mortality According to Metabolic Health and Obesity Status at Baseline in the KoGES Cohort
3.5. Transition of Metabolic Health and Obesity Phenotypes and the Risk of CVD and Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Magno, C.P.; Lane, K.T.; Hinojosa, M.W.; Lane, J.S. Association of Hypertension, Diabetes, Dyslipidemia, and Metabolic Syndrome with Obesity: Findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J. Am. Coll. Surg. 2008, 207, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef] [Green Version]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [Green Version]
- Phillips, C.M. Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev. Endocr. Metab. Disord. 2013, 14, 219–227. [Google Scholar] [CrossRef]
- Phillips, C.M. Metabolically healthy obesity across the life course: Epidemiology, determinants, and implications. Ann. N. Y. Acad. Sci. 2017, 1391, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Mongraw-Chaffin, M.; Foster, M.C.; Anderson, C.A.M.; Burke, G.L.; Haq, N.; Kalyani, R.R.; Ouyang, P.; Sibley, C.T.; Tracy, R.; Woodward, M.; et al. Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 71, 1857–1865. [Google Scholar] [CrossRef]
- Hamer, M.; Stamatakis, E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J. Clin. Endocrinol. Metab. 2012, 97, 2482–2488. [Google Scholar] [CrossRef]
- Eckel, N.; Meidtner, K.; Kalle-Uhlmann, T.; Stefan, N.; Schulze, M.B. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2016, 23, 956–966. [Google Scholar] [CrossRef]
- Zheng, R.; Zhou, D.; Zhu, Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blüher, M. Are metabolically healthy obese individuals really healthy? Endocrinology 2014, 171, R209–R219. [Google Scholar] [CrossRef]
- Lind, L.; Risérus, U.; Ärnlöv, J. Impact of the Definition of Metabolically Healthy Obesity on the Association with Incident Cardiovascular Disease. Metab. Syndr. Relat. Disord. 2020, 18, 302–307. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, L.; Zheng, R.; Zheng, Y. The prevalence, metabolic risk and effects of lifestyle intervention for metabolically healthy obesity: A systematic review and meta-analysis: A PRISMA-compliant article. Medicine 2017, 96, e8838. [Google Scholar] [CrossRef]
- Bell, J.A.; Hamer, M.; Sabia, S.; Singh-Manoux, A.; Batty, G.D.; Kivimaki, M. The Natural Course of Healthy Obesity over 20 Years. J. Am. Coll. Cardiol. 2015, 65, 101–102. [Google Scholar] [CrossRef] [Green Version]
- Eckel, N.; Li, Y.; Kuxhaus, O.; Stefan, N.; Hu, F.B.; Schulze, M.B. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90,257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 714–724. [Google Scholar] [CrossRef]
- Gao, M.; Lv, J.; Yu, C.; Guo, Y.; Bian, Z.; Yang, R.; Du, H.; Yang, L.; Chen, Y.; Li, Z.; et al. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: A cohort study. PLoS Med. 2020, 17, e1003351. [Google Scholar] [CrossRef]
- Hosseinpanah, F.; Tasdighi, E.; Barzin, M.; Mahdavi, M.; Ghanbarian, A.; Valizadeh, M.; Azizi, F. The association between transition from metabolically healthy obesity to metabolic syndrome, and incidence of cardiovascular disease: Tehran lipid and glucose study. PLoS ONE 2020, 15, e0239164. [Google Scholar] [CrossRef]
