1. Introduction
Low breast milk supply is the most frequent cause of breastfeeding failure [
1]. Identifying safe and efficient dairy products or drugs is an urgent task for scientists. Lactation is defined as the secretion of milk from the mammary gland (MG) and is influenced by a complex hormonal network [
2]. Although initially required for the morphological development and differentiation of the MG, prolactin (PRL) plays a crucial role in stimulating milk protein (MP) and lactose synthesis to promote milk synthesis [
3,
4]. Breastfeeding is arguably the best way to feed. Breast milk can provide a variety of nutritional and bioactive health factors, breastfeeding can improve the health status and immune system development of infants and breastfed infants have a lower incidence of gastrointestinal diseases and mortality [
5,
6,
7]. Amino acids (AAs) play crucial roles in the synthesis of MP in the MG. Most recently, in Wu’s review [
8], the amino acid uptake/output ratios in four different mammals showed that five AAs are associated with lactation: Val, Ile, Leu, Arg and His. Among these, in vitro experiments in mammary epithelial cells (MECs) demonstrated that Val could increase β-casein synthesis by activating the mammalian target of rapamycin (mTOR) and the Ras/ERK signaling pathway and Arg could promote cell proliferation and increase protein synthesis by activating the mTOR signaling pathway. However, the underlying mechanisms and signaling pathways by which AAs regulate milk synthesis were largely unknown until recently. Zhou et al. summarized some recent studies on the effects of EAAs on MP synthesis in dairy cows. Essential amino acids (EAAs) that can increase milk yield and MP yield include Met, Val, His, Phe, Thr, Arg and Trp. In addition, Val can increase the transcription of casein’s genes and His can promote β-casein synthesis and increase milk yield, and it tends to increase the MP yield. Moreover, Phe, Thr, Arg and Trp are positively associated with milk yield [
9]. These studies on lactation have mainly focused on dairy cows, goats, sows and ewes, with the purpose of improving the quality of dairy products, milk yield and quality. There is still a lack of research on human lactation, which needs to be developed.
The oyster is one of the main shellfish cultured in the coastal areas of Guangdong, Guangxi, Fujian and Shandong. The Hong Kong oyster (
Crassostrea hongkongensis) is an economically important invertebrate that is found in mudflats and is widely cultivated in southern China [
10]. Around 400 years ago, it was recorded in
Ben Cao Gang Mu that oyster meat cured asthenia and healed women’s blood. Around 200 years ago, it was recorded in Fu Qing-zhu Gynecology that oysters had a remarkable effect on sweating irritability. According to the Chinese Materia Medica, oysters have long been used in foods and pharmaceuticals. As they are very rich in protein, oysters are a good source for the preparation of bioactive peptides.
Studies have shown that the polypeptides from oyster enzymatic hydrolysates have various physiological activities, such as enhancing immunity [
11] and antitumor [
12,
13], anti-inflammatory [
14,
15] and antioxidative effects [
16]. There is no literature related to oyster peptides and lactation. In our previous study, an enzymatic hydrolysate of
Crassostrea hongkongensis could obviously increase hourly lactation in overloaded lactating rats and pup weight, significantly promoted the expansion and filling of the mammary gland’s acinar cavity in these rats and promoted lactation [
17]. This suggests that oysters can be used as a dietary source of exogenous peptides to promote milk secretion. More recently, Cai et al. found that the bioactive polypeptide OPH3-1 of octopus protein hydrolysate can promote the proliferation of mouse mammary epithelial cells and promote the synthesis of β-casein [
18]. Other studies found that small peptides can be absorbed in an intact form [
19,
20,
21]. Moreover, peptides appear to be more efficient than free amino acids in promoting protein synthesis in lactating mammary tissue, but little is known about the underlying mechanisms. Thus, further understanding the nutritional strategies with which to regulate milk synthesis and its underlying mechanism is important for human beings.
