Nutrition during Pregnancy and Lactation: Epigenetic Effects on Infants’ Immune System in Food Allergy
Abstract
:1. Introduction
1.1. Food Allergy
1.2. Epigenetic Mechanisms
1.2.1. DNA Methylation
1.2.2. Histone Modifications
1.2.3. MicroRNAs
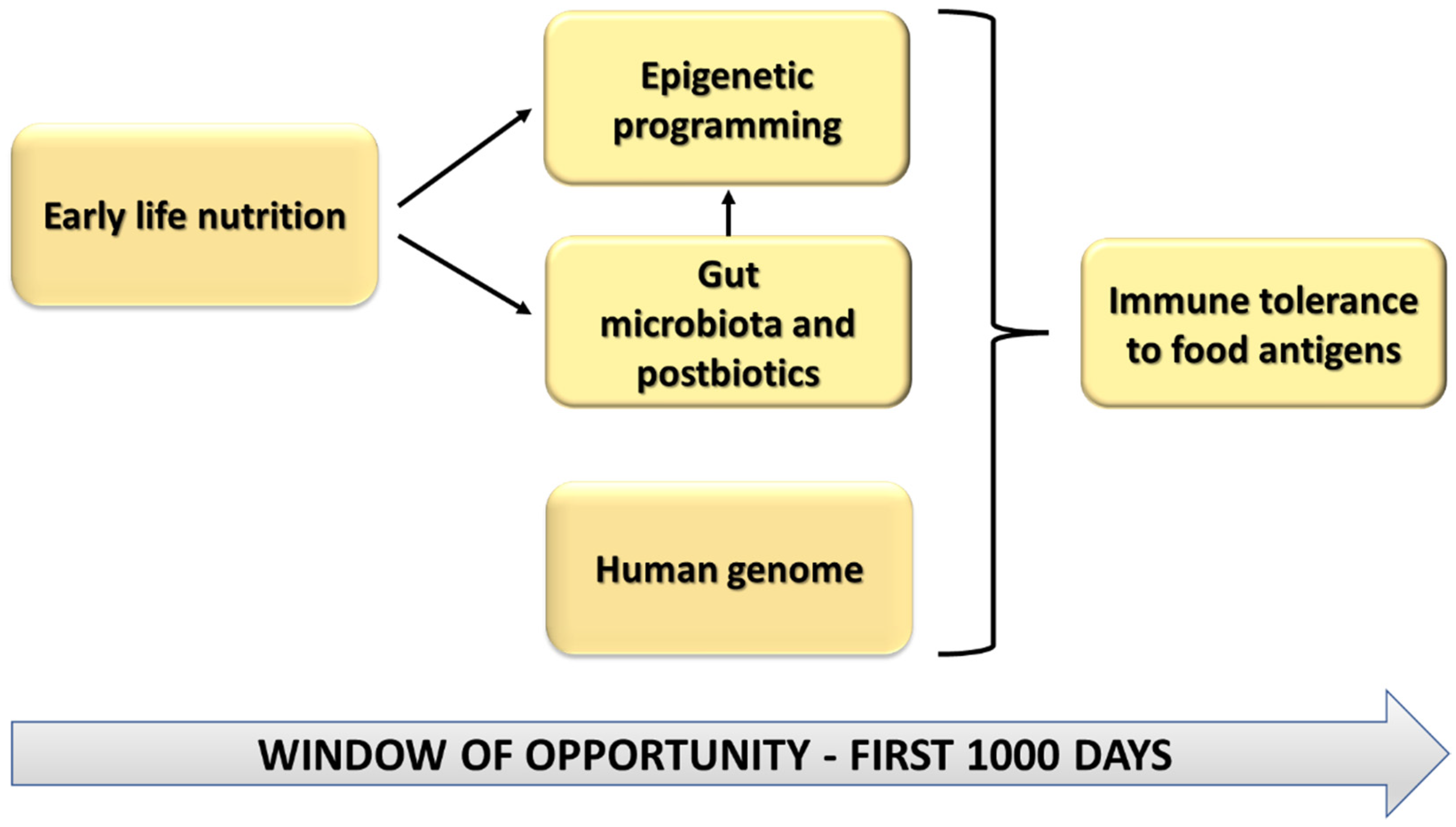
1.3. Pregnancy, Lactation and the First Year of Life: A Window of Opportunity
2. Early Life Nutrition and Epigenetic Effects in Food Allergy
2.1. Gut Microbiota
2.2. SCFAs
2.3. PUFAs
2.4. Vitamin D and A
2.5. Breastfeeding
2.6. Complementary Feeding
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACLY | ATP citrate lyase |
| ALA | Alpha-linolenic acid |
| ARA | Arachidonic acid |
| DCs | Dendritic cells |
| DHA | Docosahexaenoic acid |
| DNMT | DNA methyltransferases |
| EPA | Eicosapentaenoic acid |
| FoxP3 | Forkhead box P3 |
| GPCRs | G-protein coupled receptors |
| HDAC | Histone deacetylases |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| KDAC | Lysine deacetylase |
| LA | Linoleic acid |
| LCPUFAs | Long chain polyunsaturated fatty acids |
| miRNAs | microRNAs |
| mRNA | Messenger RNA |
| PBMCs | Peripheral blood mononuclear cells |
| PKCζ | Protein kinase C ζ |
| PUFAs | Polyunsaturated fatty acids |
| RA | Retinoic acid |
| RISC | RNA-induced silencing complex |
| SCFAs | Short-chain fatty acids |
| SNX25 | Sorting nexin 25 |
| TBX21 | T-box 21 |
| TGF-β | Transforming growth factor-beta |
| Th | T helper |
| Tregs | Regulatory T cells |
| TSLP | Thymic stromal lymphopoietin |
References
- Loh, W.; Tang, M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, R.L.; Mavoa, S.; Koplin, J.J. An Overview of Environmental Risk Factors for Food Allergy. Int. J. Environ. Res. Public Health 2022, 19, 722. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Alashkar Alhamwe, B.; Miethe, S.; Garn, H. Epigenetic Mechanisms in Allergy Development and Prevention. Handb. Exp. Pharmacol. 2022, 268, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity (Edinb.) 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, N.; Alhamwe, B.A.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and early-life nutrition, epigenetics, and allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef]
- Paparo, L.; Di Costanzo, M.; Di Scala, C.; Cosenza, L.; Leone, L.; Nocerino, R.; Canani, R.B. The influence of early life nutrition on epigenetic regulatory mechanisms of the immune system. Nutrients 2014, 6, 4706–4719. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Fu, J.; Zhou, Y. Research Progress in Atopic March. Front. Immunol. 2020, 11, 1907. [Google Scholar] [CrossRef]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2012, 38, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Li, E.; Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef] [PubMed]