- Guo, X.; Li, Z.; Zhou, Y.; Yu, S.; Yang, H.; Sun, G.; Zheng, L.; Afzal, J.; Liu, Y.; Sun, Y. The effects of transitions in metabolic health and obesity status on incident cardiovascular disease: Insights from a general Chinese population. Eur. J. Prev. Cardiol. 2020, 28, 1250–1258. [Google Scholar] [CrossRef]
- Cho, Y.K.; Lee, J.; Kim, H.S.; Park, J.-Y.; Lee, W.J.; Kim, Y.-J.; Jung, C.H. Metabolic health is a determining factor for incident colorectal cancer in the obese population: A nationwide population-based cohort study. Cancer Med. 2021, 10, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Panagiotakos, D.B.; Yannakoulia, M.; Georgousopoulou, E.; Critselis, E.; Chrysohoou, C.; Tousoulis, D.; Pitsavos, C. Transition from metabolically benign to metabolically unhealthy obesity and 10-year cardiovascular disease incidence: The ATTICA cohort study. Metab. Clin. Exp. 2019, 93, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Kang, Y.M.; Yoo, J.H.; Lee, J.; Park, J.Y.; Lee, W.J.; Kim, Y.J.; Jung, C.H. Implications of the dynamic nature of metabolic health status and obesity on risk of incident cardiovascular events and mortality: A nationwide population-based cohort study. Metab. Clin. Exp. 2019, 97, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, P.; Yap, M.; van Staveren, W.A. Body mass index and percent body fat: A meta analysis among different ethnic groups. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1998, 22, 1164–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, U.; Ernst, T.; Buchthal, S.D.; Latch, M.; Albright, C.L.; Wilkens, L.R.; Kolonel, L.N.; Murphy, S.P.; Chang, L.; Novotny, R.; et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr. Diabetes 2011, 1, e6. [Google Scholar] [CrossRef]
- Park, J.-K.; Kim, K.-S.; Lee, T.-Y.; Lee, K.-S.; Lee, D.-H.; Lee, S.-H.; Jee, S.-H.; Suh, I.; Koh, K.-W.; Ryu, S.-Y. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. J. Prev. Med. Public Health 2000, 33, 76–82. [Google Scholar]
- Song, S.O.; Jung, C.H.; Song, Y.D.; Park, C.-Y.; Kwon, H.-S.; Cha, B.S.; Park, J.-Y.; Lee, K.-U.; Ko, K.S.; Lee, B.-W. Background and Data Configuration Process of a Nationwide Population-Based Study Using the Korean National Health Insurance System. Diabetes Metab. J. 2014, 38, 395–403. [Google Scholar] [CrossRef]
- Yang, H.K.; Han, K.; Kwon, H.S.; Park, Y.M.; Cho, J.H.; Yoon, K.H.; Kang, M.I.; Cha, B.Y.; Lee, S.H. Obesity, metabolic health, and mortality in adults: A nationwide population-based study in Korea. Sci. Rep. 2016, 6, 30329. [Google Scholar] [CrossRef]
- Sung, K.C.; Ryu, S.; Cheong, E.S.; Kim, B.S.; Kim, B.J.; Kim, Y.B.; Chung, P.W.; Wild, S.H.; Byrne, C.D. All-Cause and Cardiovascular Mortality Among Koreans: Effects of Obesity and Metabolic Health. Am. J. Prev. Med. 2015, 49, 62–71. [Google Scholar] [CrossRef]
- Choi, K.M.; Cho, H.J.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Baik, S.H.; Choi, D.S.; Kim, N.H. Higher mortality in metabolically obese normal-weight people than in metabolically healthy obese subjects in elderly Koreans. Clin. Endocrinol. 2013, 79, 364–370. [Google Scholar] [CrossRef]
