Mediterranean-Type Diets as a Protective Factor for Asthma and Atopy
Abstract
:1. Introduction
2. Obstacles to the Validation of Associations
2.1. Issue No. 1: What Is a “Mediterranean Diet”?
2.2. Issue No. 2: Nutrients, Foods or Dietary Patterns?
2.3. Issue No. 3: Observation versus Intervention
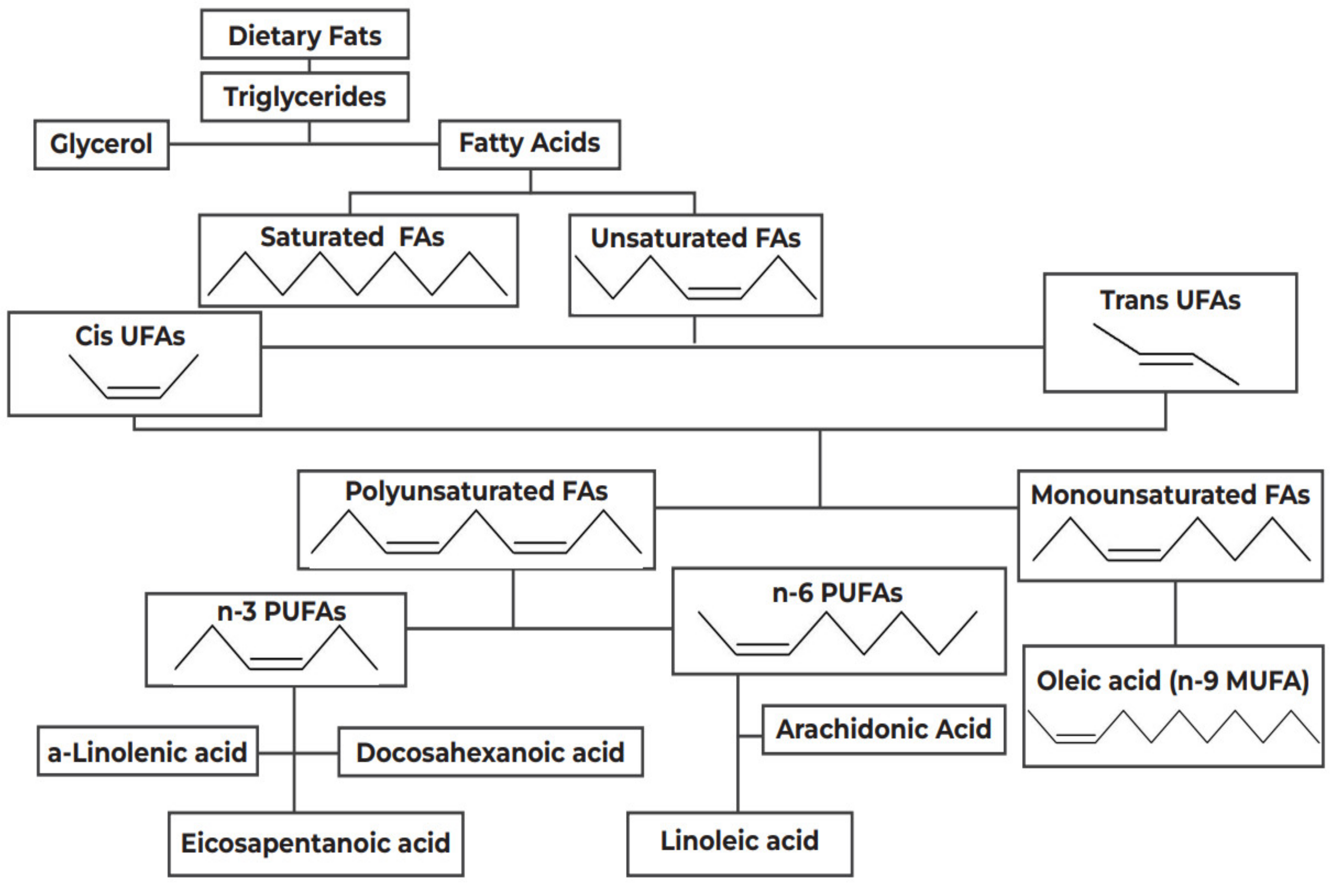
3. Evaluation of Dietary Constituents
3.1. The Lipid Hypothesis
3.2. The Antioxidant Hypothesis
3.3. The Anti-Inflammatory Hypothesis
4. Conclusions: Mediterranean-Type Dietary Pattern?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christiansen, E.S.; Kjaer, H.F.; Eller, E.; Bindslev-Jensen, C.; Høst, A.; Mortz, C.G.; Halken, S. The Prevalence of Atopic Diseases and the Patterns of Sensitization in Adolescence. Pediatric Allergy Immunol. 2016, 27, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Masilamani, M.; Li, X.-M.; Sampson, H.A. The False Alarm Hypothesis: Food Allergy Is Associated with High Dietary Advanced Glycation End-Products and Proglycating Dietary Sugars That Mimic Alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, C.; Silverberg, J.I. The Prevalence and Persistence of Atopic Dermatitis in Urban United States Children. Ann. Allergy Asthma Immunol. 2019, 123, 173–178.e1. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Miligkos, M.; Xepapadaki, P. A Current Perspective of Allergic Asthma: From Mechanisms to Management. Handb. Exp. Pharmacol. 2022, 268, 69–93. [Google Scholar] [CrossRef]
- Musaad, S.M.A.; Paige, K.N.; Teran-Garcia, M.; Donovan, S.M.; Fiese, B.H. Childhood Overweight/Obesity and Pediatric Asthma: The Role of Parental Perception of Child Weight Status. Nutrients 2013, 5, 3713–3729. [Google Scholar] [CrossRef] [Green Version]
- Murrison, L.B.; Brandt, E.B.; Myers, J.B.; Hershey, G.K.K. Environmental Exposures and Mechanisms in Allergy and Asthma Development. J. Clin. Investig. 2019, 129, 1504–1515. [Google Scholar] [CrossRef] [Green Version]
- Asher, M.I.; García-Marcos, L.; Pearce, N.E.; Strachan, D.P. Trends in Worldwide Asthma Prevalence. Eur. Respir. J. 2020, 56, 2002094. [Google Scholar] [CrossRef]
- Fortun, M.; Fortun, K.; Costelloe-Kuehn, B.; Saheb, T.; Price, D.; Kenner, A.; Crowder, J. Asthma, Culture, and Cultural Analysis: Continuing Challenges. Adv. Exp. Med. Biol. 2014, 795, 321–332. [Google Scholar] [CrossRef]
- Burney, P.G. The Causes of Asthma–Does Salt Potentiate Bronchial Activity? Discussion Paper. J. R. Soc. Med. 1987, 80, 364–367. [Google Scholar] [CrossRef] [Green Version]
- Seaton, A.; Godden, D.J.; Brown, K. Increase in Asthma: A More Toxic Environment or a More Susceptible Population? Thorax 1994, 49, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Larsen, V.; Del Giacco, S.R.; Moreira, A.; Bonini, M.; Charles, D.; Reeves, T.; Carlsen, K.-H.; Haahtela, T.; Bonini, S.; Fonseca, J.; et al. Asthma and Dietary Intake: An Overview of Systematic Reviews. Allergy 2016, 71, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, K.; Devereux, G. Diet and Asthma: Nutrition Implications from Prevention to Treatment. J. Am. Diet. Assoc. 2011, 111, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Nurmatov, U.; Devereux, G.; Sheikh, A. Nutrients and Foods for the Primary Prevention of Asthma and Allergy: Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. 2011, 127, 724–733.e30. [Google Scholar] [CrossRef] [PubMed]
- Robison, R.; Kumar, R. The Effect of Prenatal and Postnatal Dietary Exposures on Childhood Development of Atopic Disease. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 139–144. [Google Scholar] [CrossRef]
- Allan, K.; Kelly, F.J.; Devereux, G. Antioxidants and Allergic Disease: A Case of Too Little or Too Much? Clin. Exp. Allergy 2010, 40, 370–380. [Google Scholar] [CrossRef]
- Anandan, C.; Nurmatov, U.; Sheikh, A. Omega 3 and 6 Oils for Primary Prevention of Allergic Disease: Systematic Review and Meta-Analysis. Allergy 2009, 64, 840–848. [Google Scholar] [CrossRef]
- Varraso, R. Nutrition and Asthma. Curr. Allergy Asthma Rep. 2012, 12, 201–210. [Google Scholar] [CrossRef]
- Arvaniti, F.; Priftis, K.N.; Panagiotakos, D.B. Dietary Habits and Asthma: A Review. Allergy Asthma Proc. 2010, 31, e1–e10. [Google Scholar] [CrossRef]
- Perkins, T.N.; Oczypok, E.A.; Dutz, R.E.; Donnell, M.L.; Myerburg, M.M.; Oury, T.D. The Receptor for Advanced Glycation End Products Is a Critical Mediator of Type 2 Cytokine Signaling in the Lungs. J. Allergy Clin. Immunol. 2019, 144, 796–808.e12. [Google Scholar] [CrossRef] [Green Version]
- Worldwide Variations in the Prevalence of Asthma Symptoms: The International Study of Asthma and Allergies in Childhood (ISAAC). Eur. Respir. J. 1998, 12, 315–335. [CrossRef] [Green Version]
