DHA-Enriched Fish Oil Ameliorates Deficits in Cognition Associated with Menopause and the APOE4 Genotype in Rodents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Animal Model and Experimental Design
2.3. Behavioural Assessment
2.4. Biochemical Analyses
2.5. RNA Isolation and qRT-PCR
2.6. Statistical Analysis
3. Results
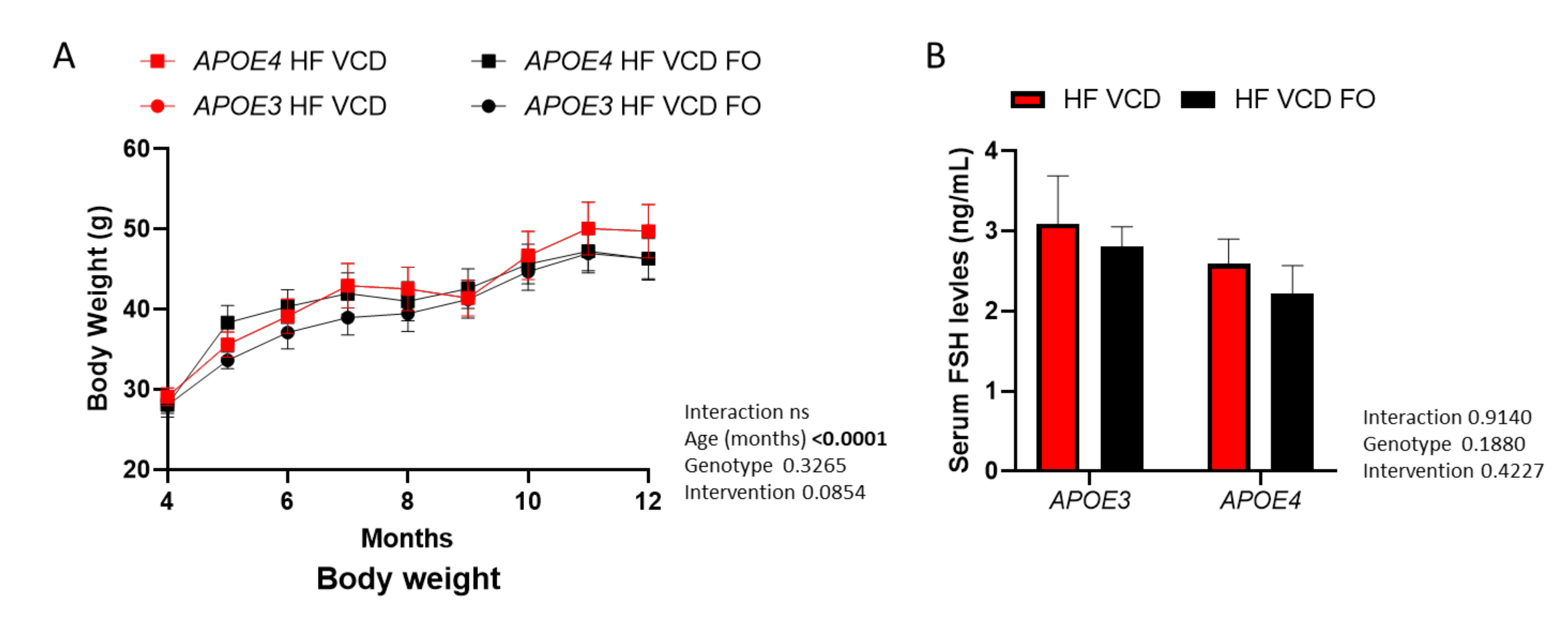
3.1. FO Supplementation or Ovarian Failure Does Not Significantly Modify Weight Gain
3.2. DHA-Rich FO Supplementation Restores APOE4-Induced Impairment in Recognition Memory
3.3. DHA-Rich FO Supplementation Increases DHA Levels in the Brain of VCD-Treated Animals
3.4. FO Supplementation Improves Brain Deficits Induced by VCD and APOE4 Genotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steyaert, J.; Deckers, K.; Smits, C.; Fox, C.; Thyrian, R.; Jeon, Y.H.; Vernooij-Dassen, M.; Köhler, S.; Interdem Taskforce on Prevention of Dementia. Putting primary prevention of dementia on everybody’s agenda. Aging Ment. Health 2021, 25, 1376–1380. [Google Scholar] [CrossRef]
- Michaelson, D.M. APOE epsilon4: The most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimers Dement. 2014, 10, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Safieh, M.; Korczyn, A.D.; Michaelson, D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019, 17, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
- Snyder, H.M.; Asthana, S.; Bain, L.; Brinton, R.; Craft, S.; Dubal, D.B.; Espeland, M.A.; Gatz, M.; Mielke, M.M.; Raber, J.; et al. Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimers Dement. 2016, 12, 1186–1196. [Google Scholar] [CrossRef]
- Neu, S.C.; Pa, J.; Kukull, W.; Beekly, D.; Kuzma, A.; Gangadharan, P.; Wang, L.S.; Romero, K.; Arneric, S.P.; Redolfi, A.; et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017, 74, 1178–1189. [Google Scholar] [CrossRef]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, M.A.; Azcoitia, I.; Gonzalez-Burgos, I.; Garcia-Segura, L.M. Signaling mechanisms mediating the regulation of synaptic plasticity and memory by estradiol. Horm. Behav. 2015, 74, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, J.; Scali, J.; Carrière, I.; Amieva, H.; Rouaud, O.; Berr, C.; Ritchie, K.; Ancelin, M.L. Impact of a premature menopause on cognitive function in later life. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1729–1739. [Google Scholar] [CrossRef]
- Karim, R.; Koc, M.; Rettberg, J.R.; Hodis, H.N.; Henderson, V.W.; St. John, J.A.; Allayee, H.; Brinton, R.D.; Mack, W.J. Apolipoprotein E4 genotype in combination with poor metabolic profile is associated with reduced cognitive performance in healthy postmenopausal women: Implications for late onset Alzheimer’s disease. Menopause 2019, 26, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Bojar, I.; Stasiak, M.; Cyniak-Magierska, A.; Raczkiewicz, D.; Lewiński, A. Cognitive Function, APOE Gene Polymorphisms, and Thyroid Status Associations in Postmenopausal Women in Poland. Dement. Geriatr. Cogn. Disord. 2016, 42, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.; Monti, M.C.; Sinforiani, E.; Cairati, M.; Guaita, A.; Montomoli, C.; Govoni, S.; Racchi, M. Estrogen receptor alpha and APOEepsilon4 polymorphisms interact to increase risk for sporadic AD in Italian females. Eur. J. Neurol. 2006, 13, 639–644. [Google Scholar] [PubMed]
