Intake of Natural, Unprocessed Tiger Nuts (Cyperus esculentus L.) Drink Significantly Favors Intestinal Beneficial Bacteria in a Short Period of Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tiger Nut Drink Preparation
2.2. Study Design and Participants
2.3. Nutritional Assessment
2.4. Biological Samples and Gut Microbiota Analysis
2.5. Statistical Analysis
3. Results
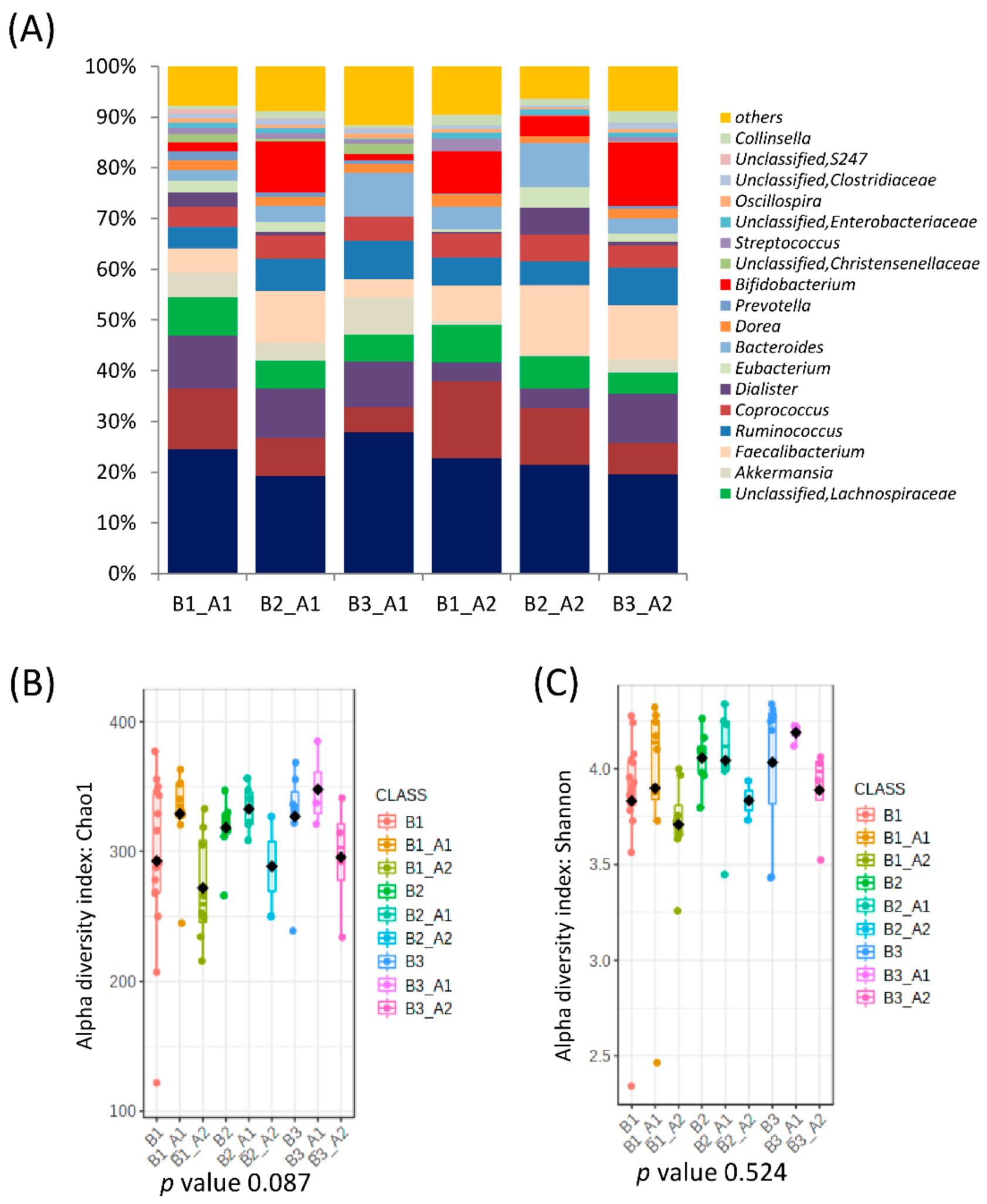
3.1. Association of Microbiota Composition with Diet and Anthropometric Data
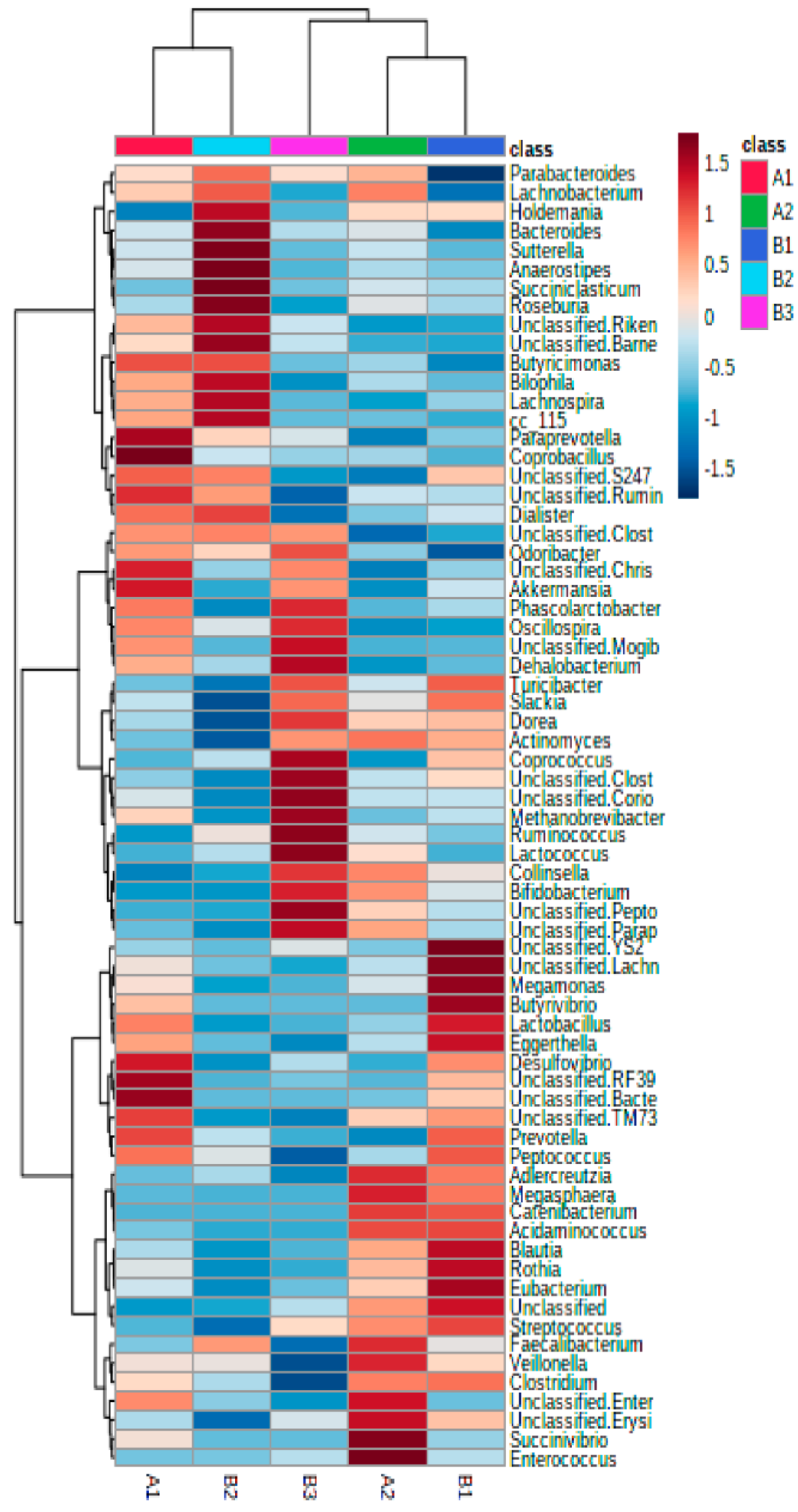
3.2. Further Cluster Analysis of Microbiota before and after Horchata Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cavanilles, A.J. Observaciones Sobre la Historia Natural, Geografia, Agricultura, Poblacion y Frutos del Reyno de Valencia, Facsimil; Cavanilles, A.J., Pereyra, P.J., López Enguídanos, T., Eds.; Imprenta Real: Madrid, Spain, 1797; Volume II, p. 338. [Google Scholar]
- Webster, T.M. Mulch type affects growth and tuber production of yellow nutsedge (Cyperus esculentus) and purple nutsedge (Cyperus rotundus). Weed Sci. 2005, 53, 834–838. [Google Scholar] [CrossRef]
- Cantalejo, M.J. Analysis of Volatile Components Derived from Raw and Roasted Earth-Almond (Cyperus esculentus L.). J. Agric. Food Chem. 1997, 45, 1853–1860. [Google Scholar] [CrossRef]
- Ezeh, O.; Gordon, M.H.; Niranjan, K. Tiger nut oil (Cyperus esculentus L.): A review of its composition and physico-chemical properties. Eur. J. Lipid Sci. Technol. 2014, 116, 783–794. [Google Scholar] [CrossRef]
- López-Marcos, M.C.; Bailina, C.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. Properties of Dietary Fibers from Agroindustrial Coproducts as Source for Fiber-Enriched Foods. Food Bioproc. Technol. 2015, 8, 2400–2408. [Google Scholar] [CrossRef]
- Navarro, J.L.; Schwartz, M.; Gasque, F.; Alberola, J.; Pérez, P.; Lafuente, B. Evolución de las características analíticas de la chufa (Cyperus esculentus L.) a lo largo del periodo de recolección. Rev. Agroquím. Tecnol. Alim. 1983, 23, 387–394. [Google Scholar]
- Sánchez-Zapata, E.; Fernández-López, J.; Angel Pérez-Alvarez, J. Tiger Nut (Cyperus esculentus) Commercialization: Health Aspects, Composition, Properties, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 366–377. [Google Scholar] [CrossRef]
