Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease
Abstract
:1. Introduction
2. Materials and Methodology
3. Occurrence and Causes of Hashimoto’s Disease
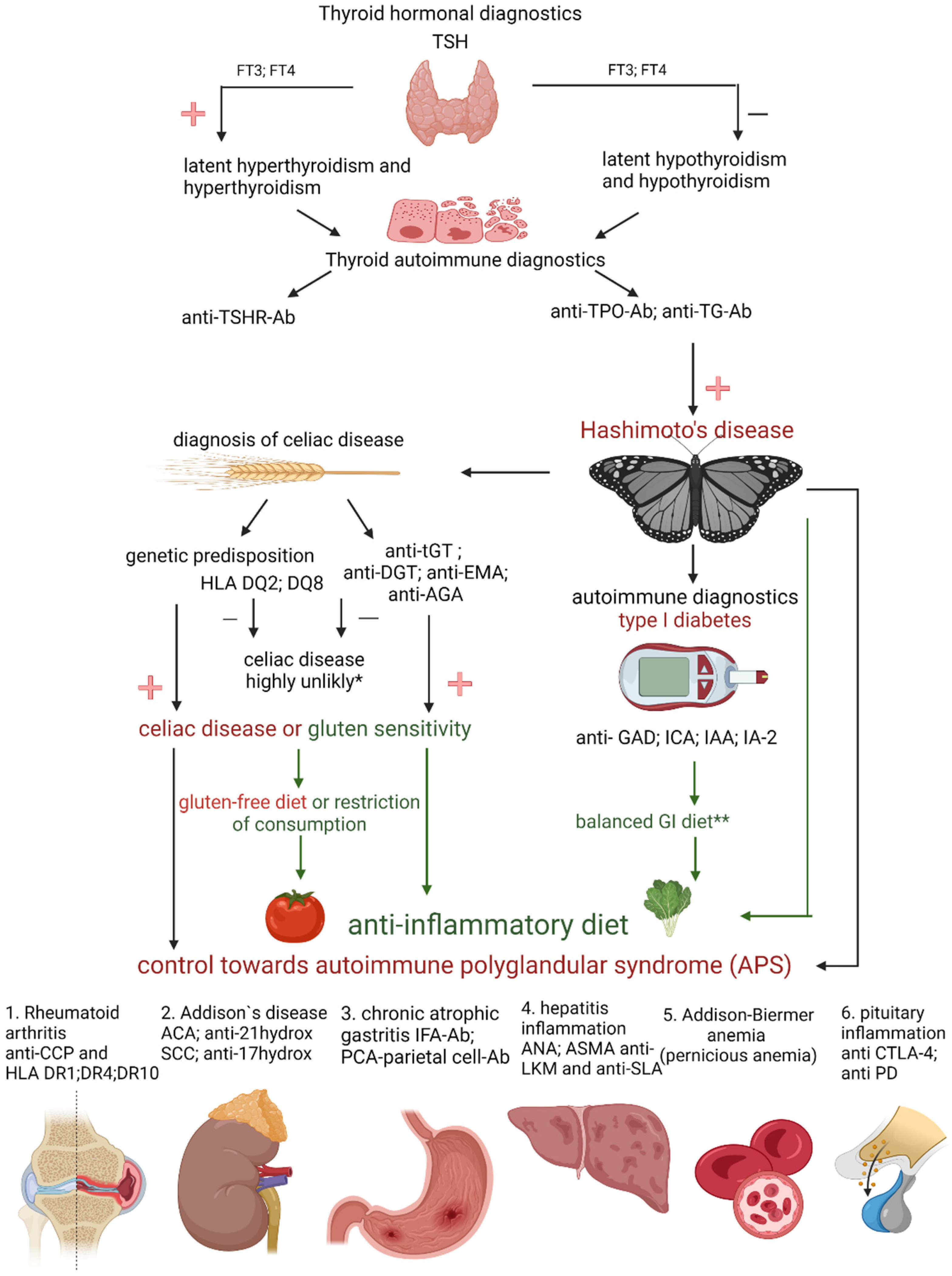
4. Diagnosis of Hashimoto’s Disease
5. Diseases Accompanying Hashimoto’s Disease
- APS 1 occurs with a frequency of 1:100,000 births, is usually diagnosed in childhood, and for its diagnosis, it is necessary to find Addison’s disease coexisting with at least one of two pathologies: hypoparathyroidism and/or mucosal and skin candidiasis [26]. Genetic factors play an important role in the development of APS. The disease is inherited in an autosomal and recessive manner. Patients struggling with autoimmune polyglandular syndrome often experience fertility problems that result from premature ovarian failure in women or primary testicular failure in men. In addition, patients may develop other autoimmune diseases such as type 1 diabetes, autoimmune hepatitis, alopecia areata, or CD [23];
- APS 2 occurs at a frequency of approximately 1:1000 births. Most often, its symptoms appear in adolescence. It is characterized by the coexistence of Addison’s disease with at least one of two diseases: autoimmune thyroid disease and/or type 1 diabetes [26]. These patients develop other autoimmune diseases, including those associated with non-endocrine diseases, e.g., celiac disease, ulcerative colitis, or neuropathy [23];
- APS 3 is diagnosed in the fourth decade of life. It is characterized by the coexistence of an autoimmune disease of the thyroid gland with another autoimmune disease(s) such as type 1 diabetes, gastritis, vitiligo, or alopecia areata [23]. Addison’s disease is not one of the components of APS 3.
6. Treatment
7. Gluten-Free Diet
8. Structure and Absorption of Gluten
9. Debatable Validity of Introducing the Gluten-Free Diet in Hashimoto’s Disease
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haahtela, T.; Holgate, S.; Pawankar, R.; Akdis, C.A.; Benjaponpitak, S.; Caraballo, L.; Demain, J.; Portnoy, J.; von Hertzen, L. The biodiversity hypothesis and allergic disease: World allergy organization position statement. World Allergy Organ. J. 2013, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bargiel, P.; Szczuko, M.; Stachowska, L.; Prowans, P.; Czapla, N.; Markowska, M.; Petriczko, J.; Kledzik, J.; Jędrzejczyk-Kledzik, A.; Palma, J.; et al. Microbiome Metabolites and Thyroid Dysfunction. J. Clin. Med. 2021, 10, 3609. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Feng, J.; Li, J.; Zhao, L.; Liu, Y.; Chen, H.; Jin, Y.; Zhu, B.; Wei, Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid 2018, 28, 175–186. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Weetman, A.P. The Pathogenesis of Hashimoto’s Thyroiditis: Further Developments in our Understanding. Horm. Metab. Res. 2015, 47, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.H.; Fu, D.G. Autoimmune thyroid disease: Mechanism, genetics and current knowledge. Eur. Rev. Med Pharmacol. Sci. 2014, 18, 3611–3618. [Google Scholar]