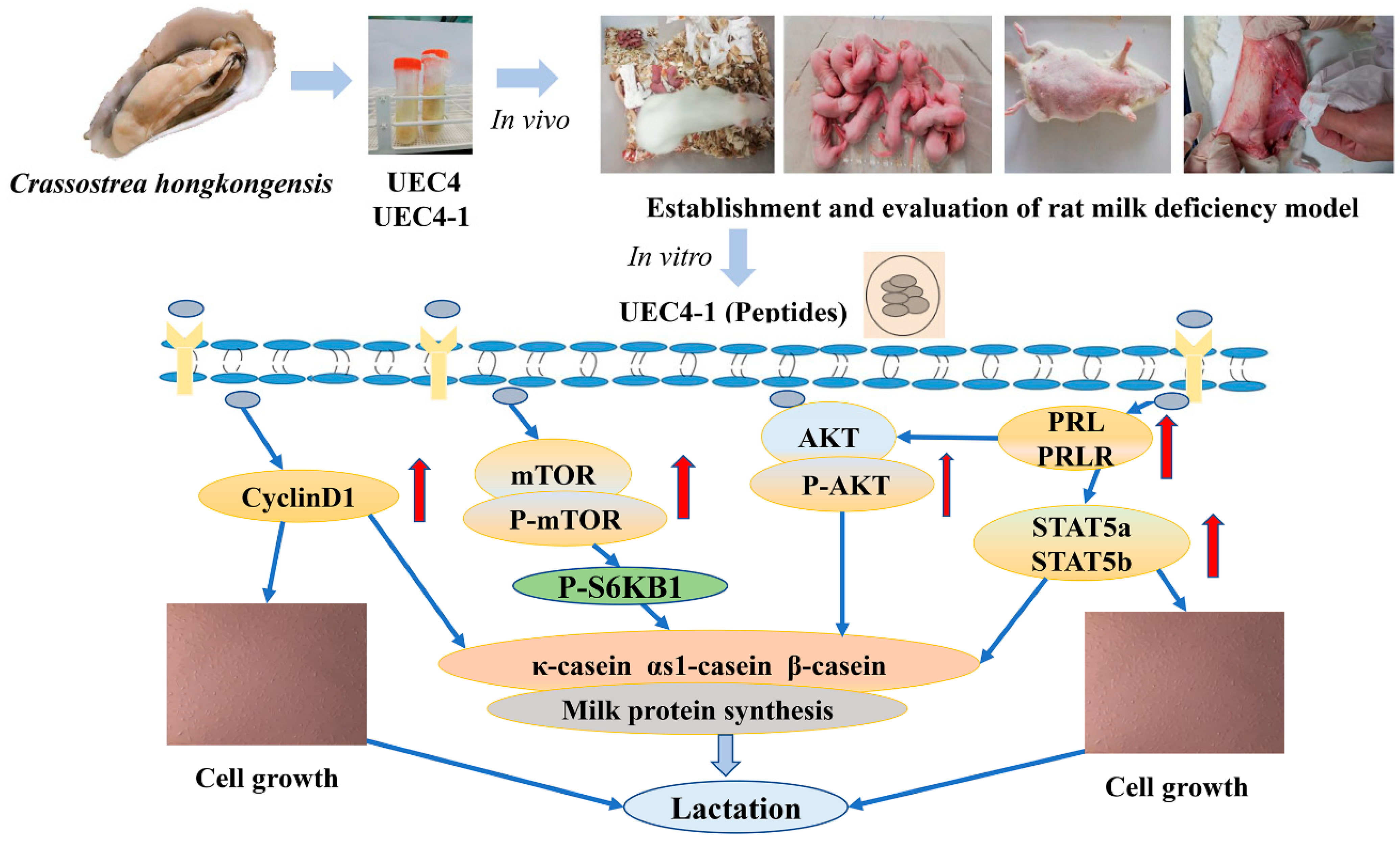
In this study, we studied the potential efficacy and mechanism of the active peptide UEC4-1 for improving postpartum hypogalactia in rats with hypogalactia. The effect of UEC4-1 on the proliferation of HMECs was studied to lay the foundation for structural analysis and research into the lactation mechanism.
2. Materials and Methods
2.1. Materials
Live Hong Kong oysters (Crassostrea hongkongensis) were obtained from the Dongfeng Aquatic Products Market (Zhanjiang, China).