- Gujar, H.; Weisenberger, D.J.; Liang, G. The roles of human DNA methyltransferases and their isoforms in shaping the epigenome. Genes (Basel) 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizuka-Koga, M.; Nakatsukasa, H.; Ito, M.; Akanuma, T.; Lu, Q.; Yoshimura, A. Induction and maintenance of regulatory T cells by transcription factors and epigenetic modifications. J. Autoimmun. 2017, 83, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Zentner, G.E.; Henikoff, S. Regulation of nucleosome dynamics by histone modifications. Nat. Struct. Mol. Biol. 2013, 20, 259–266. [Google Scholar] [CrossRef]
- Dutta, A.; Venkataganesh, H.; Love, P.E. New insights into epigenetic regulation of T cell differentiation. Cells 2021, 10, 3459. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Baskara-Yhuellou, I.; Tost, J. The impact of microRNAs on alterations of gene regulatory networks in allergic diseases. Adv. Protein Chem. Struct. Biol. 2020, 120, 237–312. [Google Scholar] [CrossRef]
- Bélanger, É.; Madore, A.M.; Boucher-Lafleur, A.M.; Simon, M.M.; Kwan, T.; Pastinen, T.; Laprise, C. Eosinophil micrornas play a regulatory role in allergic diseases included in the atopic march. Int. J. Mol. Sci. 2020, 21, 9011. [Google Scholar] [CrossRef]
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X. Early life precursors, epigenetics, and the development of food allergy. Semin. Immunopathol. 2012, 34, 655–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, H.M.; Rutten, N.B.M.M.; Boekhorst, J.; Saulnier, D.M.; Kortman, G.A.M.; Contractor, N.; Kullen, M.; Floris, E.; Harmsen, H.J.M.; Vlieger, A.M.; et al. Intestinal colonisation patterns in breastfed and formula-fed infants during the first 12 weeks of life reveal sequential microbiota signatures. Sci. Rep. 2017, 7, 8327. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F. Gut Microbiota Development: Influence of Diet from Infancy to Toddlerhood. Ann. Nutr. Metab. 2021, 77 (Suppl. 3), 21–34. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Joseph, C.L.; Sitarik, A.R.; Kim, H.; Huffnagle, G.; Fujimura, K.; Yong, G.J.M.; Levin, A.M.; Zoratti, E.; Lynch, S.; Ownby, D.R.; et al. Infant gut bacterial community composition and food-related manifestation of atopy in early childhood. Pediatr. Allergy Immunol. 2022, 33, e13704. [Google Scholar] [CrossRef]
- Loo, E.X.L.; Ong, L.; Goh, A.; Chia, A.R.; Teoh, O.H.; Colega, M.T.; Chan, Y.H.; Saw, S.M.; Kwek, K.; Gluckman, P.D.; et al. Effect of Maternal Dietary Patterns during Pregnancy on Self-Reported Allergic Diseases in the First 3 Years of Life: Results from the GUSTO Study. Int. Arch. Allergy Immunol. 2017, 173, 105–113. [Google Scholar] [CrossRef]
- Biagi, C.; Di Nunzio, M.; Bordoni, A.; Gori, D.; Lanari, M. Effect of adherence to mediterranean diet during pregnancy on children’s health: A systematic review. Nutrients 2019, 11, 997. [Google Scholar] [CrossRef] [Green Version]
- Grimshaw, K.E.C.; Maskell, J.; Oliver, E.M.; Morris, R.C.G.; Foote, K.D.; Mills, E.N.C.; Margetts, B.M.; Roberts, G. Diet and food allergy development during infancy: Birth cohort study findings using prospective food diary data. J. Allergy Clin. Immunol. 2014, 133, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; Hayglass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Padrão, P.; Teixeira, V.H.; Carvalho, P.; Delgado, L.; Lopes, C.; Severo, M.; Moreira, P. Dietary patterns and asthma prevalence, incidence and control. Clin. Exp. Allergy 2015, 45, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef] [Green Version]