- Chatzi, L.; Kogevinas, M. Prenatal and Childhood Mediterranean Diet and the Development of Asthma and Allergies in Children. Public Health Nutr. 2009, 12, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marcos, L. Mediterranean Diet as a Protection against Asthma: Still Another Brick in Building a Causative Association. Allergol. Immunopathol. 2016, 44, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Di Nunzio, M.; Bordoni, A.; Gori, D.; Lanari, M. Effect of Adherence to Mediterranean Diet during Pregnancy on Children’s Health: A Systematic Review. Nutrients 2019, 11, 997. [Google Scholar] [CrossRef] [Green Version]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo-Mora, J.J.; García-Vigara, A.; Sánchez-Sánchez, M.L.; García-Pérez, M.-Á.; Tarín, J.; Cano, A. The Mediterranean Diet: A Historical Perspective on Food for Health. Maturitas 2020, 132, 65–69. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Kelleher, A.H.; Kristo, A.S. Mediterranean Diet. Encyclopedia 2021, 1, 31. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean Diet and Its Components in Relation to All-Cause Mortality: Meta-Analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean Diet to Non-Mediterranean Countries. What Is and What Is Not the Mediterranean Diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Menotti, A.; Puddu, P.E. How the Seven Countries Study Contributed to the Definition and Development of the Mediterranean Diet Concept: A 50-Year Journey. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 245–252. [Google Scholar] [CrossRef]
- Tourlouki, E.; Matalas, A.-L.; Bountziouka, V.; Tyrovolas, S.; Zeimbekis, A.; Gotsis, E.; Tsiligianni, I.; Protopapa, I.; Protopapas, C.; Metallinos, G.; et al. Are Current Dietary Habits in Mediterranean Islands a Reflection of the Past? Results from the MEDIS Study. Ecol. Food Nutr. 2013, 52, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Belahsen, R. Nutrition Transition and Food Sustainability. Proc. Nutr. Soc. 2014, 73, 385–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konieczna, J.; Yañez, A.; Moñino, M.; Babio, N.; Toledo, E.; Martínez-González, M.A.; Sorlí, J.V.; Salas-Salvadó, J.; Estruch, R.; Ros, E.; et al. Longitudinal Changes in Mediterranean Diet and Transition between Different Obesity Phenotypes. Clin. Nutr. 2020, 39, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Closas, R.; Berenguer, A.; González, C.A. Changes in Food Supply in Mediterranean Countries from 1961 to 2001. Public Health Nutr. 2006, 9, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Apostolaki, I.; Pepa, A.; Magriplis, E.; Malisova, O.; Kapsokefalou, M. Mediterranean Diet Adherence, Social Capital and Health Related Quality of Life in the Older Adults of Crete, Greece: The MINOA Study. Mediterr. J. Nutr. Metab. 2020, 13, 149–161. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Popławski, C. Antioxidant Properties and Fatty Acid Profile of Cretan Extra Virgin Bioolive Oils: A Pilot Study. Int. J. Food Sci. 2021, 2021, 5554002. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Luczynska, M.; Kowalski, M.L.; Voutilainen, H.; Ahlström, M.; Haahtela, T.; Toskala, E.; Bockelbrink, A.; Lee, H.-H.; Vassilopoulou, E.; et al. Use of a Common Food Frequency Questionnaire (FFQ) to Assess Dietary Patterns and Their Relation to Allergy and Asthma in Europe: Pilot Study of the GA2LEN FFQ. Eur. J. Clin. Nutr. 2011, 65, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Barabasi, A.-L.; Menichetti, G.; Loscalzo, J. The Unmapped Chemical Complexity of Our Diet. Nat. Food 2019, 1, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Lv, N.; Xiao, L.; Ma, J. Dietary Pattern and Asthma: A Systematic Review and Meta-Analysis. J. Asthma Allergy 2014, 7, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Bédard, A.; Li, Z.; Ait-Hadad, W.; Camargo, C.A.J.; Leynaert, B.; Pison, C.; Dumas, O.; Varraso, R. The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research. Int. J. Environ. Res. Public Health 2021, 18, 3013. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.C.; da Silva, D.T.R.; Hennemann, M.L.; Sarmento, R.A.; Almeida, J.C.; de Tarso Roth Dalcin, P. Diet Effects in the Asthma Treatment: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-Y.; Forno, E.; Holguin, F.; Celedón, J.C. Diet and Asthma: An Update. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef] [Green Version]
- De Batlle, J.; Garcia-Aymerich, J.; Barraza-Villarreal, A.; Antó, J.M.; Romieu, I. Mediterranean Diet Is Associated with Reduced Asthma and Rhinitis in Mexican Children. Allergy 2008, 63, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Andrianasolo, R.M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Galan, P.; Varraso, R. Associations between Dietary Scores with Asthma Symptoms and Asthma Control in Adults. Eur. Respir. J. 2018, 52, 1702572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milà-Villarroel, R.; Bach-Faig, A.; Puig, J.; Puchal, A.; Farran, A.; Serra-Majem, L.; Carrasco, J.L. Comparison and Evaluation of the Reliability of Indexes of Adherence to the Mediterranean Diet. Public Health Nutr. 2011, 14, 2338–2345. [Google Scholar] [CrossRef] [Green Version]
- Papamichael, M.M.; Shrestha, S.K.; Itsiopoulos, C.; Erbas, B. The Role of Fish Intake on Asthma in Children: A Meta-Analysis of Observational Studies. Pediatr. Allergy Immunol. 2018, 29, 350–360. [Google Scholar] [CrossRef]
- Yang, H.; Xun, P.; He, K. Fish and Fish Oil Intake in Relation to Risk of Asthma: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e80048. [Google Scholar] [CrossRef] [Green Version]
- Vassilopoulou, E.; Konstantinou, G.N.; Dimitriou, A.; Manios, Y.; Koumbi, L.; Papadopoulos, N.G. The Impact of Food Histamine Intake on Asthma Activity: A Pilot Study. Nutrients 2020, 12, 3402. [Google Scholar] [CrossRef]