- Pontifex, M.G.; Martinsen, A.; Saleh, R.N.M.; Harden, G.; Tejera, N.; Müller, M.; Fox, C.; Vauzour, D.; Minihane, A.M. APOE4 genotype exacerbates the impact of menopause on cognition and synaptic plasticity in APOE-TR mice. FASEB J. 2021, 35, e21583. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhuang, P.; He, W.; Chen, J.N.; Wang, W.Q.; Freedman, N.D.; Abnet, C.C.; Wang, J.B.; Jiao, J.J. Association of fish and long-chain omega-3 fatty acids intakes with total and cause-specific mortality: Prospective analysis of 421 309 individuals. J. Intern. Med. 2018, 284, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.N.M.; Minihane, A.M. Fish, n-3 fatty acids, cognition and dementia risk: Not just a fishy tale. Proc. Nutr. Soc. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Bo, Y.; Zhang, X.; Wang, Y.; You, J.; Cui, H.; Zhu, Y.; Pang, W.; Liu, W.; Jiang, Y.; Lu, Q. The n-3 Polyunsaturated Fatty Acids Supplementation Improved the Cognitive Function in the Chinese Elderly with Mild Cognitive Impairment: A Double-Blind Randomized Controlled Trial. Nutrients 2017, 9, 54. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Q.; Chu, J.; Zeng, W.; Yang, M.; Zhu, S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1422–1436. [Google Scholar] [CrossRef] [Green Version]
- Takeyama, E.; Islam, A.; Watanabe, N.; Tsubaki, H.; Fukushima, M.; Mamun, M.A.; Sato, S.; Sato, T.; Eto, F.; Yao, I.; et al. Dietary Intake of Green Nut Oil or DHA Ameliorates DHA Distribution in the Brain of a Mouse Model of Dementia Accompanied by Memory Recovery. Nutrients 2019, 11, 2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, G.; Shi, B.; Wu, K.; Chen, S.; Gao, X.; Xiao, S.; Kang, J.X.; Li, W.; Huang, R. The protective role of endogenous n-3 polyunsaturated fatty acids in Tau Alzheimer’s disease mouse model. Int. J. Neurosci. 2019, 129, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.; Taylor, K.; Bilousova, T.; Weiland, D.; Pham, T.; Zuo, X.; Yang, F.; Chen, P.P.; Glabe, C.G.; Takacs, A.; et al. Dietary DHA supplementation in an APP/PS1 transgenic rat model of AD reduces behavioral and Abeta pathology and modulates Abeta oligomerization. Neurobiol. Dis. 2015, 82, 552–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkouch, M.; Hachem, M.; Elgot, A.; Lo Van, A.; Picq, M.; Guichardant, M.; Lagarde, M.; Bernoud-Hubac, N. The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J. Nutr. Biochem. 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N., Jr.; Frautschy, S.A.; Cole, G.M. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef] [Green Version]