- Di Costanzo, M.; De Paulis, N.; Biasucci, G. Butyrate: A link between early life nutrition and gut microbiome in the development of food allergy. Life 2021, 11, 384. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 799–809. [Google Scholar] [CrossRef]
- Cait, A.; Cardenas, E.; Dimitriu, P.A.; Amenyogbe, N.; Dai, D. Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. J. Allergy Clin. Immunol. 2019, 144, 1638–1647.e3. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Schilderink, R.; Verseijden, C.; Seppen, J.; Muncan, V.; van den Brink, G.R.; Lambers, T.T.; van Tol, E.A.; de Jonge, W.J. The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 310, G1138–G1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goverse, G.; Molenaar, R.; Macia, L.; Tan, J.; Erkelens, M.N.; Konijn, T.; Knippenberg, M.; Cook, E.C.L.; Hanekamp, D.; Veldhoen, M.; et al. Diet-Derived Short Chain Fatty Acids Stimulate Intestinal Epithelial Cells To Induce Mucosal Tolerogenic Dendritic Cells. J. Immunol. 2017, 198, 2172–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Kaga, N.; Nakanishi, Y.; Ohno, H.; Miyamoto, J.; Kimura, I.; Hori, S.; Sasaki, T.; Hiramatsu, K.; Okumura, K.; et al. Maternal High Fiber Diet during Pregnancy and Lactation Influences Regulatory T Cell Differentiation in Offspring in Mice. J. Immunol. 2017, 199, 3516–3524. [Google Scholar] [CrossRef] [Green Version]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Le Moan, N.; Grueter, C.A.; Lim, H.; Laura, R.; Stevens, R.D.; Newgard, C.B.; et al. Supression of oxidative stress and β-OHB as endogenous histone deactetylase. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Justin, R.; Thompson, C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Hoppenbrouwers, T.; Cvejić Hogervorst, J.H.; Garssen, J.; Wichers, H.J.; Willemsen, L.E.M. Long chain polyunsaturated fatty acids (LCPUFAs) in the prevention of food allergy. Front. Immunol. 2019, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-chain polyunsaturated fatty acids (LCPUFAs) and the developing immune system: A narrative review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Can early omega-3 fatty acid exposure reduce risk of childhood allergic disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef] [Green Version]
- Wendell, S.G.; Baffi, C.; Holguin, F. Fatty Acids, Inflammation, and Asthma. J. Allergy Clin. Immunol. 2014, 133, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Willemsen, L.E.M. Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur. J. Pharmacol. 2016, 785, 174–186. [Google Scholar] [CrossRef]