- McKeever, T.M.; Britton, J. Diet and Asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sarwar, K.; Litonjua, A.A. As You Eat It: Effects of Prenatal Nutrition on Asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Von Mutius, E.; Smits, H.H. Primary Prevention of Asthma: From Risk and Protective Factors to Targeted Strategies for Prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef]
- Noutsios, G.T.; Floros, J. Childhood Asthma: Causes, Risks, and Protective Factors; a Role of Innate Immunity. Swiss Med. Wkly. 2014, 144, w14036. [Google Scholar] [CrossRef]
- Muraro, A.; Halken, S.; Arshad, S.H.; Beyer, K.; Dubois, A.E.J.; Du Toit, G.; Eigenmann, P.A.; Grimshaw, K.E.C.; Hoest, A.; Lack, G.; et al. EAACI Food Allergy and Anaphylaxis Guidelines. Primary Prevention of Food Allergy. Allergy 2014, 69, 590–601. [Google Scholar] [CrossRef]
- Sonnenschein-van der Voort, A.M.M.; Jaddoe, V.W.V.; van der Valk, R.J.P.; Willemsen, S.P.; Hofman, A.; Moll, H.A.; de Jongste, J.C.; Duijts, L. Duration and Exclusiveness of Breastfeeding and Childhood Asthma-Related Symptoms. Eur. Respir. J. 2012, 39, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Erratum to Supplement—The Pregnancy and Birth to 24 Months Project: A Series of Systematic Reviews on Diet and Health. Am. J. Clin. Nutr. 2019, 110, 1041. [CrossRef]
- Güngör, D.; Nadaud, P.; LaPergola, C.C.; Dreibelbis, C.; Wong, Y.P.; Terry, N.; Abrams, S.A.; Beker, L.; Jacobovits, T.; Järvinen, K.M.; et al. Infant Milk-Feeding Practices and Food Allergies, Allergic Rhinitis, Atopic Dermatitis, and Asthma throughout the Life Span: A Systematic Review. Am. J. Clin. Nutr. 2019, 109 (Suppl. 7), 772S–799S. [Google Scholar] [CrossRef]
- Lodge, C.J.; Tan, D.J.; Lau, M.X.Z.; Dai, X.; Tham, R.; Lowe, A.J.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and Asthma and Allergies: A Systematic Review and Meta-Analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef]
- Kim, J.H. Role of Breast-Feeding in the Development of Atopic Dermatitis in Early Childhood. Allergy Asthma Immunol. Res. 2017, 9, 285–287. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Dai, R.; Lu, L.; Fan, X.; Yu, Y. Breastfeeding and Atopic Dermatitis Risk: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Dermatology 2020, 236, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D.; Rundell, K.W. Dietary Polyunsaturated Fatty Acids in Asthma- and Exercise-Induced Bronchoconstriction. Eur. J. Clin. Nutr. 2005, 59, 1335–1346. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M.J.; Hartman, T.J.; Adgent, M.; Gardner, K.; Gebretsadik, T.; Moore, P.E.; Davis, R.L.; LeWinn, K.Z.; Bush, N.R.; Tylavsky, F.; et al. Prenatal Polyunsaturated Fatty Acids and Child Asthma: Effect Modification by Maternal Asthma and Child Sex. J. Allergy Clin. Immunol. 2020, 145, 800–807.e4. [Google Scholar] [CrossRef]
- Mayor, S. High Dose Fish Oil Supplements in Late Pregnancy Reduce Asthma in Offspring, Finds Study. BMJ 2016, 356, i6861. [Google Scholar] [CrossRef] [PubMed]
- Chercoles, E.R. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. Acta Pediatr. Esp. 2017, 75, 81. [Google Scholar] [CrossRef]
- Rago, D.; Rasmussen, M.A.; Lee-Sarwar, K.A.; Weiss, S.T.; Lasky-Su, J.; Stokholm, J.; Bønnelykke, K.; Chawes, B.L.; Bisgaard, H. Fish-Oil Supplementation in Pregnancy, Child Metabolomics and Asthma Risk. EBioMedicine 2019, 46, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.; Strøm, M.; Maslova, E.; Dahl, R.; Hoffmann, H.J.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Granström, C.; Halldorsson, T.I.; et al. Fish Oil Supplementation during Pregnancy and Allergic Respiratory Disease in the Adult Offspring. J. Allergy Clin. Immunol. 2017, 139, 104–111.e4. [Google Scholar] [CrossRef] [Green Version]
- Black, P.N.; Sharpe, S. Dietary Fat and Asthma: Is There a Connection? Eur. Respir. J. 1997, 10, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Wendell, S.G.; Baffi, C.; Holguin, F. Fatty Acids, Inflammation, and Asthma. J. Allergy Clin. Immunol. 2014, 133, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of Arachidonic Acid Metabolism: A Review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.; Brüne, B. Prostanoids and Resolution of Inflammation—Beyond the Lipid-Mediator Class Switch. Front. Immunol. 2021, 12, 2838. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Fergusson, J.; Salimi, M.; Panse, I.; Ussher, J.E.; Hegazy, A.N.; Vinall, S.L.; Jackson, D.G.; Hunter, M.G.; Pettipher, R.; et al. Prostaglandin D2 and Leukotriene E4 Synergize to Stimulate Diverse TH2 Functions and TH2 Cell/Neutrophil Crosstalk. J. Allergy Clin. Immunol. 2015, 135, 1311–1358. [Google Scholar] [CrossRef] [Green Version]
- Miles, E.A.; Calder, P.C. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, G.; Ecker, J. The Opposing Effects of N-3 and n-6 Fatty Acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Rossouw, J.E.; Roberts, M.B.; Liu, S.; Johnson, K.C.; Shikany, J.M.; Manson, J.E.; Tinker, L.F.; Eaton, C.B. Theoretical Effects of Substituting Butter with Margarine on Risk of Cardiovascular Disease. Epidemiology 2017, 28, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Oliver, P.J.; Arutla, S.; Yenigalla, A.; Hund, T.J.; Parinandi, N.L. Lipid Nutrition in Asthma. Cell Biochem. Biophys. 2021, 79, 669–694. [Google Scholar] [CrossRef]