- Liontiris, M.I.; Mazokopakis, E.E. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients.Points that need more investigation. Hell J. Nucl. Med. 2017, 20, 51–56. [Google Scholar] [CrossRef]
- Sur, M.L.; Gaga, R.; Lazăr, C.; Lazea, C. Genetic and Environmental Factors in the Pathophysiology of Hashimoto’s Thyroiditis. Pediatr. Endocrinol. Rev. PER 2020, 17, 343–348. [Google Scholar] [CrossRef]
- Helmreich, D.L.; Tylee, D. Thyroid hormone regulation by stress and behavioral differences in adult male rats. Horm. Behav. 2011, 60, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimoto’s thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef]
- Puszkarz, I.; Guty, E.; Stefaniak, I.; Bonarek, A. Role of food and nutrition in pathogenesis and prevention of Hashimoto’s thyroiditis. J. Educ. Health Sport 2018, 8, 394–401. [Google Scholar] [CrossRef]
- Zaletel, K.; Gaberšček, S. Hashimoto’s Thyroiditis: From Genes to the Disease. Curr. Genom. 2011, 12, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Iddah, M.A.; Macharia, B.N. Autoimmune Thyroid Disorders. ISRN Endocrinol. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zagrodzki, P.; Kryczyk, J. The importance of selenium in the treatment of Hashimoto’s disease. Postepy. Hig. Med. Dosw. 2014, 68, 1129–1137. (In Polish) [Google Scholar] [CrossRef]
- Duntas, L.H. The Role of Iodine and Selenium in Autoimmune Thyroiditis. Horm. Metab. Res. 2015, 47, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, L.; Yao, P.; Yu, D.; Hao, L.; Sun, X. Effect of excessive iodine on immune function of lymphocytes and intervention with selenium. J. Huazhong Univ. Sci. Technol. 2007, 27, 422–425. [Google Scholar] [CrossRef]
- Lachowicz, K.; Stachoń, M.; Pałkowska-Goździk, E.; Lange, E. Fizjologiczne aspekty postępowania dietetycznego w chorobie Hashimoto. Kosmos 2019, 68, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Omiotek, Z.; Burda, A.; Wójcik, W. Method for classification of ultrasound thyroid images by decision tree induction. Pract. Inst. Elektrotechniki. 2012, 260, 57–68. (In Polish) [Google Scholar]
- Chaudhary, V.; Bano, S. Thyroid ultrasound. Indian J. Endocrinol. Metab. 2013, 17, 219–227. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ihnatowicz, P.; Drywień, M.; Wątor, P.; Wojsiat, J. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Ann. Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef]
- Kuliczkowska-Płaksej, J.; Tupikowska, M.; Zatońska, K.; Bednarek-Tupikowska, G.; Kuliczkowska-Płaksej, J. Subclinical thyroid dysfunction—Whether and when to treat? Fam. Med. Prim. Care Rev. 2013, 15, 27–33. (In Polish) [Google Scholar]
- Joubert, B.; Rostásy, K.; Honnorat, J. Immune-mediated ataxias. Neuroparasitology Trop. Neurol. 2018, 155, 313–332. [Google Scholar] [CrossRef]