- Sartorio, M.U.A.; Pendezza, E.; Coppola, S.; Paparo, L.; D’auria, E.; Zuccotti, G.V.; Berni Canani, R. Potential role of omega-3 polyunsaturated fatty acids in pediatric food allergy. Nutrients 2022, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- LARN. Dietary Reference Values of Nutrients and Energy for the Italian Population. Revision IV 2014. Available online: https://eng.sinu.it/larn/ (accessed on 28 February 2022).
- Sioen, I.; van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C.; et al. Systematic Review on N-3 and N-6 Polyunsaturated Fatty Acid Intake in European Countries in Light of the Current Recommendations-Focus on Specific Population Groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef]
- Thomas Brenna, J.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Iranpour, R.; Kelishadi, R.; Babaie, S.; Khosravi-Darani, K.; Farajian, S. Comparison of long chain polyunsaturated fatty acid content in human milk in preterm and term deliveries and its correlation with mothers’ diet. J. Res. Med. Sci. 2013, 18, 1–5. [Google Scholar]
- Palmer, D.J.; Sullivan, T.; Gold, M.S.; Prescott, S.L.; Heddle, R.; Gibson, R.A.; Makrides, M. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: Randomised controlled trial. BMJ 2012, 344, e184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harb, H.; Irvine, J.; Amarasekera, M.; Hii, C.S.; Kesper, D.A.; Ma, Y.F.; D’Vaz, N.; Renz, H.; Potaczek, D.P.; Prescott, S.L.; et al. The role of PKCζ in cord blood T-cell maturation towards Th1 cytokine profile and its epigenetic regulation by fish oil. Biosci. Rep. 2017, 37, BSR20160485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, N.; Frumento, P.; Harb, H.; Alhamwe, B.A.; Johansson, C.; Eick, L.; Alm, J.; Renz, H.; Scheynius, A.; Potaczek, D.P. Histone acetylation of immune regulatory genes in human placenta in association with materal intake of olive oil and fish consumption. Int. J. Mol. Sci. 2019, 20, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Barraza-Villarreal, A.; Hernandez-Vargas, H.; Sly, P.D.; Biessy, C.; Ramakrishnan, U.; Romieu, I.; Herceg, Z. Modulation of DNA methylation states and infant immune system by dietary supplementation with v-3 PUFA during pregnancy in an intervention study. Am. J. Clin. Nutr. 2013, 98, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, T.; Tanaka, K.; Yamashita, H.; Saneyasu, K.-I.; Tanaka, H.; Takasato, Y.; Sugiura, S.; Inagaki, N.; Ito, K. Food allergy is linked to season of birth, sun exposure, and vitamin D deficiency. Allergol. Int. 2019, 68, 172–177. [Google Scholar] [CrossRef]
- Allen, K.J.; Koplin, J.J.; Ponsonby, A.L.; Gurrin, L.C.; Wake, M.; Vuillermin, P.; Martin, P.; Matheson, M.; Lowe, A.; Robinson, M.; et al. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J. Allergy Clin. Immunol. 2013, 131, 1109–1116.e6. [Google Scholar] [CrossRef]
- Cañas, J.A.; Núñez, R.; Cruz-amaya, A.; Gómez, F.; Torres, M.J.; Palomares, F.; Mayorga, C. Epigenetics in food allergy and immunomodulation. Nutrients 2021, 13, 4345. [Google Scholar] [CrossRef]
- Fetahu, I.S.; Höbaus, J.; Kállay, E. Vitamin D and the epigenome. Front. Physiol. 2014, 5, 164. [Google Scholar] [CrossRef] [Green Version]