The normal human mammary epithelial cell line MCF-10A (item: BNCC 341931), a complete growth medium (DMEM/F12 + 5% HS + 20 ng/mL EGF + 0.5 μg/mL hydrocortisone + 10 μg/mL insulin + 1% NEAA + 1% P/S) and DMEM/F12 (1:1) basic (1×) (Gibco, C11330500BT) were purchased from Beina Chuanglian Biotechnology Co., Ltd. (Beijing, China). AKT1, αs1-casein (CSN1S1) and Cyclin D1 were sourced from Proteintech (Rosemont, IL, USA); P-AKT and p-S6K1 were obtained from Cell Signaling (Danfoss, MA, USA); S6K1, mTOR, P-mTOR, κ-casein (CSN3), β-lactoglobulin (BLG), β-casein (CSN2), prolactin (PRL), prolactin receptor (PRLR), STAT5a, STAT5b and β-actin were obtained from Affinity biosciences, OH, USA; and casein enzymatic hydrolysate was obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China).
2.2. Preparation of Oyster Peptides
According to methods previously described by our group [
17], the hydrolysates of the prepared oysters were subjected to ultrafiltration after being dissolved and diluted in distilled water. The supernatant was sequentially fractionated using 10 kDa, 5 kDa, 3 kDa and 1 kDa ultrafiltration membranes to obtain 5–10 kDa, 3–5 kDa, 1–3 kDa and <1 kDa fractions, which were given the names UEC1 to UEC4 in sequence (UEC stands for ultrafiltration components of enzymatic hydrolysates of
Crassostrea hongkongensis). Next, the filtered solution was freeze-dried and stored at −5 °C until use. The peptide preparation process and the evaluation of cell proliferation are shown in
Figure 1.
The cell proliferation rate was determined by the MTT method. The pretreated Sephadex G-15 was loaded into a 1.6 × 100 cm2 glass chromatographic column, and a quantity of UEC4 was accurately weighed. The 50 mg/mL solution was prepared with deionized water, and 5 mL of the sample solution was loaded into the column. The sample solution was eluted with deionized water at a flow rate of 0.33 mL/min. The UV detector was used to detect the components at 280 nm, and the components were collected. A sample was collected into a tube every 10 min, and 3.33 mL of the contents of each tube were frozen and dried for further use. A NaCl eluent (1 moL/L) was prepared with a 10 mmoL Tris-buffer solution, 0.50 g of the sample solution was added to 10 mL of the 10 mM Tris-buffer solution to make a final concentration of 50 mg/mL of the sample solution, and the mixture was centrifuged at 12,000× g rpm (12,800× g). The upper sample solution was passed through a 0.22 μm filter. The Superdex 30 Increase 3.2/300 pre-packed peptide column was combined with an AKTA Purifier100 protein purification system. The equilibrium solution was continuously washed, and the flow rate was 0.5 mL/min and 0.8 mL/tube. The absorbance of the elution peak was detected at 220 nm. For the RP-HPLC analysis, we took a certain amount of the best proliferation component sample, dissolved it in 1 mL of ultrapure water and prepared a 20 mg/mL concentration of the polypeptide sample solution. The sample solution was injected into a reversed-phase column and subjected to high-performance liquid chromatography. Sample volume: 1 mL; mobile phase: 5–80% (v/v) methanol containing 0.1% (v/v) trifluoroacetic acid (TFA); flow rate: 0.8 mL/min; linear elution time, 35 min; column temperature, 35 °C; eluent absorbance was detected at 220 nm.
2.3. Peptide Identification by LC-ESI-Orbitrap MS/MS
The samples of UEC4-1 were sent to the Shenzhen Huada Gene Laboratory for sample determination using an Orbitrap FUSION LUMOS mass spectrometer. The offline data were collected using the MaxQuant integrated Andromeda engine. At the spectral level, the filtration was completed with a PSM-level FDR ≤ 1%, and, at the peptide level, the filtration was further carried out with a peptide-level FDR ≤ 1%. The data were processed by searching the Uniprot protein database and the NCBI and Ensembl genome protein databases to obtain the dominant identification results.