- Bosch, L.; Alegría, A.; Farré, R. RP-HPLC Determination of Tiger Nut and Orgeat Amino Acid Contents. Food Sci. Technol. Int. 2005, 11, 33–40. [Google Scholar] [CrossRef]
- Rubert, J.; Monforte, A.; Hurkova, K.; Pérez-Martínez, G.; Blesa, J.; Navarro, J.L.; Stranka, M.; Soriano, J.M.; Hajslova, J. Untargeted metabolomics of fresh and heat treatment Tiger nut (Cyperus esculentus L.) milks reveals further insight into food quality and nutrition. J. Chromatogr. 2017, 1514, 80–87. [Google Scholar] [CrossRef]
- Parker, M.L.; Ng, A.; Smith, A.C.; Waldron, K.W. Esterified Phenolics of the Cell Walls of Chufa (Cyperus esculentus L.) Tubers and Their Role in Texture. J. Agric. Food Chem. 2000, 48, 6284–6291. [Google Scholar] [CrossRef]
- Ihenetu, S.C.; Ibe, F.C.; Inyamah, P.C. Comparative study of the properties of yellow and brown Cyperus esculentus L. World News Nat. Sci. 2021, 35, 25–37. [Google Scholar]
- Özcan, M.M.; Ghafoor, K.; Al Juhaimi, F.; Uslu, N.; Babiker, E.E.; Ahmed, I.A.M. Influence of germination on bioactive properties, phytochemicals and mineral contents of Tigernut (Cyperus esculentus L.) tuber and oils. J. Food Meas. Charact. 2021, 15, 3580–3589. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food. Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef]
- Lee-Sarwar, K. The farm-like effect: Rural exposures in early life, the microbiome, and asthma. J. Allergy Clin. Immunol. 2021, 148, 89–90. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.-S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Font Quer, P. Plantas Medicinales: El Dioscórides Renovado, 20th ed.; (First Ed. 1956); Ediciones Península: Barcelona, Spain, 2016. [Google Scholar]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Bum, E.N.; Schmutz, M.; Meyer, C.; Rakotonirina, A.; Bopelet, M.; Portet, C.; Jeker, A.; Rakotonirina, S.V.; Olpe, H.R.; Herrling, P. Anticonvulsant properties of the methanolic extract of Cyperus articulatus (Cyperaceae). J. Ethnopharmacol. 2001, 76, 145–150. [Google Scholar] [CrossRef]