- Mamareli, P.; Kruse, F.; Lu, C.-W.; Guderian, M.; Floess, S.; Rox, K.; Allan, D.S.J.; Carlyle, J.R.; Brönstrup, M.; Müller, R.; et al. Targeting Cellular Fatty Acid Synthesis Limits T Helper and Innate Lymphoid Cell Function during Intestinal Inflammation and Infection. Mucosal Immunol. 2021, 14, 164–176. [Google Scholar] [CrossRef]
- Howie, D.; Ten Bokum, A.; Necula, A.S.; Cobbold, S.P.; Waldmann, H. The Role of Lipid Metabolism in T Lymphocyte Differentiation and Survival. Front. Immunol. 2018, 8, 1949. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Nakashima, H.; Tomozawa, J.; Shimazaki, Y.; Ohyanagi, C.; Kawaguchi, N.; Ohya, S.; Kohno, S.; Nabe, T. Deficiency of N-6 Polyunsaturated Fatty Acids Is Mainly Responsible for Atopic Dermatitis-like Pruritic Skin Inflammation in Special Diet-Fed Hairless Mice. Exp. Dermatol. 2013, 22, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2021, 11, 3818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-T.; Xu, J.; Wang, Y.-M.; Xue, C.-H. Health Benefits of Dietary Marine DHA/EPA-Enriched Glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef]
- De Koning, L.; Anand, S.S. Vascular viewpoint. Vasc. Med. 2004, 9, 145–146. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The Mediterranean Diets: What Is so Special about the Diet of Greece? The Scientific Evidence. J. Nutr. 2001, 131, 3065S–3073S. [Google Scholar] [CrossRef]
- Salam, M.T.; Li, Y.-F.; Langholz, B.; Gilliland, F.D. Maternal Fish Consumption during Pregnancy and Risk of Early Childhood Asthma. J. Asthma 2005, 42, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Lumia, M.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Erkkola, M.; Uusitalo, L.; Niinistö, S.; Kenward, M.G.; Ilonen, J.; Simell, O.; et al. Dietary Fatty Acid Composition during Pregnancy and the Risk of Asthma in the Offspring. Pediatric Allergy Immunol. 2011, 22, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Torrent, M.; Garcia-Esteban, R.; Ferrer, C.; Ribas-Fitó, N.; Antó, J.M.; Sunyer, J. Maternal Fish Intake during Pregnancy and Atopy and Asthma in Infancy. Clin. Exp. Allergy 2007, 37, 518–525. [Google Scholar] [CrossRef]
- Calvani, M.; Alessandri, C.; Miceli Sopo, S.; Panetta, V.; Pingitore, G.; Tripodi, S.; Zappalà, D.; Zicari, A. Consumption of Fish, Butter and Margarine during Pregnancy and Development of Allergic Sensitizations in the Offspring: Role of Maternal Atopy. Pediatr. Allergy Immunol. 2006, 17, 94–102. [Google Scholar] [CrossRef]
- Fogarty, A.; Britton, J. The Role of Diet in the Aetiology of Asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2000, 30, 615–627. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, S.J.; Fallon, E.M.; Kalish, B.T.; Gura, K.M.; Puder, M. The Role of the ω-3 Fatty Acid DHA in the Human Life Cycle. J. Parenter. Enter. Nutr. 2013, 37, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Østerdal, M.L.; Salvig, J.D.; Mortensen, L.M.; Rytter, D.; Secher, N.J.; Henriksen, T.B. Fish Oil Intake Compared with Olive Oil Intake in Late Pregnancy and Asthma in the Offspring: 16 y of Registry-Based Follow-up from a Randomized Controlled Trial. Am. J. Clin. Nutr. 2008, 88, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Pike, K.C.; Calder, P.C.; Inskip, H.M.; Robinson, S.M.; Roberts, G.C.; Cooper, C.; Godfrey, K.M.; Lucas, J.S.A. Maternal Plasma Phosphatidylcholine Fatty Acids and Atopy and Wheeze in the Offspring at Age of 6 Years. Clin. Dev. Immunol. 2012, 2012, 474613. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish Oil Supplementation in Pregnancy Modifies Neonatal Allergen-Specific Immune Responses and Clinical Outcomes in Infants at High Risk of Atopy: A Randomized, Controlled Trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Alm, B.; Aberg, N.; Erdes, L.; Möllborg, P.; Pettersson, R.; Norvenius, S.G.; Goksör, E.; Wennergren, G. Early Introduction of Fish Decreases the Risk of Eczema in Infants. Arch. Dis. Child. 2009, 94, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafstad, P.; Nystad, W.; Magnus, P.; Jaakkola, J.J.K. Asthma and Allergic Rhinitis at 4 Years of Age in Relation to Fish Consumption in Infancy. J. Asthma 2003, 40, 343–348. [Google Scholar] [CrossRef]
- Antova, T.; Pattenden, S.; Nikiforov, B.; Leonardi, G.S.; Boeva, B.; Fletcher, T.; Rudnai, P.; Slachtova, H.; Tabak, C.; Zlotkowska, R.; et al. Nutrition and Respiratory Health in Children in Six Central and Eastern European Countries. Thorax 2003, 58, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Ellwood, P.; Asher, M.I.; Björkstén, B.; Burr, M.; Pearce, N.; Robertson, C.F. Diet and Asthma, Allergic Rhinoconjunctivitis and Atopic Eczema Symptom Prevalence: An Ecological Analysis of the International Study of Asthma and Allergies in Childhood (ISAAC) Data. ISAAC Phase One Study Group. Eur. Respir. J. 2001, 17, 436–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ju, S.-Y. Asthma and Dietary Intake of Fish, Seaweeds, and Fatty Acids in Korean Adults. Nutrients 2019, 11, 2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, B.B.; Kåre, D.L. Open Trial of Supplements of Omega 3 and 6 Fatty Acids, Vitamins and Minerals in Atopic Dermatitis. J. Dermatolog. Treat. 2006, 17, 82–85. [Google Scholar] [CrossRef]