- Calon, F.; Lim, G.P.; Morihara, T.; Yang, F.; Ubeda, O.; Salem, N., Jr.; Frautschy, S.A.; Cole, G.M. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2005, 22, 617–626. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Rioux-Perreault, C.; Fortier, M.; Tremblay-Mercier, J.; Zhang, Y.; Lawrence, P.; Vohl, M.C.; Perron, P.; Lorrain, D.; Brenna, J.T.; et al. Disturbance in uniformly 13C-labelled DHA metabolism in elderly human subjects carrying the apoE epsilon4 allele. Br. J. Nutr. 2013, 110, 1751–1759. [Google Scholar] [CrossRef] [Green Version]
- Yassine, H.N.; Croteau, E.; Rawat, V.; Hibbeln, J.R.; Rapoport, S.I.; Cunnane, S.C.; Umhau, J.C. DHA brain uptake and APOE4 status: A PET study with [1-11C]-DHA. Alzheimers Res. Ther. 2017, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Martinsen, A.; Tejera, N.; Vauzour, D.; Harden, G.; Dick, J.; Shinde, S.; Barden, A.; Mori, T.A.; Minihane, A.M. Altered SPMs and age-associated decrease in brain DHA in APOE4 female mice. FASEB J. 2019, 33, 10315–10326. [Google Scholar] [CrossRef] [Green Version]
- Nock, T.G.; Chouinard-Watkins, R.; Plourde, M. Carriers of an apolipoprotein E epsilon 4 allele are more vulnerable to a dietary deficiency in omega-3 fatty acids and cognitive decline. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1068–1078. [Google Scholar] [CrossRef]
- Yassine, H.N.; Braskie, M.N.; Mack, W.J.; Castor, K.J.; Fonteh, A.N.; Schneider, L.S.; Harrington, M.G.; Chui, H.C. Association of Docosahexaenoic Acid Supplementation With Alzheimer Disease Stage in Apolipoprotein E epsilon4 Carriers: A Review. JAMA Neurol. 2017, 74, 339–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, P.M.; Christensen, J.H.; Ewe, M.; Aardestrup, I.V.; Schmidt, E.B. The incorporation of marine n-3 PUFA into platelets and adipose tissue in pre- and postmenopausal women: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2010, 104, 318–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Kim, T.H.; Park, Y. Association between erythrocyte levels of n-3 polyunsaturated fatty acids and depression in postmenopausal women using or not using hormone therapy. Menopause 2016, 23, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, L.A.; Ouyang, P.; Golden, S.H.; Szklo, M.; Gapstur, S.M.; Vaidya, D.; Liu, K. Do sex hormones or hormone therapy modify the relation of n-3 fatty acids with incident depressive symptoms in postmenopausal women? The MESA Study. Psychoneuroendocrinology 2017, 75, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Zhuang, Y.; Gomez-Pinilla, F. High-fat diet transition reduces brain DHA levels associated with altered brain plasticity and behaviour. Sci. Rep. 2012, 2, 431. [Google Scholar] [CrossRef] [Green Version]
- Knouff, C.; Hinsdale, M.E.; Mezdour, H.; Altenburg, M.K.; Watanabe, M.; Quarfordt, S.H.; Sullivan, P.M.; Maeda, N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J. Clin. Investig. 1999, 103, 1579–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, P.M.; Mezdour, H.; Aratani, Y.; Knouff, C.; Najib, J.; Reddick, R.L.; Quarfordt, S.H.; Maeda, N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 1997, 272, 17972–17980. [Google Scholar] [CrossRef] [Green Version]