- Rakotonirina, V.S.; Bum, E.N.; Rakotonirina, A.; Bopelet, M. Sedative properties of the decoction of the rhizome of Cyperus articulatus. Fitoterapia 2001, 72, 22–29. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y. New definition of resistant starch types from the gut microbiota perspectives–a review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–11. [Google Scholar] [CrossRef]
- Panlasigui, L.N.; Thompson, L.U.; Juliano, B.O.; Perez, C.M.; Yiu, S.H.; Greenberg, G.R. Rice varieties with similar amylose content differ in starch digestibility and glycemic response in humans. Am. J. Clin. Nutr. 1991, 54, 871–877. [Google Scholar] [CrossRef]
- Yang, X.; Darko, K.O.; Huang, Y.; He, C.; Yang, H.; He, S.; Li, J.; Li, J.; Hocher, B.; Yin, Y. Resistant Starch Regulates Gut Microbiota: Structure, Biochemistry and Cell Signalling. Cell Physiol. Biochem. 2017, 42, 306–318. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Śliżewska, K. Efficiency of Resistant Starch and Dextrins as Prebiotics: A Review of the Existing Evidence and Clinical Trials. Nutrients 2021, 13, 3808. [Google Scholar] [CrossRef]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef]
- Keenan, M.J.; Janes, M.; Robert, J.; Martin, R.J.; Raggio, A.M.; McCutcheon, K.L.; Pelkman, C.; Tulley, R.; Goita, M.; Durham, H.A.; et al. Resistant starch from high amylose maize (HAM-RS2) reduces body fat and increases gut bacteria in ovariectomized (OVX) rats. Obesity 2013, 21, 981–984. [Google Scholar] [CrossRef] [Green Version]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucl. Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.-J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome–environment interactions. Bioinformatics 2016, 33, 782–783. [Google Scholar] [CrossRef] [Green Version]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candida activity of Brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef]
- Weenen, H.; Nkunya, M.H.; Bray, D.H.; Mwasumbi, L.B.; Kinabo, L.S.; Kilimali, V.A.; Wijnberg, J.B. Antimalarial compounds containing an alpha, beta-unsaturated carbonyl moiety from Tanzanian medicinal plants. Planta Med. 1990, 56, 371–373. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Calatayud, M.; Romo-Vaquero, M.; Espín, J.C.; Selma, M.V.; Collado, M.C. Urolithin Metabotypes Can Determine the Modulation of Gut Microbiota in Healthy Individuals by Tracking Walnuts Consumption over Three Days. Nutrients 2019, 11, 2483. [Google Scholar] [CrossRef] [Green Version]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Solch, R.J.; Aigbogun, J.O.; Voyiadjis, A.G.; Talkington, G.M.; Darensbourg, R.M.; O’Connell, S.; Pickett, K.M.; Perez, S.R.; Maraganore, D.M. Mediterranean diet adherence, gut microbiota, and Alzheimer’s or Parkinson’s disease risk: A systematic review. J. Neurol. Sci. 2022, 434, 120166. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Manek, R.V.; Builders, P.F.; Kolling, W.M.; Emeje, M.; Kunle, O.O. Physicochemical and Binder Properties of Starch Obtained from Cyperus esculentus. AAPS Pharm. Sci. Tech. 2012, 13, 379–388. [Google Scholar] [CrossRef]
- Akonor, P.T.; Tortoe, C.; Oduro-Yeboah, C.; Saka, E.A.; Ewool, J. Physicochemical, Microstructural, and Rheological Characterization of Tigernut (Cyperus esculentus) Starch. Int. J. Food Sci. 2019, 2019, 3830651. [Google Scholar] [CrossRef] [Green Version]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M.L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2013, 83, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-T.; Hu, G.-A.; Zhu, L.; Zhao, Z.-C.; Yun, J.; Gao, M.-J.; Zhan, X.-B. In vitro digestion and fecal fermentation of highly resistant starch rice and its effect on the gut microbiota. Food Chem. 2021, 361, 130095. [Google Scholar] [CrossRef]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.; Bergeron, N.; Krauss, R.M.; Jansson, J.K.; Tiedje, J.M.; Drake, H.L. Metagenomic Insights into the Degradation of Resistant Starch by Human Gut Microbiota. Appl. Environ. Microbiol. 2018, 84, e01562-18. [Google Scholar] [CrossRef] [Green Version]
- Haudum, C.; Lindheim, L.; Ascani, A.; Trummer, C.; Horvath, A.; Münzker, J.; Obermayer-Pietsch, B. Impact of Short-Term Isoflavone Intervention in Polycystic Ovary Syndrome (PCOS) Patients on Microbiota Composition and Metagenomics. Nutrients 2020, 12, 1622. [Google Scholar] [CrossRef]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [Green Version]
- Statista. Market Value of Soy Milk Worldwide From 2018 to 2025 (in Billion U.S. Dollars) [Graph]. Available online: https://www.statista.com/statistics/896138/global-soy-milk-market-value/ (accessed on 19 June 2019).