- Søyland, E.; Funk, J.; Rajka, G.; Sandberg, M.; Thune, P.; Rustad, L.; Helland, S.; Middelfart, K.; Odu, S.; Falk, E.S. Dietary Supplementation with Very Long-Chain n-3 Fatty Acids in Patients with Atopic Dermatitis. A Double-Blind, Multicentre Study. Br. J. Dermatol. 1994, 130, 757–764. [Google Scholar] [CrossRef]
- Hodge, L.; Salome, C.M.; Hughes, J.M.; Liu-Brennan, D.; Rimmer, J.; Allman, M.; Pang, D.; Armour, C.; Woolcock, A.J. Effect of Dietary Intake of Omega-3 and Omega-6 Fatty Acids on Severity of Asthma in Children. Eur. Respir. J. 1998, 11, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Abdo-Sultan, M.K.; Abd-El-Lateef, R.S.; Kamel, F.Z. Efficacy of Omega-3 Fatty Acids Supplementation versus Sublingual Immunotherapy in Patients with Bronchial Asthma. Egypt. J. Immunol. 2019, 26, 79–89. [Google Scholar]
- Mickleborough, T.D.; Murray, R.L.; Ionescu, A.A.; Lindley, M.R. Fish Oil Supplementation Reduces Severity of Exercise-Induced Bronchoconstriction in Elite Athletes. Am. J. Respir. Crit. Care Med. 2003, 168, 1181–1189. [Google Scholar] [CrossRef]
- Thien, F.C.; Mencia-Huerta, J.M.; Lee, T.H. Dietary Fish Oil Effects on Seasonal Hay Fever and Asthma in Pollen-Sensitive Subjects. Am. Rev. Respir. Dis. 1993, 147, 1138–1143. [Google Scholar] [CrossRef]
- Kremmyda, L.-S.; Vlachava, M.; Noakes, P.S.; Diaper, N.D.; Miles, E.A.; Calder, P.C. Atopy Risk in Infants and Children in Relation to Early Exposure to Fish, Oily Fish, or Long-Chain Omega-3 Fatty Acids: A Systematic Review. Clin. Rev. Allergy Immunol. 2011, 41, 36–66. [Google Scholar] [CrossRef]
- Mihrshahi, S.; Peat, J.K.; Webb, K.; Tovey, E.R.; Marks, G.B.; Mellis, C.M.; Leeder, S.R. The Childhood Asthma Prevention Study (CAPS): Design and Research Protocol of a Randomized Trial for the Primary Prevention of Asthma. Control. Clin. Trials 2001, 22, 333–354. [Google Scholar] [CrossRef]
- Gunaratne, A.W.; Makrides, M.; Collins, C.T. Maternal Prenatal and/or Postnatal n-3 Long Chain Polyunsaturated Fatty Acids (LCPUFA) Supplementation for Preventing Allergies in Early Childhood. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Muley, P.; Shah, M.; Muley, A. Omega-3 Fatty Acids Supplementation in Children to Prevent Asthma: Is It Worthy?—A Systematic Review and Meta-Analysis. J. Allergy 2015, 2015, 312052. [Google Scholar] [CrossRef] [Green Version]
- Zambalde, É.P.; Teixeira, M.M.; Favarin, D.C.; de Oliveira, J.R.; Magalhães, M.L.; Cunha, M.M.; Silva, W.C.J.; Okuma, C.H.; Rodrigues, V.J.; Levy, B.D.; et al. The Anti-Inflammatory and pro-Resolution Effects of Aspirin-Triggered RvD1 (AT-RvD1) on Peripheral Blood Mononuclear Cells from Patients with Severe Asthma. Int. Immunopharmacol. 2016, 35, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.R.; Johns, C.B.; Palumbo, M.L.; Murphy, K.C.; Cahill, K.N.; Laidlaw, T.M. Dietary Fatty Acid Modification for the Treatment of Aspirin-Exacerbated Respiratory Disease: A Prospective Pilot Trial. J. Allergy Clin. Immunol. Pract. 2018, 6, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Standl, M.; Demmelmair, H.; Koletzko, B.; Heinrich, J. Cord Blood LC-PUFA Composition and Allergic Diseases during the First 10 Yr. Results from the LISAplus Study. Pediatric Allergy Immunol. 2014, 25, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.-M.; Chiang, B.-L.; Wang, L.-C. Maternal Nutritional Status and Development of Atopic Dermatitis in Their Offspring. Clin. Rev. Allergy Immunol. 2021, 61, 128–155. [Google Scholar] [CrossRef]
- Galli, E.; Picardo, M.; Chini, L.; Passi, S.; Moschese, V.; Terminali, O.; Paone, F.; Fraioli, G.; Rossi, P. Analysis of Polyunsaturated Fatty Acids in Newborn Sera: A Screening Tool for Atopic Disease? Br. J. Dermatol. 1994, 130, 752–756. [Google Scholar] [CrossRef]
- Horrobin, D.F. Essential Fatty Acid Metabolism and Its Modification in Atopic Eczema. Am. J. Clin. Nutr. 2000, 71 (Suppl. 1), 367S–372S. [Google Scholar] [CrossRef]
- Mayser, P.; Mayer, K.; Mahloudjian, M.; Benzing, S.; Krämer, H.-J.; Schill, W.-B.; Seeger, W.; Grimminger, F. A Double-Blind, Randomized, Placebo-Controlled Trial of n-3 versus n-6 Fatty Acid-Based Lipid Infusion in Atopic Dermatitis. J. Parenter. Enter. Nutr. 2002, 26, 151–158. [Google Scholar] [CrossRef]
- Almqvist, C.; Garden, F.; Xuan, W.; Mihrshahi, S.; Leeder, S.R.; Oddy, W.; Webb, K.; Marks, G.B. Omega-3 and Omega-6 Fatty Acid Exposure from Early Life Does Not Affect Atopy and Asthma at Age 5 Years. J. Allergy Clin. Immunol. 2007, 119, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.C.; et al. EAACI Position Paper: Influence of Dietary Fatty Acids on Asthma, Food Allergy, and Atopic Dermatitis. Allergy 2019, 74, 1429–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Lin, L.; Shi, B.; Jing, J.; Cai, L. The Effects of Early Life Polyunsaturated Fatty Acids and Ruminant Trans Fatty Acids on Allergic Diseases: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Weiland, S.K.; von Mutius, E.; Hüsing, A.; Asher, M.I. Intake of Trans Fatty Acids and Prevalence of Childhood Asthma and Allergies in Europe. ISAAC Steering Committee. Lancet 1999, 353, 2040–2041. [Google Scholar] [CrossRef]