- Thériault, P.; ElAli, A.; Rivest, S. High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget 2016, 7, 67808–67827. [Google Scholar] [CrossRef] [Green Version]
- Chouinard-Watkins, R.; Vandal, M.; Léveillé, P.; Pinçon, A.; Calon, F.; Plourde, M. Docosahexaenoic acid prevents cognitive deficits in human apolipoprotein E epsilon 4-targeted replacement mice. Neurobiol. Aging 2017, 57, 28–35. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Metherel, A.H.; Chen, C.T.; Shaikh, S.R.; Nadjar, A.; Joffre, C.; Layé, S. Brain eicosapentaenoic acid metabolism as a lead for novel therapeutics in major depression. Brain Behav. Immun. 2020, 85, 21–28. [Google Scholar] [CrossRef]
- Sharma, V.; McNeill, J.H. To scale or not to scale: The principles of dose extrapolation. Br. J. Pharmacol. 2009, 157, 907–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, L.H.; Enroth-Cugell, C. Tests of the mouse visual system. Mamm. Genome 2000, 11, 531–536. [Google Scholar] [CrossRef]
- Davis, K.E.; Eacott, M.J.; Easton, A.; Gigg, J. Episodic-like memory is sensitive to both Alzheimer’s-like pathological accumulation and normal ageing processes in mice. Behav. Brain Res. 2013, 254, 73–82. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018, 141, e58593. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Ramiro, I.; Rushbrook, S.; Ipharraguerre, I.R.; Bevan, D.; Davies, S.; Tejera, N.; Mena, P.; de Pascual-Teresa, S.; Del Rio, D.; et al. n-3 Fatty acids combined with flavan-3-ols prevent steatosis and liver injury in a murine model of NAFLD. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 69–78. [Google Scholar] [CrossRef]
- Day, P.E.L.; Chambers, K.F.; Winterbone, M.S.; García-Blanco, T.; Vauzour, D.; Kroon, P.A. Validation of control genes and a standardised protocol for quantifying gene expression in the livers of C57BL/6 and ApoE−/− mice. Sci. Rep. 2018, 8, 8081. [Google Scholar] [CrossRef]
- Zhong, N.; Weisgraber, K.H. Understanding the association of apolipoprotein E4 with Alzheimer disease: Clues from its structure. J. Biol. Chem. 2009, 284, 6027–6031. [Google Scholar] [CrossRef] [Green Version]
- Frautschy, S.A.; Cole, G.M. Why Pleiotropic Interventions are Needed for Alzheimer’s Disease. Mol. Neurobiol. 2010, 41, 392–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bour, A.; Grootendorst, J.; Vogel, E.; Kelche, C.; Dodart, J.C.; Bales, K.; Moreau, P.H.; Sullivan, P.M.; Mathis, C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav. Brain Res. 2008, 193, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.P. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease. FASEB J. 2018, 33, 1554–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef]