2.4. Animal Experiment
2.4.1. Animals
Specific-pathogen-free (SPF) healthy SD rats (5–6 weeks old) were purchased from Zhuhai Baishitong Biotechnology Co., Ltd. (Zhuhai, China; animal production license number: SCXK (Guangdong) 2020-0051; certificate number: 44822700004877). There were 90 female rats and 45 male rats. The body weights of the female rats were 150–180 g, and those of the male rats were 180–200 g. The rats were housed separately in standard cages with wood chips and were allowed access to food and water ad libitum in the animal room of the School of Food Science and Technology, Guangdong Ocean University, China (No. IACUC-20190107-02). The system was set to maintain the temperature between 22 and 24 °C and the humidity between 55% and 65%. After 3 weeks of adaptive feeding, sexually mature unfertilized female and male rats at a female:male ratio of 2:1 per cage were mated to breed new pups. After delivery, the number of pups per litter was counted and recorded.
2.4.2. Experimental Design and Treatments
According to methods previously described by other researchers and the dosage determined by our group [
17,
22,
23], bromocriptine mesylate (C
32H
40BrN
5O
5·CH
4SO
3; Da:750.70), a prolactin central inhibitor, was used to establish the postpartum hypogalactia model. Within 24 h of giving birth, 72 female rats and their offspring (12 pups per litter) were randomly divided into nine groups (8 rats in each group): the normal group (NG), the model group (MG), the Buxueshengmi granules (a lactation agent purchased from Jiuzhitang Co., Ltd., Changsha, China) group (BXSMG), the UEC4 high-dose group (UEC4-H), the UEC4 medium-dose group (UEC4-M), the UEC4 low-dose group (UEC4–L), the UEC4-1 high-dose group (UEC4-1-H), the UEC4-1 medium-dose group (UEC4-1-M) and the UEC4-1 low-dose group (UEC4-1-L). From the second day after delivery, the mother rats of each group were gavaged once per day for 7 days. The experimental details are shown in
Table 1. The lactating mothers and pups were weighed once a day, and the lactation amount of the mothers and the average body weight gain of the pups during the period were calculated. Any rat deaths were recorded every day.
2.4.3. Net Weights of the Rat Pups and Mammary Gland Parameters
The weight gain per litter was calculated as W
G = W
(x) − W
(1). Here, W
(x) is the weight of all the pups in the litter on the 7th day, and W
(1) is the weight of all the pups in the litter on the 1st day. On the 8th day, postpartum rats were killed by cervical dislocation, and the pups were weighed. The index weight (I.W.) of each mammary gland was calculated according to Matousek et al. [
24]; here, I.W. = organ weight (g)/body weight (g) × 100.
2.4.4. Prolactin (PRL), Prolactin Receptor (PRLR) Levels and Mammary Gland Structure
According to the method of Dong et al. [
22], the blood samples were clotted at room temperature and centrifuged at 3000×
g rpm for 15 min at 4 °C, and the serum was stored at −80 °C for the follow-up assays. Mammary gland tissue (100 mg) samples were rinsed with PBS, homogenized in 1 mL of PBS, stored overnight at −80 °C and then centrifuged at 5000×
g rpm for 5 min at 4 °C. The supernatant was removed, aliquoted and stored at −80 °C. The collection experiment of the above samples was carried out and completed on 24 August 2021. The serum and tissue homogenates were analyzed using rat prolactin PRL and PRLR ELISA kits (Guangzhou Dingguo Biotechnology Co., Ltd., Guangzhou, China).
The paraformaldehyde-treated mammary gland tissues were dehydrated and embedded in paraffin. Paraffin sections (5 μm) were prepared and stained with hematoxylin and eosin (HE) and observed and photographed under an optical microscope (DMI4000B intelligent inverted fluorescence microscope, Leica, Wetzlar, Germany).
2.5. Cell Cultures
The MCF-10A cells were suspended with an appropriate amount of complete medium and transferred into a T25 culture flask. The cells were cultured in a 5% CO
2 incubator at 37 °C. The culture medium was changed every 48 h. The cells were digested with 0.25% trypsin and passaged at 1:2. After several passages, the cells grew well. According to our previous experimental study, the cells (1 × 10
5 cells/mL) were seeded in 96-well plates and were administered various concentrations of the sample for 48 h. The final concentrations of UEC1–UEC4 and F1–F4 were 0, 12.5, 25, 50, 100, 200 and 400 μg/mL; those of S1–S4 and P1–P4 were 0, 12.5, 25, 50, 100 and 200 μg/mL. The content of UEC4-1 was 0, 12.5, 25, 50, 100 and 200 μg/mL. A control group of 50 μg/mL casein hydrolysate was set. The MTT method was used to evaluate the proliferation rate of the MCF-10A cells. The proliferation rate was calculated using the following equation:
2.6. Establishment of the Cell Model for Evaluating the Lactation Effect of UEC4-1
2.6.1. Cell Cycle Synchronization
The cells were starved for 24 h in serum-free medium [
25]. MCF-10A cells were inoculated in 6-well plates with a complete medium. When the cell density reached about 50%, the samples were cultured in serum-free DMEM/F12. The normal culture was set as the control, and the cells in the normal culture and starvation culture were collected separately. A cycle analysis was carried out by flow cytometry (FACSCantoTM II, BD Biosciences, San Jose, CA, USA) to detect the synchronization effect.