- Kim, N.H.; Seo, J.A.; Cho, H.; Seo, J.H.; Yu, J.H.; Yoo, H.J.; Kim, S.G.; Choi, K.M.; Baik, S.H.; Choi, D.S.; et al. Risk of the Development of Diabetes and Cardiovascular Disease in Metabolically Healthy Obese People: The Korean Genome and Epidemiology Study. Medicine 2016, 95, e3384. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Rotondi, M.; Sui, X.; Blair, S.N.; Ardern, C.I. Individuals with obesity but no other metabolic risk factors are not at significantly elevated all-cause mortality risk in men and women. Clin. Obes. 2018, 8, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, D.; Zheng, D.; Amsalu, E.; Wang, A.; Tao, L.; Guo, J.; Li, X.; Wang, W.; Guo, X. Metabolically healthy obese phenotype and risk of cardiovascular disease: Results from the China Health and Retirement Longitudinal Study. Arch. Gerontol. Geriatr. 2019, 82, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-M.M.; White, A.J.; Nichols, H.B.; O’Brien, K.M.; Weinberg, C.R.; Sandler, D.P. The association between metabolic health, obesity phenotype and the risk of breast cancer. Int. J. Cancer 2017, 140, 2657–2666. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Kim, S.; Kim, H.; Cha, C.; Chung, M.S. Associations between obesity, metabolic health, and the risk of breast cancer in East Asian women. Br. J. Cancer 2021, 125, 1718–1725. [Google Scholar] [CrossRef]
- Shin, C.M.; Han, K.; Lee, D.H.; Choi, Y.J.; Kim, N.; Park, Y.S.; Yoon, H. Association Among Obesity, Metabolic Health, and the Risk for Colorectal Cancer in the General Population in Korea Using the National Health Insurance Service–National Sample Cohort. Dis. Colon Rectum 2017, 60, 1192–1200. [Google Scholar] [CrossRef]
- Oh, C.M.; Jun, J.K.; Suh, M. Risk of cancer mortality according to the metabolic health status and degree of obesity. Asian Pac. J. Cancer Prev. 2014, 15, 10027–10031. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Han, B.G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Regional Office for the Western Pacific. In The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Seo, M.H.; Lee, W.-Y.; Kim, S.S.; Kang, J.-H.; Kang, J.-H.; Kim, K.K.; Kim, B.-Y.; Kim, Y.-H.; Kim, W.-J.; Kim, E.M.; et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef]
- Seidell, J.C.; Verschuren, W.M.M.; van Leer, E.M.; Kromhout, D. Overweight, Underweight, and Mortality: A Prospective Study of 48287 Men and Women. Arch. Intern. Med. 1996, 156, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Excess Deaths Associated with Underweight, Overweight, and Obesity. JAMA 2005, 293, 1861–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskaran, K.; dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Akinyemiju, T.; Moore, J.X.; Pisu, M.; Judd, S.E.; Goodman, M.; Shikany, J.M.; Howard, V.J.; Safford, M.; Gilchrist, S.C. A Prospective Study of Obesity, Metabolic Health, and Cancer Mortality. Obesity 2018, 26, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Moore, L.L.; Chadid, S.; Singer, M.R.; Kreger, B.E.; Denis, G.V. Metabolic Health Reduces Risk of Obesity-Related Cancer in Framingham Study Adults. Cancer Epidemiol. Prev. Biomark. 2014, 23, 2057–2065. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Won, Y.-J.; Park, Y.R.; Jung, K.-W.; Kong, H.-J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res. Treat 2020, 52, 335–350. [Google Scholar] [CrossRef]