- Husebye, E.S.; Anderson, M.S.; Kämpe, O. Autoimmune Polyendocrine Syndromes. N. Engl. J. Med. 2018, 378, 1132–1141. [Google Scholar] [CrossRef]
- Ulrich, J.; Goerges, J.; Keck, C.; Müller-Wieland, D.; Diederich, S.; Janssen, O.E. Impact of Autoimmune Thyroiditis on Reproductive and Metabolic Parameters in Patients with Polycystic Ovary Syndrome. Exp. Clin. Endocrinol. Diabetes 2018, 126, 198–204. [Google Scholar] [CrossRef]
- Siegmann, E.M.; Müller, H.H.O.; Luecke, C. Association of Depression and Anxiety Disorders with Autoimmune Thyroiditis—A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Kahaly, G.J.; Frommer, L.; Schuppan, D. Celiac Disease and Glandular Autoimmunity. Nutrients 2018, 10, 814. [Google Scholar] [CrossRef] [Green Version]
- Ihnatowicz, P.; Wątor, P.; Drywień, M.E. The importance of gluten exclusion in the management of Hashimoto’s thyroiditis. Ann. Agric. Environ. Med. 2021, 28, 558–568. [Google Scholar] [CrossRef]
- Oxentenko, A.S.; Rubio-Tapia, A. Celiac Disease. Mayo Clin. Proc. 2019, 94, 2556–2571. [Google Scholar] [CrossRef] [Green Version]
- Kaličanin, D.; Brčić, L.; Barić, A.; Zlodre, S.; Barbalić, M.; Lovrić, V.T.; Punda, A.; Perica, V.B. Evaluation of Correlations Between Food-Specific Antibodies and Clinical Aspects of Hashimoto’s Thyroiditis. J. Am. Coll. Nutr. 2019, 38, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Michałowska, J.; Pastusiak, K.; Bogdański, P. Controversies on gluten. Varia Medica 2018, 2, 13–19. (In Polish) [Google Scholar]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, F.; Di Lorenzo, C.; Biella, S.; Bani, C.; Restani, P. Ancient and Modern Cereals as Ingredients of the Gluten-Free Diet: Are They Safe Enough for Celiac Consumers? Foods 2021, 10, 906. [Google Scholar] [CrossRef]
- Ruiz-Carnicer, Á.; Comino, I.; Segura, V.; Ozuna, C.V.; Moreno, M.d.L.; López-Casado, M.Á.; Torres, M.I.; Barro, F.; Sousa, C. Celiac Immunogenic Potential of α-Gliadin Epitope Variants from Triticum and Aegilops Species. Nutrients 2019, 11, 220. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas-Torres, F.; Cabrera-Chávez, F.; Figueroa-Salcido, O.; Ontiveros, N. Non-Celiac Gluten Sensitivity: An Update. Medicine 2021, 57, 52. [Google Scholar] [CrossRef]
- Clarindo, M.V.; Possebon, A.T.; Soligo, E.M.; Uyeda, H.; Ruaro, R.T.; Empinotti, J.C. Dermatitis herpetiformis: Pathophysiology, clinical presentation, diagnosis and treatment. An. Bras. Dermatol. 2014, 89, 865–877. [Google Scholar] [CrossRef]
- Bonciani, D.; Verdelli, A.; Bonciolini, V.; D’Errico, A.; Antiga, E.; Fabbri, P.; Caproni, M. Dermatitis Herpetiformis: From the Genetics to the Development of Skin Lesions. Clin. Dev. Immunol. 2012, 2012, 239691. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPS Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef] [Green Version]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Myszkowska-Ryciak, J.; Harton, A.; Gajewska, D. Analysis of nutritional value and costs of gluten-free diet compared to standard food ration. Medycyna Ogólna i Nauki o Zdrowiu 2015, 21, 312–316. [Google Scholar] [CrossRef]