2.6.2. Cell Cycle Analysis
The experimental groups were as follows: control group, 0 μg/mL; sample groups, 12.5, 25 and 50 μg/mL.
MCF-10A cells with a growth abundance of 80% were subcultured in a 6 cm dish at a rate of 3 × 105/well. After the cells were cultured and had adhered to the wall, the cell culture medium was carefully poured out, and the cells were washed. According to the grouping, the cell culture medium, containing different concentrations of the samples, was added. After 48 h of continuous culture, the cells were washed twice with PBS. After trypsin digestion, the cells were resuspended in PBS. After washing and centrifugation, 70% glacial ethanol was immediately added, and the samples were stored at 4 °C overnight. After the cells were fixed, the cell suspension of the previous day was centrifuged and vortexed to remove the ethanol fixative, and the cells were washed twice with cold PBS. The supernatant was removed, and a propidium iodide (PI) staining solution containing RNase was added. The cells were stained at room temperature in the dark for 15–20 min. The fluorescence intensity in each group was detected by flow cytometry, and the experiment was repeated three times.
2.6.3. Cell Migration Experiment
MCF-10A cells were digested with trypsin. After complete medium was used to inactivate the trypsin, the cells were resuspended and counted. A Transwell chamber (an 8 μm pore-diameter product from Coming) was inoculated with 1 × 104 cells/well. The Transwell chamber was filled with serum-free DMEM/F12 medium. The 24-well plates were filled with a medium containing different concentrations of UEC4-1. After incubation for 16 h, the chamber was taken out. The medium was discarded, and the cells on the inside of the chamber were wiped with cotton swabs. The outer membrane was washed with PBS on both sides and fixed with 4% formaldehyde for 15–20 min. The cells were stained with 0.1% crystal violet and observed under a microscope.
2.7. Real-Time PCR Analysis of Gene Expression
Total RNA was extracted from MCF-10A cells using TRIzol (Thermo, Waltham, MA, USA) reagent. A Hifair
® II 1st Strand cDNA Synthesis Kit (Takara) was used for reverse transcription to synthesize cDNA. According to the instructions of the kit, RNAiso Plus (Takara, Shiga, Japan) and Hieff
® qPCR SYBR Green Master Mix (Low Rox Plus) were used for real-time fluorescence quantitative PCR. Quantitative PCR was performed on ABIViia7 real-time PCR equipment (BIO-RAD T100, Hercules, CA, USA) under the following conditions: denaturation at 95 °C for 10 s, pre-denaturation at 95 °C for 5 s, and annealing at 60 °C for 30 s and 40 cycles. The dissolution curve was collected at 95 °C for 15 s, 60 °C for 60 s and 95 °C for 15 s. The PCR primer series is shown in
Table 2. The relative expression levels of the target genes were normalized to β-actin and were calculated by the 2
−ΔΔCt relative quantitative method. The experiment was repeated three times. The results for different treatments of the same sample were analyzed by t-tests with the SPSS 22.0 statistical analysis software, and an analysis of variance was carried out between multiple sets of data.