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and Gut Microbiota: Interaction and Implication for Human Health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef]
- Taylor, P.W. Interactions of Tea-Derived Catechin Gallates with Bacterial Pathogens. Molecules 2020, 25, 1986. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Takagi, S.; Ando, T.; Yoneyama, H.; Ito, K.; Mizugai, H.; Isogai, E. Antimicrobial activity of tea catechin against canine oral bacteria and the functional mechanisms. J. Vet. Med. Sci. 2016, 78, 1439–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Xiao, X.; Li, K.; Li, X.; Yu, K.; Liao, X.; Shi, B. Prevention of Bacterial Colonization Based on Self-Assembled Metal–Phenolic Nanocoating from Rare-Earth Ions and Catechin. ACS Appl. Mater. Interface 2020, 12, 22237–22245. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Fernandes, A.P.; Holmgren, A.; Li, X.; Zhong, L. Bacterial thioredoxin and thioredoxin reductase as mediators for epigallocatechin 3-gallate-induced antimicrobial action. FEBS J. 2016, 283, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, K.-I.; Nihei, K.-I.; Masuoka, N. Non-antibiotic antibacterial activity of dodecyl gallate. Bioorg. Med. Chem. 2003, 11, 573–580. [Google Scholar] [CrossRef]


| All (n = 31) | Cluster B1 (n = 16) | Cluster B2 (n = 8) | Cluster B3 (n = 7) | p-Value | |
|---|---|---|---|---|---|
| Clinical Characteristics | |||||
| Age (years) | 37.9 ± 11.2 | 35.6 ± 12.7 | 39.0 ± 8.9 | 42.0 ± 10.0 | 0.451 |
| Gender (Female %) | 15 (48.39) | 8 (50.02) | 8 (37.50) | 4 (57.14) | 0.737 |
| BMI (kg/m2) | 23.2 ± 3.3 | 23.2 ± 3.7 | 24.4 ± 2.8 | 21.7 ± 2.3 | 0.294 |
| MD score | 8.6 ± 1.8 | 8.6 ± 2.0 | 8.5 ± 2.1 | 8.8 ± 1.2 | 0.925 |
| Dietary intakes | |||||
| Energy (kcal) | 2959.5 ± 623.1 | 2907.2 ± 473.3 | 2801.1 ± 599.8 | 3260.1 ± 902.8 | 0.334 |
| Total protein (g/day) | 116.9 ± 27.6 | 112.1 ± 20.1 | 111.3 ± 24.7 | 134.4 ± 40.2 | 0.164 |
| Animal protein (g/day) | 74.4 ± 21.7 | 72.6 ± 17.8 | 69.4 ± 19.4 | 84.3 ± 31.3 | 0.386 |
| Vegetal protein (g/day) | 42.5 ± 14.9 | 39.5 ± 10.5 | 41.8 ± 14.2 | 50.1 ± 22.7 | 0.300 |
| Lipids (g/day) | 133.1 ± 27.8 | 134.8 ± 24.9 | 126.1 ± 21.9 | 137.4 ± 40.8 | 0.706 |
| Cholesterol (g/day) | 389.0 ± 109.5 | 386.8 ± 78.1 | 366.3 ± 112.7 | 420.0 ± 167.7 | 0.649 |
| SFA | 36.3 ± 10.2 | 37.2 ± 9.9 | 33.2 ± 8.7 | 37.6 ± 13.1 | 0.633 |
| MUFA | 64.4 ± 12.9 | 64.9 ± 13.2 | 62.5 ± 11.4 | 65.7 ± 15.7 | 0.879 |
| PUFA | 22.2 ± 9.0 | 22.5 ± 9.0 | 20.9 ± 7.7 | 23.1 ± 11.4 | 0.889 |