- Kuhnt, K.; Degen, C.; Jahreis, G. Evaluation of the Impact of Ruminant Trans Fatty Acids on Human Health: Important Aspects to Consider. Crit. Rev. Food Sci. Nutr. 2016, 56, 1964–1980. [Google Scholar] [CrossRef]
- Jiménez-Cepeda, A.; Dávila-Said, G.; Orea-Tejeda, A.; González-Islas, D.; Elizondo-Montes, M.; Pérez-Cortes, G.; Keirns-Davies, C.; Castillo-Aguilar, L.F.; Verdeja-Vendrell, L.; Peláez-Hernández, V.; et al. Dietary Intake of Fatty Acids and Its Relationship with FEV1/FVC in Patients with Chronic Obstructive Pulmonary Disease. Clin. Nutr. ESPEN 2019, 29, 92–96. [Google Scholar] [CrossRef]
- Kompauer, I.; Demmelmair, H.; Koletzko, B.; Bolte, G.; Linseisen, J.; Heinrich, J. Association of Fatty Acids in Serum Phospholipids with Lung Function and Bronchial Hyperresponsiveness in Adults. Eur. J. Epidemiol. 2008, 23, 175–190. [Google Scholar] [CrossRef]
- Wood, L.G. Diet, Obesity, and Asthma. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. 5), S332–S338. [Google Scholar] [CrossRef]
- Trak-Fellermeier, M.A.; Brasche, S.; Winkler, G.; Koletzko, B.; Heinrich, J. Food and Fatty Acid Intake and Atopic Disease in Adults. Eur. Respir. J. 2004, 23, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Allan, K.M.; Prabhu, N.; Craig, L.C.A.; McNeill, G.; Kirby, B.; McLay, J.; Helms, P.J.; Ayres, J.G.; Seaton, A.; Turner, S.W.; et al. Maternal Vitamin D and E Intakes during Pregnancy Are Associated with Asthma in Children. Eur. Respir. J. 2015, 45, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, E.; Vardaka, E.; Efthymiou, D.; Pitsios, C. Early Life Triggers for Food Allergy That in Turn Impacts Dietary Habits in Childhood. Allergol. Immunopathol. 2021, 49, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, F.; Pistelli, R.; Sestini, P.; Fortes, C.; Renzoni, E.; Rusconi, F.; Dell’Orco, V.; Ciccone, G.; Bisanti, L. Consumption of Fresh Fruit Rich in Vitamin C and Wheezing Symptoms in Children. SIDRIA Collaborative Group, Italy (Italian Studies on Respiratory Disorders in Children and the Environment). Thorax 2000, 55, 283–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemilä, H. The Effect of Vitamin C on Bronchoconstriction and Respiratory Symptoms Caused by Exercise: A Review and Statistical Analysis. Allergy Asthma Clin. Immunol. 2014, 10, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemilä, H. Vitamin C and Asthma. J. Allergy Clin. Immunol. 2014, 134, 1216. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, A.; Lewis, S.A.; Scrivener, S.L.; Antoniak, M.; Pacey, S.; Pringle, M.; Britton, J. Oral Magnesium and Vitamin C Supplements in Asthma: A Parallel Group Randomized Placebo-Controlled Trial. Clin. Exp. Allergy 2003, 33, 1355–1359. [Google Scholar] [CrossRef]
- Kaur, B.; Rowe, B.H.; Stovold, E. Vitamin C Supplementation for Asthma. Cochrane Database Syst. Rev. 2009, 2013, CD000993. [Google Scholar] [CrossRef]
- Vollbracht, C.; Raithel, M.; Krick, B.; Kraft, K.; Hagel, A.F. Intravenous Vitamin C in the Treatment of Allergies: An Interim Subgroup Analysis of a Long-Term Observational Study. J. Int. Med. Res. 2018, 46, 3640–3655. [Google Scholar] [CrossRef]
- Riccioni, G.; Barbara, M.; Bucciarelli, T.; di Ilio, C.; D’Orazio, N. Antioxidant Vitamin Supplementation in Asthma. Ann. Clin. Lab. Sci. 2007, 37, 96–101. [Google Scholar]
- Wilkinson, M.; Hart, A.; Milan, S.J.; Sugumar, K. Vitamins C and E for Asthma and Exercise-Induced Bronchoconstriction. Cochrane database Syst. Rev. 2014, 2014, CD010749. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Averill, S.H.; Lajiness, J.D. Asthma, Allergy and Vitamin E: Current and Future Perspectives. Free Radic. Biol. Med. 2021, 179, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Pearson, P.J.K.; Lewis, S.A.; Britton, J.; Fogarty, A. Vitamin E Supplements in Asthma: A Parallel Group Randomised Placebo Controlled Trial. Thorax 2004, 59, 652–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaffari, J.; Farid Hossiani, R.; Khalilian, A.; Nahanmoghadam, N.; Salehifar, E.; Rafatpanah, H. Vitamin e Supplementation, Lung Functions and Clinical Manifestations in Children with Moderate Asthma: A Randomized Double Blind Placebo-Controlled Trial. Iran. J. Allergy. Asthma. Immunol. 2014, 13, 98–103. [Google Scholar] [PubMed]
- Nwaru, B.I.; Virtanen, S.M.; Alfthan, G.; Karvonen, A.M.; Genuneit, J.; Lauener, R.P.; Dalphin, J.-C.; Hyvärinen, A.; Pfefferle, P.; Riedler, J.; et al. Serum Vitamin E Concentrations at 1 Year and Risk of Atopy, Atopic Dermatitis, Wheezing, and Asthma in Childhood: The PASTURE Study. Allergy 2014, 69, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Abdala-Valencia, H.; Hartert, T. Two Faces of Vitamin E in the Lung. Am. J. Respir. Crit. Care Med. 2013, 188, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Tobias, T.A.M.; Wood, L.G.; Rastogi, D. Carotenoids, Fatty Acids and Disease Burden in Obese Minority Adolescents with Asthma. Clin. Exp. Allergy 2019, 49, 838–846. [Google Scholar] [CrossRef]
- Bai, Y.-J.; Dai, R.-J. Serum Levels of Vitamin A and 25-Hydroxyvitamin D3 (25OHD3) as Reflectors of Pulmonary Function and Quality of Life (QOL) in Children with Stable Asthma: A Case-Control Study. Medicine 2018, 97, e9830. [Google Scholar] [CrossRef]
