A Randomised, Double-Blind, Placebo-Controlled Crossover Trial of Resveratrol Supplementation for Prophylaxis of Hormonal Migraine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
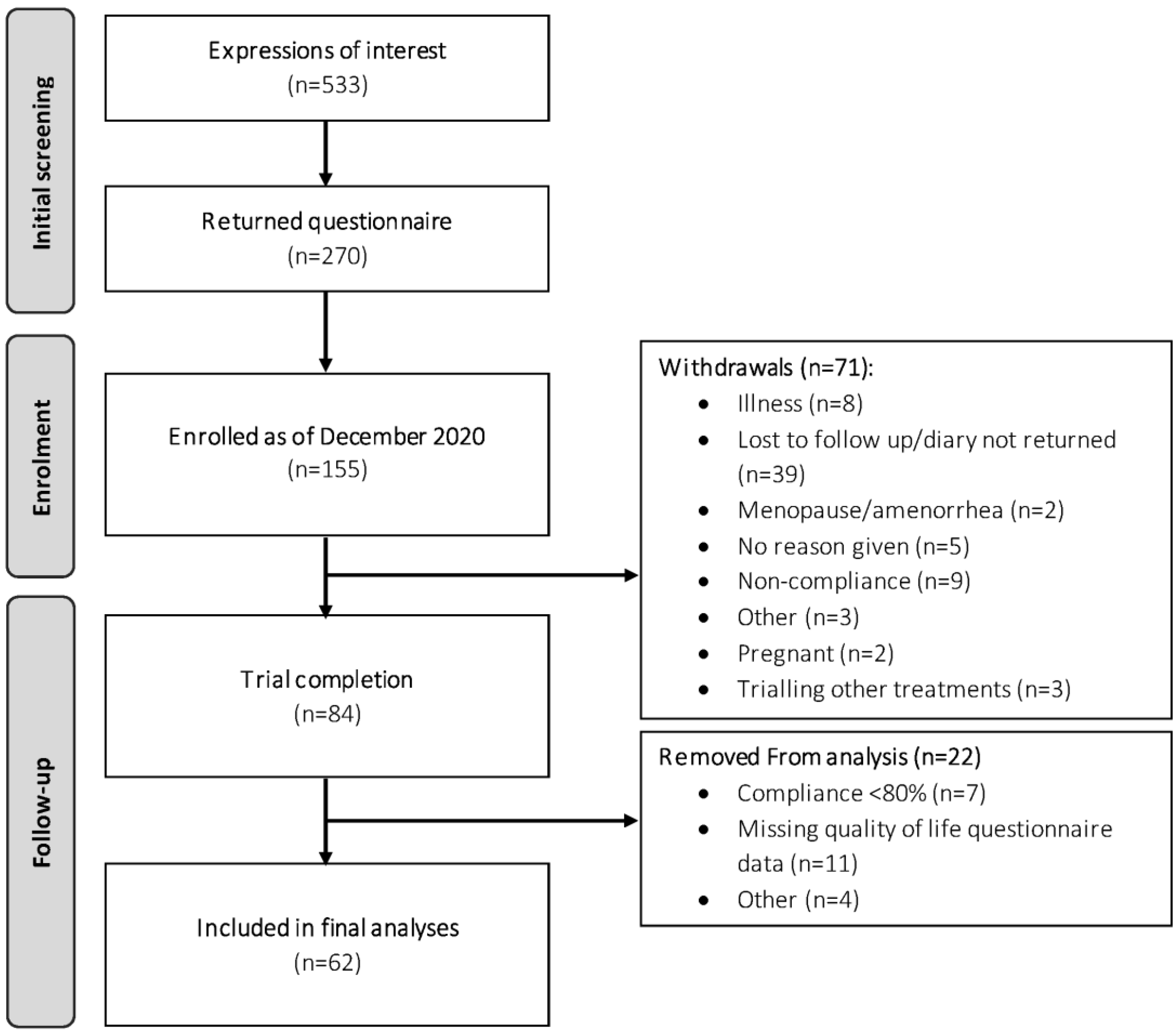
2.2. Study Population
2.3. Intervention and Treatment Regimen
2.4. Study Outcomes
2.4.1. Primary Outcome: Hormonal Migraine Burden Index
2.4.2. Secondary Outcomes
2.5. Sample Size
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Compliance
3.3. Primary Outcome
3.4. Secondary Outcomes
3.4.1. Visual Analogue Scale
3.4.2. Migraine-Related Disability and Quality of Life
3.4.3. Responders and Non-Responders
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steiner, T.J.; Stovner, L.J.; Birbeck, G.L. Migraine: The seventh disabler. J. Headache Pain 2013, 14, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Headache Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/headache-disorders (accessed on 14 January 2022).