- Poole, A.; Song, Y.; Brown, H.; Hart, P.H.; Zhang, G. (Brad) Cellular and molecular mechanisms of vitamin D in food allergy. J. Cell. Mol. Med. 2018, 22, 3270–3277. [Google Scholar] [CrossRef] [Green Version]
- Junge, K.M.; Bauer, T.; Geissler, S.; Hirche, F.; Thürmann, L.; Bauer, M.; Trump, S.; Bieg, M.; Weichenhan, D.; Gu, L.; et al. Increased Vitamin D levels at birth and in early infancy increase offspring allergy risk-Evidence for involvement of epigenetic mechanisms. J. Allergy Clin. Immunol. 2016, 137, 610–613. [Google Scholar] [CrossRef]
- Comeau, M.R.; Ziegler, S.F. The influence of TSLP on the allergic response. Mucosal Immunol. 2010, 3, 138–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, T. TSLP expression: Cellular sources, triggers, and regulatory mechanisms. Allergol. Int. 2012, 61, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Wang, X.; Shi, H.; Su, S.; Harshfield, G.A.; Gutin, B.; Snieder, H.; Dong, Y. A genome-wide methylation study of severe vitamin D deficiency in African American adolescents. J. Pediatr. 2013, 162, 1004–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.M.; Gillespie, S.L.; Thiele, D.K.; Ralph, J.L.; Ohm, J.E. Effects of Maternal Vitamin D Supplementation on the Maternal and Infant Epigenome. Breastfeed. Med. 2018, 13, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.T.; Wang, L.; Wei, Z.Z.; Liu, B.; Liu, X.Y.; Yu, X.D. Vitamin D deficiency during pregnancy affects the function of Th1/Th2 cells and methylation of IFN-γ gene in offspring rats. Immunol. Lett. 2019, 212, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Song, Y.; O’Sullivan, M.; Pereira, G.; Loh, R.; Zhang, G. The Implications of DNA Methylation on Food Allergy. Int. Arch. Allergy Immunol. 2017, 173, 183–192. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, Y.; Shen, X.; Yang, K.; Cai, W. Maternal and early-life vitamin D deficiency enhances allergireaction in an ovalbumin-sensitized BALB/c mouse model. Food Nutr. Res. 2018, 62, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Weisse, K.; Winkler, S.; Hirche, F.; Herberth, G.; Hinz, D.; Bauer, M.; Röder, S.; Rolle-Kampczyk, U.; von Bergen, M.; Olek, S.; et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy 2013, 68, 220–228. [Google Scholar] [CrossRef]
- Kim, C.H. Control of innate and adaptive lymphocytes by the RAR-retinoic acid axis. Immune Netw. 2018, 18, e1. [Google Scholar] [CrossRef] [Green Version]
- Maeta, A.; Matsushima, M.; Katahira, R.; Takahashi, K. Retinoic acid ameliorates the severity of food allergy under allergen exposure in a mouse model with food allergy. J. Nutr. Sci. Vitaminol. (Tokyo) 2020, 66, 375–380. [Google Scholar] [CrossRef]
- Badolati, I.; Sverremark-Ekström, E.; van der Heiden, M. Th9 cells in allergic diseases: A role for the microbiota? Scand. J. Immunol. 2020, 91, e12857. [Google Scholar] [CrossRef] [PubMed]
- Breastfeeding-WHO World Health Organization. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 28 February 2022).