- Johnson, W.; Bell, J.A.; Robson, E.; Norris, T.; Kivimäki, M.; Hamer, M. Do worse baseline risk factors explain the association of healthy obesity with increased mortality risk? Whitehall II Study. Int. J. Obes. 2019, 43, 1578–1589. [Google Scholar] [CrossRef] [Green Version]
- Hamer, M.; Johnson, W.; Bell, J.A. Improving risk estimates for metabolically healthy obesity and mortality using a refined healthy reference group. Eur. J. Endocrinol. 2017, 177, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.Y.; Wang, M.Y.; Lin, Y.S.; Lin, C.J.; Lo, K.; Chang, I.J.; Cheng, T.Y.; Tsai, S.Y.; Chen, H.H.; Lin, C.Y.; et al. The Association between Metabolically Healthy Obesity, Cardiovascular Disease, and All-Cause Mortality Risk in Asia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 1320. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.R.; Brancati, F.L.; Yeh, H.-C. Trends in the prevalence of type 2 diabetes in Asians versus whites: Results from the United States National Health Interview Survey, 1997–2008. Diabetes Care 2011, 34, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Leahy, J.L. Pathogenesis of Type 2 Diabetes Mellitus. Arch. Med. Res. 2005, 36, 197–209. [Google Scholar] [CrossRef] [PubMed]



| MHN | MUN | MHO | MUO | p-Value | |
|---|---|---|---|---|---|
| n | 9813 | 8711 | 3278 | 6666 | - |
| Weighted n (weighted %) | 6,703,404 (36.4) | 5,133,971 (27.9) | 2,335,099 (12.7) | 4,238,314 (23.0) | - |
| Lifestyle and socio-economic factors | |||||
| Age, years | 51.2 ± 0.1 | 58.8 ± 0.2 | 50.6 ± 0.2 | 56.5 ± 0.2 | <0.001 |
| Male, n (%) | 3900 (44.4) | 3935 (50.0) | 1435 (51.5) | 2979 (52.5) | <0.001 |
| Residential area, % Urban Rural | 69.8 30.2 | 68.7 31.3 | 66.8 33.2 | 67.2 32.8 | 0.009 |
| Education level, % ≤Elementary school graduate Middle school graduate High school graduate ≥University graduate | 18.6 13.6 38.9 28.9 | 36.9 15.5 29.8 17.8 | 20.6 15.6 37.2 26.7 | 35.0 15.3 29.4 20.3 | <0.001 |
| Household income level, % Low (<₩2,000,000) High (≥₩2,000,000) | 37.8 62.2 | 51.0 49.0 | 39.7 60.3 | 48.3 51.7 | <0.001 |
| Current smoker, n (%) | 1894 (22.8) | 1632 (22.8) | 603 (23.1) | 1110 (21.4) | 0.325 |
| Current drinker, n (%) | 4938 (54.2) | 4138 (53.6) | 1704 (56.2) | 3247 (55.4) | 0.093 |
| Metabolic equivalent of task | 2235 ± 51 | 2040 ± 52 | 2296 ± 83 | 2070 ± 61 | <0.001 |
| Metabolic health risks and obesity status | |||||
| BMI, kg/m2 | 22.05 ± 0.02 | 22.56 ± 0.02 | 27.04 ± 0.04 | 27.54 ± 0.04 | <0.001 |
| Diabetes, n (%) | - | 2037 (23.4) | - | 1793 (26.4) | <0.001 |
| Hypertension, n (%) | - | 6219 (69.2) | - | 5272 (78.0) | <0.001 |
| Dyslipidemia, n (%) | - | 3458 (40.1) | - | 2760 (41.1) | <0.001 |
| Number of metabolic health risks | - | 1.33 ± 0.01 | - | 1.45 ± 0.01 | <0.001 |