- Ciborowska, H.; Rudnicka, A. Dietetyka. Żywienie Zdrowego i Chorego Człowieka. IV, Rozszerzone i Uaktualnione; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2014; pp. 542–545. (In Polish) [Google Scholar]
- Caminero, A.; Nistal, E.; Herrán, A.R.; Pérez-Andrés, J.; Vaquero, L.; Vivas, S. Chapter 13—Gluten Metabolism in Humans: Involvement of the Gut Microbiota. In Wheat and Rice in Disease Prevention and Health; Academic Press: San Diego, CA, USA, 2014; pp. 157–170. [Google Scholar]
- Minelli, R.; Gaiani, F.; Kayali, S.; Di Mario, F.; Fornaroli, F.; Leandro, G.; Nouvenne, A.; Vincenzi, F.; Angelis, G.L.D. Thyroid and celiac disease in pediatric age: A literature review. Acta Biomed 2018, 89, 11–16. [Google Scholar] [PubMed]
- Rasheed, J.; Hassan, R.; Khalid, M.; Zafar, F. Frequency of autoimmune thyroiditis in children with Celiac disease and effect of gluten free diet. Pak. J. Med Sci. 2020, 36, 1280–1284. [Google Scholar] [CrossRef]
- Escarnot, E.; Gofflot, S.; Sinnaeve, G.; Dubois, B.; Bertin, P.; Mingeot, D. Reactivity of gluten proteins from spelt and bread wheat accessions towards A1 and G12 antibodies in the framework of celiac disease. Food Chem. 2018, 268, 522–532. [Google Scholar] [CrossRef]
- Cebolla, Á.; de Lourdes Moreno, M.; Coto, L.; Sousa, C. Gluten Immunogenic Peptides as Standard for the Evaluation of Potential Harmful Prolamin Content in Food and Human Specimen. Nutrients 2018, 10, 1927. [Google Scholar] [CrossRef] [Green Version]
- Riseh, S.H.; Farhang, M.A.; Mobasseri, M.; Jafarabadi, M.A. The Relationship between Thyroid Hormones, Antithyroid Antibodies, Anti-Tissue Transglutaminase and Anti-Gliadin Antibodies in Patients with Hashimoto’s Thyroiditis. Acta Endocrinol. 2017, 13, 174–179. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2018, 127, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Ventura, A.; Neri, E.; Ughi, C.; Leopaldi, A.; Città, A.; Not, T. Gluten-dependent diabetes-related and thyroid-related autoantibodies in patients with celiac disease. J. Pediatr. 2000, 137, 263–265. [Google Scholar] [CrossRef]
- Antvorskov, J.C.; Fundova, P.; Buschard, K.; Funda, D.P. Dietary gluten alters the balance of pro-inflammatory and anti-inflammatory cytokines in T cells of BALB/c mice. Immunology 2013, 138, 23–33. [Google Scholar] [CrossRef]
- Kus, K.; Zielińska, K.; Zaprutko, T.; Ratajczak, P.; Nowakowska, E. Hashimoto’s disease—The effectiveness of a gluten-free diet. Pol. Prz. Nauk Zdr. 2016, 4, 370–376. [Google Scholar]