2.8. Western Blotting
The cell lysate was added into the cell culture dish, the bottom of the culture dish was scraped repeatedly with a cell scraper so that the cells were fully broken and dissolved in the cell lysate, and the cracking process was carried out at a low temperature. The cell lysate was carefully extracted, transferred to a 1.5 mL centrifuge tube and ultrasonicated for 15 s three times. The total protein concentration was detected using the BCA Protein Quantification Kit, and the cell lysate was subjected to boiling water bath denaturation for 10 min. To determine the total protein concentration, the cells were loaded and frozen at −20 °C for further use. The samples were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a PVDF membrane at a low temperature. The hybrid membrane was removed, rinsed with TBST for 5 min, blocked with a 5% skim milk powder solution at room temperature for 2 h, rinsed with TBST for 8 min, incubated with the appropriate primary antibody dilution concentration overnight (4 °C), rinsed three times with TBST for 8 min and incubated with a diluted solution of the corresponding secondary antibody at 37 °C for 2 h. Finally, the hybrid membrane was developed by chemical fluorescence imaging. The IPP software version 6.0 was used for the quantitative analysis.
2.9. Statistical Analysis
All the quantitative data are presented as the means ± standard deviations (mean ± S.D). The data were analyzed using SPSS version 22.0. The experimental values were evaluated by a one-way ANOVA following the LSD method for multiple comparisons among the groups; p-values below 0.05 and 0.01 were considered significant and very significant, respectively.
4. Discussion
Recent studies have shown that small peptides play an important role in the mechanism of milk-protein synthesis. The concentration and amino acid composition of small peptides are also important factors affecting milk-protein synthesis, while little is known about the underlying mechanisms. The present study demonstrated that the cell proliferation of MCF-10A cells treated with the ultrafiltration component UEC4 was significantly increased after the fractionation purification of this component. Then, the UEC4-1 fraction significantly promoted the proliferation of MCF-10A cells. These results showed that UEC4-1 promoted the proliferation of MCF-10A cells in a concentration-dependent manner. Concentrations lower than 50 μg/mL could not meet the needs of cell growth, while concentrations higher than at 50 μg/mL inhibited cell growth. Similarly, some studies have shown that dipeptides and tripeptides (Met–Met and Thr–Phe–Phe) have higher absorptive efficiency than free amino acids, but only appropriate concentrations of small peptides can promote the growth, proliferation and milk-protein secretion in BMECs [
20,
21,
26,
27]. For example, the addition of Met–Met at 80 μg/mL best promoted the synthesis of casein in BMECs, and too low or too high a concentration may inhibit growth and affect casein’s gene expression [
21].
In order to study the effect of UEC4-1 on lactation and its regulatory mechanism, a model for evaluating the effect of polypeptides on lactation was established using the normal line of MCF-10A HMEC cells. Cell proliferation, migration and the cell cycle process were reported [
25]. The results from the present study show that UEC4-1 significantly promoted the proliferation of MCF-10A cells, cell transition from the G1 phase to the S phase and the migration of cells. The successful establishment of this model laid a foundation for the next step of screening the regulatory molecules of the polypeptide lactation-related signal pathway. Furthermore, our data elucidated that, compared with the control, 50 μg/mL UEC4-1 could significantly increase the gene expression of cyclin D1 in MCF-10A cells, promote the proliferation of MCF-10A cells and stimulate the synthesis of lactoprotein in HMECs. Similarly, Wang et al. found that the addition of 80 μg/mL Met–Met promoted the transition of BMEC cells from the G1 phase to the S phase, increased the expression of cyclin D1, promoted the proliferation of BMECs and stimulated the synthesis of lactoprotein in MECs [
21].
The data from the present study indicate that, with an increase in the UEC4-1 concentration, 50 µg/mL of UEC4-1 significantly increased the mRNA expression of three caseins, CSN2, CSN1S1 and CSN3. The protein expressions of CSN1S1, CSN2 and CSN3 were significantly higher than those in the control group. Moreover, UEC4-1 positively regulated the synthesis of milk protein in HMECs. The treatment of HMECs with 50 µg/mL UEC4-1 significantly increased the mRNA abundance of the signaling pathway genes mTOR, AKT and STAT5. At the level of phosphorylation, mTOR, AKT and S6KB1 were significantly increased when compared with the cells without UEC4-1 (the control group). Therefore, we speculate that UEC4-1 may increase casein mRNA expression by activating mTOR and phosphorylating its downstream molecule S6KB1, thereby regulating the expression and phosphorylation of mTOR pathway proteins. Cai et al. studied the effect of bioactive peptide fractions (OPH3-1) on β-casein expression in mouse mammary epithelial cells (HC11). OPH3-1 significantly stimulated cell proliferation and β-casein synthesis in HC11 cells and enhanced the mRNA abundance of the JAK2–STAT5 and mTOR signaling molecules. These data were consistent with β-casein upregulation [
18]. Yang et al. showed that Met–Met promoted α
s1-casein synthesis in cultured bovine mammary gland explants, and this stimulation may have been mediated by enhanced intracellular substrate availability and by the activation of the JAK2–STAT5 and mTOR signaling pathways [
20]. At the translation level, mTOR regulated cell growth and protein synthesis by binding mRNA and phosphorylating the eukaryotic initiation factor Eif4b-binding protein ribosomal 6S kinase S6K1 [
28,
29]. In addition, Wang et al. elucidated the properties of the peptide transporter and its effects on β-casein synthesis in BMECs, which included Met–Met being taken up by PepT2 and enhancing cell proliferation and β-casein synthesis by activating the JAK2–STAT5 and mTOR signaling pathways in BMECs [
21].