| Weighted Total (n) | Weighted Events (n) | Weighted Follow-Up (PY) | Weighted Incidence Rate (per 1000 PY) | HR (95% CI) | |
|---|---|---|---|---|---|
| Cardiovascular mortality | |||||
| MHN | 6,703,404 | 34,649 | 53,880,020 | 0.64 | 1.00 (ref) |
| MUN | 5,133,971 | 102,600 | 39,875,741 | 2.57 | 1.77 (1.28–2.43) |
| MHO | 2,335,099 | 6503 | 18,997,511 | 0.34 | 0.81 (0.42–1.56) |
| MUO | 4,238,314 | 45,969 | 33,735,295 | 1.36 | 1.43 (1.01–2.01) |
| Cancer mortality | |||||
| MHN | 6,703,404 | 94,389 | 53,880,020 | 1.75 | 1.00 (ref) |
| MUN | 5,133,971 | 119,614 | 39,875,741 | 3.00 | 0.91 (0.72–1.15) |
| MHO | 2,335,099 | 13,718 | 18,997,511 | 0.72 | 0.53 (0.34–0.82) |
| MUO | 4,238,314 | 77,409 | 33,735,295 | 2.29 | 0.93 (0.72–1.19) |
| All-cause mortality | |||||
| MHN | 6,703,404 | 241,938 | 53,880,020 | 4.49 | 1.00 (ref) |
| MUN | 5,133,971 | 427,964 | 39,875,741 | 10.73 | 1.19 (1.03–1.38) |
| MHO | 2,335,099 | 40,672 | 18,997,511 | 2.14 | 0.65 (0.49–0.87) |
| MUO | 4,238,314 | 202,375 | 33,735,295 | 6.00 | 0.92 (0.78–1.08) |
| MHN | MUN | MHO | MUO | p-Value | |
|---|---|---|---|---|---|
| n (%) | 2937 (39.0) | 1356 (18.0) | 1579 (21.0) | 1658 (22.0) | - |
| Lifestyle and socio-economic factors | |||||
| Age, years | 50.3 ± 0.2 | 54.9 ± 0.2 | 49.6 ± 0.2 | 53.6 ± 0.2 | <0.001 |
| Male, n (%) | 1382 (47.1) | 737 (54.4) | 670 (42.4) | 769 (46.4) | <0.001 |
| Residential area, % Urban Rural | 48.1 51.9 | 58.0 42.0 | 44.0 56.1 | 54.0 46.0 | <0.001 |
| Education level, % ≤Elementary school graduate Middle school graduate High school graduate ≥University graduate | 27.0 24.2 36.1 12.7 | 38.5 21.4 25.6 14.5 | 27.6 24.9 33.7 13.8 | 40.0 20.7 25.3 14.0 | <0.001 |
| Household income level, % Low (<₩2,000,000) High (≥₩2,000,000) | 61.5 38.5 | 69.0 31.0 | 58.3 41.7 | 66.0 34.0 | <0.001 |
| Current smoker, n (%) | 767 (26.4) | 374 (27.9) | 345 (22.2) | 335 (20.5) | <0.001 |
| Current drinker, n (%) | 1407 (48.3) | 666 (49.6) | 737 (47.2) | 768 (46.7) | 0.371 |
| Metabolic equivalent of task | 9749 ± 119 | 10456 ± 186 | 9301 ± 154 | 9848 ± 158 | <0.001 |
| Metabolic health risks and obesity status | |||||
| BMI, kg/m2 | 22.34 ± 0.03 | 22.79 ± 0.05 | 27.15 ± 0.05 | 27.65 ± 0.05 | <0.001 |
| Diabetes, n (%) | - | 265 (19.5) | - | 311 (18.8) | <0.001 |
| Hypertension, n (%) | - | 1001 (73.8) | - | 1310 (79.0) | <0.001 |
| Dyslipidemia, n (%) | - | 355 (26.2) | - | 432 (26.1) | <0.001 |
| Number of metabolic health risks | - | 1.20 ± 0.01 | - | 1.24 ± 0.01 | <0.001 |
| Total (n) | Events (n) | Follow-Up (Person-Years) | Incidence Rate (per 1000 Person-Years) | HR (95% CI) | |
|---|---|---|---|---|---|
| Cardiovascular disease incidence | |||||
| MHN | 2937 | 183 | 30,729 | 5.96 | 1.00 (ref) |
| MUN | 1356 | 172 | 13,390 | 12.85 | 1.69 (1.36–2.09) |
| MHO | 1579 | 118 | 16,512 | 7.15 | 1.36 (1.07–1.73) |
| MUO | 1658 | 224 | 16,710 | 13.41 | 1.94 (1.59–2.38) |
| Cardiovascular mortality | |||||
| MHN | 2937 | 31 | 51,329 | 0.6 | 1.00 (ref) |
| MUN | 1356 | 38 | 23,303 | 1.63 | 1.64 (0.99–2.70) |
| MHO | 1579 | 11 | 27,814 | 0.4 | 1.01 (0.50–2.03) |
| MUO | 1658 | 39 | 28,791 | 1.35 | 2.01 (1.23–3.29) |
| Cancer incidence | |||||