- Marquez, H.A.; Cardoso, W.V. Vitamin A-Retinoid Signaling in Pulmonary Development and Disease. Mol. Cell. Pediatr. 2016, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Morales, E.; Romieu, I.; Guerra, S.; Ballester, F.; Rebagliato, M.; Vioque, J.; Tardón, A.; Rodriguez Delhi, C.; Arranz, L.; Torrent, M.; et al. Maternal Vitamin D Status in Pregnancy and Risk of Lower Respiratory Tract Infections, Wheezing, and Asthma in Offspring. Epidemiology 2012, 23, 64–71. [Google Scholar] [CrossRef]
- Bountouvi, E.; Douros, K.; Papadopoulou, A. Can Getting Enough Vitamin D during Pregnancy Reduce the Risk of Getting Asthma in Childhood? Front. Pediatr. 2017, 5, 87. [Google Scholar] [CrossRef]
- Jensen, M.E.; Murphy, V.E.; Gibson, P.G.; Mattes, J.; Camargo, C.A.J. Vitamin D Status in Pregnant Women with Asthma and Its Association with Adverse Respiratory Outcomes during Infancy. J. Matern.-Fetal Neonatal Med. 2019, 32, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Christodoulou, I.; Rohde, G.; Agache, I.; Almqvist, C.; Bruno, A.; Bonini, S.; Bont, L.; Bossios, A.; Bousquet, J.; et al. Viruses and Bacteria in Acute Asthma Exacerbations—A GA2 LEN-DARE Systematic Review. Allergy 2011, 66, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Guibas, G.V.; Tsolia, M.; Christodoulou, I.; Stripeli, F.; Sakkou, Z.; Papadopoulos, N.G. Distinction between Rhinovirus-Induced Acute Asthma and Asthma-Augmented Influenza Infection. Clin. Exp. Allergy 2018, 48, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Brustad, N.; Eliasen, A.U.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. High-Dose Vitamin D Supplementation During Pregnancy and Asthma in Offspring at the Age of 6 Years. JAMA 2019, 321, 1003–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary Factors during Pregnancy and Atopic Outcomes in Childhood: A Systematic Review from the European Academy of Allergy and Clinical Immunology. Pediatric Allergy Immunol. 2020, 31, 889–912. [Google Scholar] [CrossRef]
- Lu, M.; Litonjua, A.A.; O’Connor, G.T.; Zeiger, R.S.; Bacharier, L.; Schatz, M.; Carey, V.J.; Weiss, S.T.; Mirzakhani, H. Effect of Early and Late Prenatal Vitamin D and Maternal Asthma Status on Offspring Asthma or Recurrent Wheeze. J. Allergy Clin. Immunol. 2021, 147, 1234–1241.e3. [Google Scholar] [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza A in Schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Xystrakis, E.; Kusumakar, S.; Boswell, S.; Peek, E.; Urry, Z.; Richards, D.F.; Adikibi, T.; Pridgeon, C.; Dallman, M.; Loke, T.K.; et al. Reversing the Defective Induction of IL-10-Secreting Regulatory T Cells in Glucocorticoid-Resistant Asthma Patients. J. Clin. Investig. 2006, 116, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Majak, P.; Olszowiec-Chlebna, M.; Smejda, K.; Stelmach, I. Vitamin D Supplementation in Children May Prevent Asthma Exacerbation Triggered by Acute Respiratory Infection. J. Allergy Clin. Immunol. 2011, 127, 1294–1296. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Itsiopoulos, C.; Lambert, K.; Katsardis, C.; Tsoukalas, D.; Erbas, B. Sufficient Vitamin D Status Positively Modified Ventilatory Function in Asthmatic Children Following a Mediterranean Diet Enriched with Fatty Fish Intervention Study. Nutr. Res. 2020, 82, 99–109. [Google Scholar] [CrossRef]
- Ali, N.S.; Nanji, K. A Review on the Role of Vitamin D in Asthma. Cureus 2017, 9, e1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willers, S.M.; Devereux, G.; Craig, L.C.A.; McNeill, G.; Wijga, A.H.; Abou El-Magd, W.; Turner, S.W.; Helms, P.J.; Seaton, A. Maternal Food Consumption during Pregnancy and Asthma, Respiratory and Atopic Symptoms in 5-Year-Old Children. Thorax 2007, 62, 772–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, D.; Halken, S.; Singh, C.; Muraro, A.; Angier, E.; Arasi, S.; Arshad, H.; Beyer, K.; Boyle, R.; du Toit, G.; et al. Preventing Food Allergy in Infancy and Childhood: Systematic Review of Randomised Controlled Trials. Pediatric Allergy Immunol. 2020, 31, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Kjaer, T.M.R.; Fruekilde, M.-B.; Michaelsen, K.F.; Frøkiaer, H. Fish Oil Supplementation of Lactating Mothers Affects Cytokine Production in 2 1/2-Year-Old Children. Lipids 2005, 40, 669–676. [Google Scholar] [CrossRef]
- Denburg, J.A.; Hatfield, H.M.; Cyr, M.M.; Hayes, L.; Holt, P.G.; Sehmi, R.; Dunstan, J.A.; Prescott, S.L. Fish Oil Supplementation in Pregnancy Modifies Neonatal Progenitors at Birth in Infants at Risk of Atopy. Pediatr. Res. 2005, 57, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Newson, R.B.; Shaheen, S.O.; Henderson, A.J.; Emmett, P.M.; Sherriff, A.; Calder, P.C. Umbilical Cord and Maternal Blood Red Cell Fatty Acids and Early Childhood Wheezing and Eczema. J. Allergy Clin. Immunol. 2004, 114, 531–537. [Google Scholar] [CrossRef]
- Noakes, P.S.; Vlachava, M.; Kremmyda, L.-S.; Diaper, N.D.; Miles, E.A.; Erlewyn-Lajeunesse, M.; Williams, A.P.; Godfrey, K.M.; Calder, P.C. Increased Intake of Oily Fish in Pregnancy: Effects on Neonatal Immune Responses and on Clinical Outcomes in Infants at 6 Mo. Am. J. Clin. Nutr. 2012, 95, 395–404. [Google Scholar] [CrossRef]