- Alessandri, J.M.; Extier, A.; Al-Gubory, K.H.; Langelier, B.; Baudry, C.; LePoupon, C.; Lavialle, M.; Guesnet, P. Ovariectomy and 17β-estradiol alter transcription of lipid metabolism genes and proportions of neo-formed n-3 and n-6 long-chain polyunsaturated fatty acids differently in brain and liver. J. Nutr. Biochem. 2011, 22, 820–827. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Pinçon, A.; Coulombe, J.D.; Spencer, R.; Massenavette, L.; Plourde, M. A Diet Rich in Docosahexaenoic Acid Restores Liver Arachidonic Acid and Docosahexaenoic Acid Concentrations in Mice Homozygous for the Human Apolipoprotein E epsilon4 Allele. J. Nutr. 2016, 146, 1315–1321. [Google Scholar] [CrossRef] [Green Version]
- Vandal, M.; Alata, W.; Tremblay, C.; Rioux-Perreault, C.; Salem, N., Jr.; Calon, F.; Plourde, M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J. Neurochem. 2014, 129, 516–526. [Google Scholar] [CrossRef]
- Marin, R.; Diaz, M. Estrogen Interactions With Lipid Rafts Related to Neuroprotection. Impact of Brain Ageing and Menopause. Front. Neurosci. 2018, 12, 128. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Shimada, T.; Sugioka, K.; Yamasaki, H.; Fujii, Y.; Ishibashi, Y.; Oka, J.; Shido, O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J. Neurochem. 2002, 81, 1084–1091. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tanabe, Y.; Fujii, Y.; Kikuta, T.; Shibata, H.; Shido, O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J. Nutr. 2005, 135, 549–555. [Google Scholar] [CrossRef]
- Abdullah, L.; Evans, J.E.; Emmerich, T.; Crynen, G.; Shackleton, B.; Keegan, A.P.; Luis, C.; Tai, L.; LaDu, M.J.; Mullan, M.; et al. APOE ε4 specific imbalance of arachidonic acid and docosahexaenoic acid in serum phospholipids identifies individuals with preclinical Mild Cognitive Impairment/Alzheimer’s Disease. Aging 2017, 9, 964–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zárate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemoto, H.; Yasugi, S.; Tsuda, S.; Yoda, M.; Ishiguro, T.; Kaba, N.; Itoh, T. Protective Effect of Nervonic Acid Against 6-Hydroxydopamine-Induced Oxidative Stress in PC-12 Cells. J. Oleo. Sci. 2021, 70, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Cui, Y.; Zhang, J. Nervonic acid amends motor disorder in a mouse model of Parkinson’s disease. Transl. Neurosci. 2021, 12, 237–246. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Kalueff, A.V.; Song, C. Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates BDNF function in a rodent model of neuroinflammation induced by central interleukin-1beta administration. Eur. J. Nutr. 2018, 57, 1781–1791. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef]
- Maioli, S.; Leander, K.; Nilsson, P.; Nalvarte, I. Estrogen receptors and the aging brain. Essays Biochem. 2021, 65, 913–925. [Google Scholar] [CrossRef]
- Wang, J.M.; Irwin, R.W.; Brinton, R.D. Activation of estrogen receptor alpha increases and estrogen receptor beta decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 16983–16988. [Google Scholar] [CrossRef] [Green Version]