| MHN | 2937 | 134 | 31,000 | 4.32 | 1.00 (ref) |
| MUN | 1356 | 59 | 13,965 | 4.22 | 0.94 (0.69–1.29) |
| MHO | 1579 | 63 | 16,867 | 3.74 | 0.88 (0.65–1.20) |
| MUO | 1658 | 86 | 17,585 | 4.89 | 1.00 (0.75–1.33) |
| Cancer mortality | |||||
| MHN | 2937 | 81 | 51,329 | 1.58 | 1.00 (ref) |
| MUN | 1356 | 50 | 23,303 | 2.15 | 1.03 (0.71–1.49) |
| MHO | 1579 | 28 | 27,814 | 1.01 | 0.77 (0.49–1.21) |
| MUO | 1658 | 51 | 28,791 | 1.77 | 1.02 (0.71–1.48) |
| All-cause mortality | |||||
| MHN | 2937 | 185 | 51,329 | 3.6 | 1.00 (ref) |
| MUN | 1356 | 170 | 23,303 | 7.3 | 1.41 (1.13–1.75) |
| MHO | 1579 | 63 | 27,814 | 2.27 | 0.83 (0.62–1.12) |
| MUO | 1658 | 149 | 28,791 | 5.18 | 1.30 (1.04–1.62) |
| Metabolic Health and Obesity Status | Total (n) | Events (n) | Follow-Up (Person-Years) | Incidence Rate (per 1000 Person-Years) | HR (95% CI) | |
|---|---|---|---|---|---|---|
| Baseline | 4th Follow-Up | |||||
| Participants with stable metabolic and obesity status | ||||||
| MHN | MHN | 1615 | 90 | 19,412 | 4.64 | 1.00 (ref) |
| MUN | MUN | 805 | 41 | 9326 | 4.40 | 0.91 (0.62–1.34) |
| MHO | MHO | 610 | 25 | 7392 | 3.38 | 0.76 (0.48–1.18) |
| MUO | MUO | 986 | 59 | 11,795 | 5.00 | 0.92 (0.65–1.31) |
| Participants with changing metabolic and obesity status | ||||||
| MHN | MHN | 1615 | 90 | 19,412 | 4.64 | 1.00 (ref) |
| MUN | 626 | 27 | 7243 | 3.73 | 0.80 (0.52–1.23) | |
| MHO | 141 | 10 | 1687 | 5.93 | 1.37 (0.71–2.64) | |
| MUO | 97 | 1 | 1131 | 0.88 | 0.18 (0.02–1.27) | |
| MUN | MHN | 204 | 12 | 2361 | 5.08 | 1.16 (0.61–2.22) |
| MUN | 805 | 41 | 9326 | 4.40 | 1.00 (ref) | |
| MHO | 21 | 0 | 256 | 0 | - | |
| MUO | 109 | 2 | 1256 | 1.59 | 0.34 (0.08–1.41) | |
| MHO | MHN | 181 | 14 | 2171 | 6.45 | 1.95 (1.02–3.76) |
| MUN | 98 | 6 | 1084 | 5.53 | 1.54 (0.63–3.78) | |
| MHO | 610 | 25 | 7392 | 3.38 | 1.00 (ref) | |
| MUO | 441 | 16 | 5421 | 2.95 | 0.80 (0.42–1.51) | |
| MUO | MHN | 42 | 3 | 563 | 5.33 | 1.42 (0.44–4.56) |
| MUN | 221 | 12 | 2571 | 4.67 | 1.00 (0.52–1.91) | |
| MHO | 132 | 9 | 1709 | 5.27 | 1.07 (0.51–2.25) | |
| MUO | 986 | 59 | 11,795 | 5.00 | 1.00 (ref) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kwak, S.-Y.; Park, D.; Kim, G.-E.; Park, C.Y.; Shin, M.-J. Prolonged or Transition to Metabolically Unhealthy Status, Regardless of Obesity Status, Is Associated with Higher Risk of Cardiovascular Disease Incidence and Mortality in Koreans. Nutrients 2022, 14, 1644. https://doi.org/10.3390/nu14081644
Lee J, Kwak S-Y, Park D, Kim G-E, Park CY, Shin M-J. Prolonged or Transition to Metabolically Unhealthy Status, Regardless of Obesity Status, Is Associated with Higher Risk of Cardiovascular Disease Incidence and Mortality in Koreans. Nutrients. 2022; 14(8):1644. https://doi.org/10.3390/nu14081644
Chicago/Turabian StyleLee, Juhee, So-Young Kwak, Dahyun Park, Ga-Eun Kim, Clara Yongjoo Park, and Min-Jeong Shin. 2022. "Prolonged or Transition to Metabolically Unhealthy Status, Regardless of Obesity Status, Is Associated with Higher Risk of Cardiovascular Disease Incidence and Mortality in Koreans" Nutrients 14, no. 8: 1644. https://doi.org/10.3390/nu14081644