- Woldeamanuel, Y.W.; Cowan, R.P. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J. Neurol. Sci. 2017, 372, 307–315. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, E.A. Menstrual migraine: Therapeutic approaches. Ther. Adv. Neurol. Disord. 2009, 2, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Sacco, S.; Ricci, S.; Degan, D.; Carolei, A. Migraine in women: The role of hormones and their impact on vascular diseases. J. Headache Pain 2012, 13, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Olesen, J. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Dzator, J.S.A.; Howe, P.R.C.; Griffiths, L.R.; Coupland, K.G.; Wong, R.H.X. Cerebrovascular Function in Hormonal Migraine: An Exploratory Study. Front. Neurol. 2021, 12, 694980. [Google Scholar] [CrossRef]
- Miguel, C.A.; Noya-Riobó, M.V.; Mazzone, G.L.; Villar, M.J.; Coronel, M.F. Antioxidant, anti-inflammatory and neuroprotective actions of resveratrol after experimental nervous system insults. Special focus on the molecular mechanisms involved. Neurochem. Int. 2021, 150, 105188. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Howe, P.R.C.; Wong, R.H.X. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Förstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol Acts as a Mixed Agonist/Antagonist for Estrogen Receptors α and β. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Goschorska, M.; Gutowska, I.; Baranowska-Bosiacka, I.; Barczak, K.; Chlubek, D. The Use of Antioxidants in the Treatment of Migraine. Antioxidants 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, E.J.; Drummond, P.D.; Lee-Visser, J.L.A. Reduction in Migraine and Headache Frequency and Intensity with Combined Antioxidant Prophylaxis (N-acetylcysteine, Vitamin E, and Vitamin C): A Randomized Sham-Controlled Pilot Study. Pain Pract. 2020, 20, 737–747. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Frith, A.; Ellis, J.; Aspinall, L.; Hackshaw, A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 2006, 67, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Vetvik, K.G.; MacGregor, E.A. Menstrual migraine: A distinct disorder needing greater recognition. Lancet Neurol. 2021, 20, 304–315. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Treatment allocation by minimisation. BMJ 2005, 330, 843. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.H.X.; Raederstorff, D.; Howe, P.R.C. Acute Resveratrol Consumption Improves Neurovascular Coupling Capacity in Adults with Type 2 Diabetes Mellitus. Nutrients 2016, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Xia, N.; Förstermann, U.; Li, H. Resveratrol and Endothelial Nitric Oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef]
- Wangberg, S.C.; Bergmo, T.S.; Johnsen, J.-A.K. Adherence in Internet-based interventions. Patient Prefer. Adherence 2008, 2, 57–65. [Google Scholar]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrante, F.; Fusco, E.; Calabresi, P.; Cupini, L.M. Phyto-oestrogens in the Prophylaxis of Menstrual Migraine. Clin. Neuropharmacol. 2004, 27, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.E.; Olson, R.D.; Cusack, B.J. Randomized, controlled trial of phytoestrogen in the prophylactic treatment of menstrual migraine. Biomed. Pharmacother. 2002, 56, 283–288. [Google Scholar] [CrossRef]
- Viereck, V.; Emons, G.; Wuttke, W. Black cohosh: Just another phytoestrogen? Trends Endocrinol. Metab. 2005, 16, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Roberts, R.L.; Elkins, G. Complementary and Alternative Medicine for Menopause. J. Evid.-Based Integr. Med. 2019, 24, 2515690X19829380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, A.; Goadsby, P.J. The Trigeminovascular System in Humans: Pathophysiologic Implications for Primary Headache Syndromes of the Neural Influences on the Cerebral Circulation. J. Cereb. Blood Flow Metab. 1999, 19, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, R. Understanding migraine: Potential role of neurogenic inflammation. Ann. Indian Acad. Neurol. 2016, 19, 175–182. [Google Scholar] [CrossRef]