- Verduci, E.; Banderali, G.; Barberi, S.; Radaelli, G.; Lops, A.; Betti, F.; Riva, E.; Giovannini, M. Epigenetic effects of human breast milk. Nutrients 2014, 6, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- McDade, T.W.; Metzger, M.W.; Chyu, L.; Duncan, G.J.; Garfield, C.; Adam, E.K. Long-term effects of birth weight and breastfeeding duration on inflammation in early adulthood. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saarinen, U.M.; Kajosaari, M. Breastfeeding as prophylaxis against atopic disease: Prospective. Lancet 1995, 346, 1065–1069. [Google Scholar] [CrossRef]
- Muraro, A.; Dreborg, S.; Halken, S.; Høst, A.; Niggemann, B.; Aalberse, R.; Arshad, S.H.; Von Berg, A.; Carlsen, K.H.; Duschén, K.; et al. Dietary prevention of allergic diseases in infants and small children. Part II: Evaluation of methods in allergy prevention studies and sensitization markers. Definitions and diagnostic criteria of allergic diseases. Pediatr. Allergy Immunol. 2004, 15, 196–205. [Google Scholar] [CrossRef]
- Lodge, C.J.; Lowe, A.J.; Milanzi, E.; Bowatte, G.; Abramson, M.J.; Tsimiklis, H.; Axelrad, C.; Robertson, B.; Darling, A.E.; Svanes, C.; et al. Human milk oligosaccharide profiles and allergic disease up to 18 years. J. Allergy Clin. Immunol. 2021, 147, 1041–1048. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Ren, F.; Zhu, S.; Ren, Y.; Jia, B.; Li, Y.P.; Shi, Y.; Chang, Z. SNX25 regulates TGF-β signaling by enhancing the receptor degradation. Cell. Signal. 2011, 23, 935–946. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Micrornas in breastmilk and the lactating breast: Potential immunoprotectors and developmental regulators for the infant and the mother. Int. J. Environ. Res. Public Health 2015, 12, 13981–14020. [Google Scholar] [CrossRef] [Green Version]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as bioactive human milk protective component against food allergy. Allergy 2021, 76, 1398–1415. [Google Scholar] [CrossRef]
- Comberiati, P.; Costagliola, G.; D’Elios, S.; Peroni, D. Prevention of food allergy: The significance of early introduction. Medicina 2019, 55, 323. [Google Scholar] [CrossRef] [Green Version]
- Tham, E.H.; Shek, L.P.; Van Bever, H.P.; Vichyanond, P.; Ebisawa, M.; Wong, G.W.; Lee, B.W.; Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI). Early introduction of allergenic foods for the prevention of food allergy from an Asian perspective-An Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) consensus statement. Pediatr. Allergy Immunol. 2018, 29, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.A.; Smith, J.; Vale, S.; Campbell, D.E. The Australasian Society of Clinical Immunology and Allergy infant feeding for allergy prevention guidelines. Med. J. Aust. 2019, 210, 89–93. [Google Scholar] [CrossRef] [PubMed]

| Pregnancy | Lactation | Infants 6–12 Months | |
|---|---|---|---|
| Vitamin D | 15 µg | 15 µg | 10 µg |
| Vitamin A | 1000 µg | 700 µg | 450 µg |
| LCPUFAs | EPA + DHA 250 mg + DHA 100–200 mg | EPA + DHA 250 mg + DHA 100–200 mg | EPA + DHA 250 mg + DHA 100 mg |
| Fibers | 12.6–16.7 g/1000 kcal | 12.6–16.7 g/1000 kcal | 8.4 g/1000 kcal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Costanzo, M.; De Paulis, N.; Capra, M.E.; Biasucci, G. Nutrition during Pregnancy and Lactation: Epigenetic Effects on Infants’ Immune System in Food Allergy. Nutrients 2022, 14, 1766. https://doi.org/10.3390/nu14091766
Di Costanzo M, De Paulis N, Capra ME, Biasucci G. Nutrition during Pregnancy and Lactation: Epigenetic Effects on Infants’ Immune System in Food Allergy. Nutrients. 2022; 14(9):1766. https://doi.org/10.3390/nu14091766
Chicago/Turabian StyleDi Costanzo, Margherita, Nicoletta De Paulis, Maria Elena Capra, and Giacomo Biasucci. 2022. "Nutrition during Pregnancy and Lactation: Epigenetic Effects on Infants’ Immune System in Food Allergy" Nutrients 14, no. 9: 1766. https://doi.org/10.3390/nu14091766