- Sladojevic, N.; Stamatovic, S.M.; Johnson, A.M.; Choi, J.; Hu, A.; Dithmer, S.; Blasig, I.E.; Keep, R.F.; Andjelkovic, A.V. Claudin-1-Dependent Destabilization of the Blood-Brain Barrier in Chronic Stroke. J. Neurosci. 2019, 39, 743–757. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, A.J. Docosahexaenoic acid and the brain- what is its role? Asia Pac. J. Clin. Nutr. 2019, 28, 675–688. [Google Scholar] [PubMed]
- Mizuno, T.M.; Lew, P.S.; Jhanji, G. Regulation of the Fructose Transporter Gene Slc2a5 Expression by Glucose in Cultured Microglial Cells. Int. J. Mol. Sci. 2021, 22, 12668. [Google Scholar] [CrossRef] [PubMed]
- Baufeld, C.; Osterloh, A.; Prokop, S.; Miller, K.R.; Heppner, F.L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016, 132, 361–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Fatty Acid % Total | APOE3 | APOE4 | |||||
|---|---|---|---|---|---|---|---|
| HF VCD | HF VCD FO | HF VCD | HF VCD FO | Genotype | Intervention | Interaction | |
| Total n-3 PUFA | 11.6 ± 0.70 | 13.6 ± 0.40 | 11.8 ± 0.50 | 13.5 ± 0.50 | 0.9991 | 0.0022 | 0.8036 |
| 20:5 n-3 (EPA) | 0.03 ± 0.01 | 0.06 ± 0.03 | 0.03 ± 0.01 | 0.09 ± 0.03 | 0.9242 | 0.3191 | 0.2855 |
| 22:5 n-3 | 0.10 ± 0.05 | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.04 ± 0.01 | 0.2796 | 0.3508 | 0.9236 |
| 22:6 n-3 (DHA) | 11.2 ± 0.50 | 13.5 ± 0.40 | 11.7 ± 0.50 | 13.4 ± 0.50 | 0.7449 | 0.0006 | 0.5554 |
| Total n-6 | 12.6 ± 0.40 | 11.1 ± 0.30 | 13.2 ± 0.60 | 11.5 ± 0.40 | 0.2726 | 0.0036 | 0.8050 |
| 18:2 n-6 | 0.43 ± 0.07 | 0.43 ± 0.04 | 0.39 ± 0.06 | 0.43 ± 0.05 | 0.7696 | 0.7616 | 0.6878 |
| 20:2 n-6 | 0.08 ± 0.03 | 0.10 ± 0.05 | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.7328 | 0.6002 | 0.9964 |
| 20:3 n-6 | 0.21 ± 0.02 | 0.42 ± 0.03 | 0.23 ± 0.02 | 0.40 ± 0.05 | 0.9581 | <0.0001 | 0.5492 |
| 20:4 n-6 (AA) | 8.10 ± 0.39 b | 7.5 ± 0.28 b | 8.67 ± 0.50 | 7.88 ± 0.26 | 0.2471 | 0.0892 | 0.7692 |
| 22:4 n-6 | 3.23 ± 0.14 | 2.57 ± 0.07 | 3.07 ± 0.15 | 2.64 ± 0.15 | 0.7537 | 0.0005 | 0.3814 |
| 22:5 n-6 | 0.54 ± 0.09 | 0.02 ± 0.01 | 0.77 ± 0.10 | 0.03 ± 0.02 | 0.1017 | <0.0001 | 0.1236 |
| DHA:AA | 1.39 ± 0.05 | 1.79 ± 0.02 | 1.36 ± 0.03 | 1.76 ± 0.04 | 0.3425 | <0.0001 | 0.9501 |
| SFA | 35.5 ± 0.90 | 37.0 ± 0.60 | 36.9 ± 1.20 | 36.6 ± 0.80 | 0.5870 | 0.5145 | 0.3146 |
| 14:0 | 0.24 ± 0.04 | 0.09 ± 0.03 | 0.18 ± 0.05 | 0.10 ± 0.03 | 0.5783 | 0.0052 | 0.2971 |
| 16:0 | 14.70 ± 0.70 | 16.40 ± 0.40 | 17.30 ± 0.90 | 16.30 ± 0.70 | 0.1000 | 0.6241 | 0.0809 |
| 18:0 | 18.90 ± 0.50 | 19.60 ± 0.30 | 18.30 ± 0.60 | 19.40 ± 0.30 | 0.4033 | 0.0600 | 0.6637 |
| 20:0 | 0.41 ± 0.03 | 0.38 ± 0.02 | 0.33 ± 0.03 | 0.35 ± 0.04 | 0.0966 | 0.7664 | 0.4637 |
| 22:0 | 0.38 ± 0.05 | 0.25 ± 0.09 | 0.19 ± 0.06 | 0.25 ± 0.09 | 0.2206 | 0.7141 | 0.2283 |
| 24:0 | 0.78 ± 0.18 | 0.26 ± 0.13 | 0.53 ± 0.14 | 0.39 ± 0.11 | 0.6621 | 0.0285 | 0.1980 |
| MFA | 30.5 ± 0.8 | 30.0 ± 0.8 | 27.9 ± 1.4 | 30.2 ± 0.9 | 0.2229 | 0.1262 | 0.0984 |
| 16:1 n-9 | 0.22 ± 0.06 | 0.36 ± 0.05 | 0.37 ± 0.06 | 0.37 ± 0.06 | 0.1805 | 0.2057 | 0.2327 |