| Characteristics | All Participants (n = 62) | Participants Initially Allocated to | ||
|---|---|---|---|---|
| Placebo (n = 31) | Resveratrol (n = 31) | p Value | ||
| Age (years) | 37.5 ± 0.8 | 37.4 ± 1.1 | 37.6 ± 1.1 | 0.887 |
| Years with hormonal migraine † | 11.4 ± 1.0 | 11.5 ± 1 | 11.3 ± 14 | 0.915 |
| Menstrual cycle length † | 28.4 ± 0.2 | 28.4 + 0.4 | 28.3 + 0.3 | 0.877 |
| Headache impact test-6™ | 63.5 ± 0.7 | 63.9 ± 1.2 | 63.1 ± 0.9 | 0.395 |
| Migraine disability assessment | ||||
| Total score | 19.6 ± 1.8 | 18 ± 1.9 | 21.1 ± 3.2 | 0.433 |
| Item A | 11.8 ± 0.9 | 11.2 ± 1.2 | 12.4 ± 1.4 | 0.575 |
| Item B | 6.6 ± 0.2 | 6.5 ± 0.3 | 6.7 ± 0.3 | 0.511 |
| Migraine-specific quality of life | ||||
| Emotional function domain | 48.8 ± 3.5 | 48.4 ± 5.4 | 49.2 ± 4.6 | 0.914 |
| Role function preventive domain | 60.3 ± 3.1 | 61.9 ± 4.2 | 58.7 ± 4.7 | 0.626 |
| Role function restrictive domain | 44.1 ± 3.0 | 46.3 ± 4.5 | 41.9 ± 4.0 | 0.496 |
| Estimated HMBI (migraine days/month) | 3.1 ± 0.2 | 3.0 ± 0.2 | 3.1 ± 0.3 | 0.862 |
| Contraception use (yes) | 16 (26%) | 8 (26%) | 8 (26%) | 1.000 |
| Smoking history (yes) | 10 (16%) | 4 (13%) | 6 (19%) | 0.490 |
| Guessed allocation correctly (%) ‡ | 35 (56%) | 15 (48%) | 20 (65%) | 0.205 |
| Characteristics | Crossover Comparison | |||
|---|---|---|---|---|
| Placebo (n = 62) | Resveratrol (n = 62) | Treatment Difference (Resveratrol–Placebo) | p-Value | |
| Raw number of hormonal migraines per phase | 1.03 ± 0.13 | 1.07 ± 0.14 | 0.032 ± 0.177 | 0.917 |
| Hormonal migraine burden index | 0.56 ± 0.09 | 0.56 ± 0.09 | −0.003 ± 0.099 | 0.895 |
| Characteristics | Crossover Comparison | |||
|---|---|---|---|---|
| Placebo (n = 62) | Resveratrol (n = 62) | Treatment Difference (Resveratrol–Placebo) | p-Value | |
| Headache impact test-6™ | 59.4 ± 0.9 | 58.7 ± 1.1 | −0.7 ± 0.9 | 0.491 |
| Migraine disability assessment | ||||
| Total score | 15.6 ± 2.3 | 12.6 ± 1.8 | −3.0 ± 2.4 | 0.367 |
| Item A | 9.9 ± 1.0 | 8.7 ± 0.8 | −1.3 ± 0.9 | 0.341 |
| Item B | 5.2 ± 0.2 | 5.4 ± 0.2 | 0.2 ± 0.3 | 0.587 |
| Migraine-specific quality of life | ||||
| Emotional function | 62.9 ± 3.8 | 67.1 ± 3.6 | 4.2 ± 4.2 | 0.253 |
| Role function preventive | 69.9 ± 3.5 | 75.3 ± 3.3 | 5.4 ± 3.3 | 0.106 |
| Role function restrictive | 57.8 ± 3.6 | 62.7 ± 3.6 | 4.9 ± 3.6 | 0.239 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzator, J.S.A.; Howe, P.R.C.; Coupland, K.G.; Wong, R.H.X. A Randomised, Double-Blind, Placebo-Controlled Crossover Trial of Resveratrol Supplementation for Prophylaxis of Hormonal Migraine. Nutrients 2022, 14, 1763. https://doi.org/10.3390/nu14091763
Dzator JSA, Howe PRC, Coupland KG, Wong RHX. A Randomised, Double-Blind, Placebo-Controlled Crossover Trial of Resveratrol Supplementation for Prophylaxis of Hormonal Migraine. Nutrients. 2022; 14(9):1763. https://doi.org/10.3390/nu14091763
Chicago/Turabian StyleDzator, Jemima S. A., Peter R. C. Howe, Kirsten G. Coupland, and Rachel H. X. Wong. 2022. "A Randomised, Double-Blind, Placebo-Controlled Crossover Trial of Resveratrol Supplementation for Prophylaxis of Hormonal Migraine" Nutrients 14, no. 9: 1763. https://doi.org/10.3390/nu14091763
APA StyleDzator, J. S. A., Howe, P. R. C., Coupland, K. G., & Wong, R. H. X. (2022). A Randomised, Double-Blind, Placebo-Controlled Crossover Trial of Resveratrol Supplementation for Prophylaxis of Hormonal Migraine. Nutrients, 14(9), 1763. https://doi.org/10.3390/nu14091763