| Carbohydrates (g/day) | 308.5 ± 95.5 | 298.6 ± 82.1 | 288.6 ± 93.8 | 353.9 ± 123.9 | 0.362 |
| Polysaccharides (g/day) | 172.4 ± 75.7 | 166.2 ± 65.8 | 166.8 ± 78.2 | 193.1 ± 100.5 | 0.728 |
| Total dietary fiber (g/day) | 33.1 ± 12.1 | 31.6 ± 7.9 | 31.5 ± 10.9 | 38.6 ± 19.7 | 0.414 |
| Vitamin A (mcg/day) | 1243.3 ± 520.8 | 1312.5 ± 507.9 | 1024.6 ± 518.9 | 1335.2 ± 557.7 | 0.398 |
| Retinoids (mcg/day) | 325.5 ± 132.7 | 341.4 ± 128.6 | 286.4 ± 117.9 | 333.1 ± 166.4 | 0.635 |
| Carotenoids (mcg/day) | 5499.37 ± 3018.4 | 5818.7 ± 3220.7 | 4422.0 ± 3135.2 | 6000.77 ± 2458.3 | 0.514 |
| Vitamin D (mcg/day) | 3.3 ± 1.5 | 3.6 ± 1.9 | 3.2 ± 1.1 | 2.7 ± 1.0 | 0.406 |
| Vitamin E (mg/day) | 18.7 ± 6.1 | 19.0 ± 6.5 | 17.6 ± 4.5 | 19.3 ± 7.3 | 0.845 |
| Thiamine (mg/day) | 2.0 ± 0.8 | 1.9 ± 0.5 | 2.0 ± 0.7 | 2.4 ± 1.3 | 0.408 |
| Riboflavin (mg/day) | 2.1 ± 0.4 | 2.1 ± 0.5 | 1.9 ± 0.3 | 2.3 ± 0.5 | 0.224 |
| Niacin (mg/day) | 27.8 ± 7.2 | 27.4 ± 5.5 | 26.2 ± 6.8 | 30.98 ± 10.8 | 0.430 |
| Vitamin B6 (mg/day) | 2.8 ± 0.8 | 2.8 ± 0.7 | 2.8 ± 0.9 | 3.11 ± 1.2 | 0.766 |
| Folic acid (mcg/day) | 539.3 ± 197.1 | 532.2 ± 174.6 | 505.1 ± 185.8 | 594.9 ± 269.0 | 0.679 |
| Vitamin B12 (mcg/day) | 8.6 ± 3.3 | 8.3 ± 3.0 | 7.7 ± 2.0 | 10.5 ± 4.7 | 0.228 |
| Vitamin C (mg/day) | 270.3 ± 146.0 | 240.1 ± 104.1 | 256.0 ± 127.1 | 355.6 ± 222.4 | 0.212 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selma-Royo, M.; García-Mantrana, I.; Collado, M.C.; Perez-Martínez, G. Intake of Natural, Unprocessed Tiger Nuts (Cyperus esculentus L.) Drink Significantly Favors Intestinal Beneficial Bacteria in a Short Period of Time. Nutrients 2022, 14, 1709. https://doi.org/10.3390/nu14091709
Selma-Royo M, García-Mantrana I, Collado MC, Perez-Martínez G. Intake of Natural, Unprocessed Tiger Nuts (Cyperus esculentus L.) Drink Significantly Favors Intestinal Beneficial Bacteria in a Short Period of Time. Nutrients. 2022; 14(9):1709. https://doi.org/10.3390/nu14091709
Chicago/Turabian StyleSelma-Royo, Marta, Izaskun García-Mantrana, M. Carmen Collado, and Gaspar Perez-Martínez. 2022. "Intake of Natural, Unprocessed Tiger Nuts (Cyperus esculentus L.) Drink Significantly Favors Intestinal Beneficial Bacteria in a Short Period of Time" Nutrients 14, no. 9: 1709. https://doi.org/10.3390/nu14091709
APA StyleSelma-Royo, M., García-Mantrana, I., Collado, M. C., & Perez-Martínez, G. (2022). Intake of Natural, Unprocessed Tiger Nuts (Cyperus esculentus L.) Drink Significantly Favors Intestinal Beneficial Bacteria in a Short Period of Time. Nutrients, 14(9), 1709. https://doi.org/10.3390/nu14091709