Prolactin (PRL) is a polypeptide hormone synthesized in and released from the anterior pituitary gland. It is known that PRL plays an essential role in milk synthesis and secretion during lactation. which is needed for the onset and maintenance of lactation. Moreover, the PRL concentration was also positively correlated with the mammary gland wet weight in gilts and the milk yield of sows [
30]. Our in vivo data showed, at high-dose (0.6 g/kg of UEC4-1), significantly increased PRL concentration in the serum as well as significantly increased PRL and PRLR concentration in breast tissue. These results indicate that UEC4-1-H could effectively increase the lactation yield and breast development in lactating rats induced by bromocriptine. The effect was better than that in the Buxueshengmi granule group.
To explore the mechanism of lactation regulation, the in vitro data from the present study demonstrated that 50 μg/mL of UEC4-1 significantly increased the mRNA abundance of PRL and PRLR compared with the control. The possible mechanism may be through the activation of AKT and STAT5, promoting the proliferation of HMECs and stimulating the expression of PRLR. A study reported that pituitary PRL binding to PRLR could activate JAK2–STAT5 signaling, which is required for lobuloalveolar development in the mammary gland during pregnancy [
31]. Lin et al. showed that quercetin may promote the proliferation of primary MECs and stimulate the expression of PRLR through AKT activation in vitro [
32]. Another study found that the major pathway mediating prolactin action is the Janus kinase 2 (JAK2)/signal transducer and activator of transcription (STAT) pathway, which is activated by the long form of the prolactin receptor (PRLR). In addition to the JAK/STAT pathway, under some conditions, PRL may activate the signal transduction cascade (phosphatidylinositol-4,5-bisphosphate 3-kinase [PI3K]) of the phosphoinositol-3-kinase/protein kinase B (or Akt) pathway as well as the mitogen-activated protein kinase or extracellular regulated kinase pathways [
33].
In summary, the results indicate that the components of UEC4-1 were unmodified peptides with amino acid sequences of seven amino acids each: Val–Gly–Arg–Thr–Asn–Ser–His (VGRTNSH), Val–Gly–Thr–Glu–His–Arg–Lys (VGTEHRK), Asn–His–Ile–Ser–Trp–Ala–Ala (NHISWAA), Ser–Tyr–Lys–Cys–Arg–Asn–Ser (SYKCRNS), Phe–Pro–Val–Ala–Leu–Gly (FPVALMG) and Val–Gly–Met–Ile–Gly–Phe–Leu (VGMIGFL). According to various factors, the UEC4-1 component was determined to be a polypeptide with VGRTNSH as the main component (70.72%). Yang et al. and Zhou et al. have shown that dipeptides and tripeptides containing restricted amino acids such as methionine (Met), lysine (Lys), threonine (Thr) and phenylalanine (Phe) can promote the synthesis of milk protein in dairy cows MECs [
20,
27]. Wu et al. showed that Leu, Ile and Val could activate the mTOR signaling pathway, enhancing milk-protein synthesis [
8]. The polypeptides of UEC42-1 have the structural characteristics of amino acids that promote lactation.
Interest in the role of peptides in milk-protein synthesis is increasing. Although the role of peptides in milk-protein synthesis is clearly established, little is known about the underlying mechanisms. Therefore, it was necessary to study the lactation mechanism for oyster peptides in this study.