- Konieczny, S.; Lange, E.; Krusiec, J. Wpływ diet eliminacyjnych na jakość życia osób z wybranymi chorobami autoimmunologicznymi. Kosmos 2019, 68, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Abbott, R.D.; Sadowski, A.; Alt, A.G. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus 2019, 11, e4556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pobłocki, J.; Pańka, T.; Szczuko, M.; Telesiński, A.; Syrenicz, A. Whether a Gluten-Free Diet Should Be Recommended in Chronic Autoimmune Thyroiditis or Not?—A 12-Month Follow-Up. J. Clin. Med. 2021, 10, 3240. [Google Scholar] [CrossRef] [PubMed]
- Passali, M.; Josefsen, K.; Frederiksen, J.L.; Antvorskov, J.C. Current Evidence on the Efficacy of Gluten-Free Diets in Multiple Sclerosis, Psoriasis, Type 1 Diabetes and Autoimmune Thyroid Diseases. Nutrients 2020, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Szostak-Węgierek, D.; Bednarczuk, T.; Respondek, W.; Traczyk, I.; Cukrowska, B.; Ostrowska, L.; Włodarek, D.; Jeznach-Steinhagen, A.; Bierła, J.; Lange, E.; et al. The validity of gluten-free diet in Hashimoto’s thyroiditis: Statement of the Expert Committee of the Section of Medical Dietetics of the Polish Society for Parenteral, Enteral Nutrition and Metabolism (POLSPEN). Adv. Clin. Nutr. 2018, 47, 33–47. [Google Scholar]
- Ravaglia, G.; Forti, P.; Maioli, F.; Volta, U.; Arnone, G.; Pantieri, G.; Talerico, T.; Muscari, A.; Zoli, M. Increased prevalence of coeliac disease in autoimmune thyroiditis is restricted to aged patients. Exp. Gerontol. 2003, 38, 589–595. [Google Scholar] [CrossRef]
- Roy, A.; Laszkowska, M.; Sundström, J.; Lebwohl, B.; Green, P.H.R.; Kämpe, O.; Ludvigsson, J.F. Prevalence of Celiac Disease in Patients with Autoimmune Thyroid Disease: A Meta-Analysis. Thyroid 2016, 26, 880–890. [Google Scholar] [CrossRef] [Green Version]
- Carroccio, A.; D’Alcamo, A.; Cavataio, F.; Soresi, M.; Seidita, A.; Sciumè, C.; Geraci, G.; Iacono, G.; Mansueto, P. High Proportions of People with Nonceliac Wheat Sensitivity Have Autoimmune Disease or Antinuclear Antibodies. Gastroenterology 2015, 149, 596.e1–603.e1. [Google Scholar] [CrossRef] [Green Version]
- Losurdo, G.; Principi, M.; Iannone, A.; Giangaspero, A.; Piscitelli, D.; Ierardi, E.; Di Leo, A.; Barone, M. Predictivity of Autoimmune Stigmata for Gluten Sensitivity in Subjects with Microscopic Enteritis: A Retrospective Study. Nutrients 2018, 10, 2001. [Google Scholar] [CrossRef] [Green Version]
- Zettinig, G.; Weissel, M.; Flores, J.; Dudczak, R.; Vogelsang, H. Dermatitis herpetiformis is associated with atrophic but not with goitrous variant of Hashimoto’s thyroiditis. Eur. J. Clin. Investig. 2000, 30, 53–57. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Number of Patients (n) | Duration of the GFD Diet | Age and Gender W = Women M = Men | Exclusion Criteria | Inclusion Criteria | Effect | Comment |
|---|---|---|---|---|---|---|---|
| Riseh 2017 [49] | n = 82 HD group n = 40 CG group n = 42 | GFD was notapplied | 20–50 years W | GFD before the intervention celiac disease, diabetes, cardiovascular diseases | – | in HD higher levels of anti-tissue transglutaminase and anti-gliadin IgA antibodies were observed | these studies confirmed the increased risk of CD in patients with Hashimoto’s disease and the frequent occurrence of its asymptomatic form in this group of patients |