| 16:1 n-7 | 0.42 ± 0.01 | 0.48 ± 0.04 | 0.46 ± 0.04 | 0.50 ± 0.02 | 0.4596 | 0.1600 | 0.6908 |
| 18:1 n-9 | 21.20 ± 0.80 | 20.60 ± 0.40 | 19.30 ± 1.20 | 20.20 ± 0.40 | 0.1337 | 0.8116 | 0.3281 |
| 18:1 n-7 | 3.58 ± 0.16 | 3.77 ± 0.12 | 3.39 ± 0.13 | 3.59 ± 0.11 | 0.1694 | 0.1470 | 0.9972 |
| 20:1 n-9 | 3.04 ± 0.19 | 2.73 ± 0.16 | 2.21 ± 0.21 | 2.82 ± 0.31 | 0.1248 | 0.5351 | 0.0603 |
| 22:1 n-9 | 0.21 ± 0.03 | 0.18 ± 0.04 | 0.18 ± 0.02 | 0.18 ± 0.04 | 0.7174 | 0.6213 | 0.6748 |
| 24:1 n-9 | 1.96 ± 0.18 a | 1.90 ± 0.16 a | 1.14 ± 0.24 b | 2.03 ± 0.23 a | 0.1094 | 0.058 | 0.0345 |
| Total DMA | 9.82 ± 0.47 | 8.23 ± 0.51 | 8.61 ± 0.44 | 8.48 ± 0.72 | 0.9861 | 0.1982 | 0.1098 |
| Gene | Category | APOE3 | APOE4 | Genotype | Intervention | Interaction | ||
|---|---|---|---|---|---|---|---|---|
| HF VCD | HF VCD FO | HF VCD | HF VCD FO | |||||
| ERα | Oestrogen receptor | 1.04 ± 0.15 | 0.91 ± 0.12 | 1.04 ± 0.15 | 1.06 ± 0.09 | 0.5836 | 0.6634 | 0.5687 |
| Zo-1 | Tight Junction | 1.04 ± 0.13 | 0.92 ± 0.05 | 1.30 ± 0.12 | 0.97 ± 0.12 | 0.1762 | 0.0669 | 0.3045 |
| Glut-1 | Metabolic/Bioenergetic | 1.01 ± 0.06 | 0.80 ± 0.06 | 0.87 ± 0.06 | 0.93 ± 0.08 | 0.9528 | 0.2605 | 0.0610 |
| Glut-3 | Metabolic/Bioenergetic | 1.01 ± 0.08 | 0.801 ± 0.09 | 1.11 ± 0.07 | 0.94 ± 0.08 | 0.1463 | 0.0243 | 0.8017 |
| Chrebp | Metabolic/Bioenergetic | 1.01 ± 0.08 | 0.81 ± 0.08 | 1.11 ± 0.04 | 0.81 ± 0.06 | 0.4712 | 0.0018 | 0.4648 |
| Gsk3b | Metabolic/Bioenergetic | 1.01 ± 0.06 | 0.80 ± 0.06 | 1.12 ± 0.04 | 0.81 ± 0.10 | 0.3549 | 0.0012 | 0.4690 |
| Aldob | Metabolic/Bioenergetic | 1.04 ± 0.13 | 0.81 ± 0.11 | 1.4 ± 0.11 | 1.01 ± 0.10 | 0.0237 | 0.0132 | 0.4912 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontifex, M.G.; Martinsen, A.; Saleh, R.N.M.; Harden, G.; Fox, C.; Muller, M.; Vauzour, D.; Minihane, A.-M. DHA-Enriched Fish Oil Ameliorates Deficits in Cognition Associated with Menopause and the APOE4 Genotype in Rodents. Nutrients 2022, 14, 1698. https://doi.org/10.3390/nu14091698
Pontifex MG, Martinsen A, Saleh RNM, Harden G, Fox C, Muller M, Vauzour D, Minihane A-M. DHA-Enriched Fish Oil Ameliorates Deficits in Cognition Associated with Menopause and the APOE4 Genotype in Rodents. Nutrients. 2022; 14(9):1698. https://doi.org/10.3390/nu14091698
Chicago/Turabian StylePontifex, Matthew G., Anneloes Martinsen, Rasha N. M. Saleh, Glenn Harden, Chris Fox, Michael Muller, David Vauzour, and Anne-Marie Minihane. 2022. "DHA-Enriched Fish Oil Ameliorates Deficits in Cognition Associated with Menopause and the APOE4 Genotype in Rodents" Nutrients 14, no. 9: 1698. https://doi.org/10.3390/nu14091698
APA StylePontifex, M. G., Martinsen, A., Saleh, R. N. M., Harden, G., Fox, C., Muller, M., Vauzour, D., & Minihane, A.-M. (2022). DHA-Enriched Fish Oil Ameliorates Deficits in Cognition Associated with Menopause and the APOE4 Genotype in Rodents. Nutrients, 14(9), 1698. https://doi.org/10.3390/nu14091698