| Krysiak 2019 [50] | n = 34 GFD group n = 16 CG group n = 18 | 6 months | 20–45 years W | GFD before the intervention + TRAb CD diabetes other chronic diseases | Euthyroid (0.4–4.5 mU/L) + anti-TPO (>100 U/mL) the reduced echogenicity | no change TSH and fT3 and fT4 ↧ anty-TPO and anty-TG | because no small intestinal biopsy was performed, it is possible that patients might have had subclinical (asymptomatic) coeliac disease |
| Ventura 2020 [51] | n = 180 CD n = 90 W including 11 person with HDCG group n = 90 | 6, 12, 24 months | age at diagnosis 10.1 years CD W = 61; M = 29 CG W = 60; M = 30 mean age 20.5 years | – | biopsy-confirmed CD | ↧ anty-TPO no abnormality was found in serum levels of thyroid hormones or thyroid-stimulating hormone | GFD started early may prevent the other autoimmune diseases frequently associated with CD |
| Kus 2016 [53] | n = 156 all with HD | survey research | 18–60 years W = 139 (89%); M = 17 (11%) | – | 75% respondents with HD were treated with levothyroxine | the respondents stated no symptoms of the digestive system after GFD | taking measurements in different places by the patient makes it impossible to compare the results accurately and is unreliable |
| Konieczny 2019 [54] | n = 209 HD group n = 81 CD n = 118 | survey research | 18–60 years HD W = 81, CD W = 106; M = 12 | – | – | respondents found a reduction in the incidence of digestive problems | biochemical studies have not been performed |
| Pobłocki 2021 [56] | n = 92 | 3, 6, 12 months | 18–55 years W | GFD malabsorption syndromes, bariatric surgery diabetes, hypertension, coronary artery disease, active inflammation, use of gluco-corticosteroids | diagnosed cAITD ultrasound image anty-TPO anty-TG | level of TSH, fT3, fT4, anti-TPO and anti-TG-no differences were found after 3 and 6 months ↥ fT4 and ↧ TSH after 12 months | the authors ruled out the influence of the gluten-free diet on thyroid parameters in people with Hashimoto’s without CD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczuko, M.; Syrenicz, A.; Szymkowiak, K.; Przybylska, A.; Szczuko, U.; Pobłocki, J.; Kulpa, D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients 2022, 14, 1727. https://doi.org/10.3390/nu14091727
Szczuko M, Syrenicz A, Szymkowiak K, Przybylska A, Szczuko U, Pobłocki J, Kulpa D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients. 2022; 14(9):1727. https://doi.org/10.3390/nu14091727
Chicago/Turabian StyleSzczuko, Małgorzata, Anhelli Syrenicz, Katarzyna Szymkowiak, Aleksandra Przybylska, Urszula Szczuko, Jakub Pobłocki, and Danuta Kulpa. 2022. "Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease" Nutrients 14, no. 9: 1727. https://doi.org/10.3390/nu14091727