- Mensink-Bout, S.M.; Voortman, T.; Dervishaj, M.; Reiss, I.K.M.; De Jongste, J.C.; Jaddoe, V.W.V.; Duijts, L. Associations of Plasma Fatty Acid Patterns during Pregnancy with Respiratory and Allergy Outcomes at School Age. Nutrients 2020, 12, 3057. [Google Scholar] [CrossRef]
- Leermakers, E.T.M.; Sonnenschein-Van Der Voort, A.M.M.; Heppe, D.H.M.; De Jongste, J.C.; Moll, H.A.; Franco, O.H.; Hofman, A.; Jaddoe, V.W.V.; Duijts, L. Maternal Fish Consumption during Pregnancy and Risks of Wheezing and Eczema in Childhood: The Generation R Study. Eur. J. Clin. Nutr. 2013, 67, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Björkstén, B. Serum Levels of Phospholipid Fatty Acids in Mothers and Their Babies in Relation to Allergic Disease. Eur. J. Pediatr. 1998, 157, 298–303. [Google Scholar] [CrossRef]
- Miliku, K.; Richelle, J.; Becker, A.B.; Simons, E.; Moraes, T.J.; Stuart, T.E.; Mandhane, P.J.; Sears, M.R.; Subbarao, P.; Field, C.J.; et al. Sex-Specific Associations of Human Milk Long-Chain Polyunsaturated Fatty Acids and Infant Allergic Conditions. Pediatric Allergy Immunol. 2021, 32, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Duchén, K. Are Human Milk Polyunsaturated Fatty Acids (PUFA) Related to Atopy in the Mother and Her Child? Allergy 2001, 56, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H. Breastfeeding, Childhood Asthma, and Allergic Disease. Ann. Nutr. Metab. 2017, 70 (Suppl. 2), 26–36. [Google Scholar] [CrossRef] [PubMed]
- Soto-Ramírez, N.; Karmaus, W.; Zhang, H.; Liu, J.; Billings, D.; Gangur, V.; Amrol, D.; da Costa, K.-A.; Davis, S.; Goetzl, L. Fatty Acids in Breast Milk Associated with Asthma-like Symptoms and Atopy in Infancy: A Longitudinal Study. J. Asthma 2012, 49, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Wold, A.E.; Sandberg, A.-S. Low Breast Milk Levels of Long-Chain n-3 Fatty Acids in Allergic Women, despite Frequent Fish Intake. Clin. Exp. Allergy 2011, 41, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iranpour, R.; Kelishadi, R.; Babaie, S.; Khosravi-Darani, K.; Farajian, S. Comparison of Long Chain Polyunsaturated Fatty Acid Content in Human Milk in Preterm and Term Deliveries and Its Correlation with Mothers’ Diet. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 1–5. [Google Scholar]
- Bobiński, R.; Mikulska, M.; Mojska, H.; Simon, M. Comparison of the Fatty Acid Composition of Transitional and Mature Milk of Mothers Who Delivered Healthy Full-Term Babies, Preterm Babies and Full-Term Small for Gestational Age Infants. Eur. J. Clin. Nutr. 2013, 67, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Grunewald, M.; Hellmuth, C.; Kirchberg, F.F.; Mearin, M.L.; Auricchio, R.; Castillejo, G.; Korponay-Szabo, I.R.; Polanco, I.; Roca, M.; Vriezinga, S.L.; et al. Variation and Interdependencies of Human Milk Macronutrients, Fatty Acids, Adiponectin, Insulin, and IGF-II in the European PreventCD Cohort. Nutrients 2019, 11, 2034. [Google Scholar] [CrossRef] [Green Version]
- Siziba, L.P.; Lorenz, L.; Stahl, B.; Mank, M.; Marosvölgyi, T.; Decsi, T.; Rothenbacher, D.; Genuneit, J. Changes in Human Milk Fatty Acid Composition during Lactation: The Ulm SPATZ Health Study. Nutrients 2019, 11, 2842. [Google Scholar] [CrossRef] [Green Version]
- Stoney, R.M.; Woods, R.K.; Hosking, C.S.; Hill, D.J.; Abramson, M.J.; Thien, F.C.K. Maternal Breast Milk Long-Chain n-3 Fatty Acids Are Associated with Increased Risk of Atopy in Breastfed Infants. Clin. Exp. Allergy 2004, 34, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Mori, T.A.; Dunstan, J.A.; Taylor, A.L.; Thornton, C.A.; Croft, K.D.; Beilin, L.J.; Prescott, S.L. Fish Oil Supplementation in Pregnancy Lowers F2-Isoprostanes in Neonates at High Risk of Atopy. Free. Radic. Res. 2004, 38, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.H.; Nielsen, P.K.; Michaelsen, K.F.; Lund, P.; Lauritzen, L. The Composition of Polyunsaturated Fatty Acids in Erythrocytes of Lactating Mothers and Their Infants. Matern. Child Nutr. 2006, 2, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Krauss-Etschmann, S.; Hartl, D.; Rzehak, P.; Heinrich, J.; Shadid, R.; Del Carmen Ramírez-Tortosa, M.; Campoy, C.; Pardillo, S.; Schendel, D.J.; Decsi, T.; et al. Decreased Cord Blood IL-4, IL-13, and CCR4 and Increased TGF-Beta Levels after Fish Oil Supplementation of Pregnant Women. J. Allergy Clin. Immunol. 2008, 121, 464–470.e6. [Google Scholar] [CrossRef]
- Prescott, S.L.; Dunstan, J.A. Prenatal Fatty Acid Status and Immune Development: The Pathways and the Evidence. Lipids 2007, 42, 801–810. [Google Scholar] [CrossRef]
- Mickleborough, T.D.; Lindley, M.R. Omega-3 Fatty Acids: A Potential Future Treatment for Asthma? Expert Rev. Respir. Med. 2013, 7, 577–580. [Google Scholar] [CrossRef]
- Veselinović, A.; Petrović, S.; Žikić, V.; Subotić, M.; Jakovljević, V.; Jeremić, N.; Vučić, V. Neuroinflammation in Autism and Supplementation Based on Omega-3 Polyunsaturated Fatty Acids: A Narrative Review. Medicina 2021, 57, 893. [Google Scholar] [CrossRef]
- De Matos, O.G.; Amaral, S.S.; Pereira da Silva, P.E.M.; Perez, D.A.; Alvarenga, D.M.; Ferreira, A.V.M.; Alvarez-Leite, J.; Menezes, G.B.; Cara, D.C. Dietary Supplementation with Omega-3-PUFA-Rich Fish Oil Reduces Signs of Food Allergy in Ovalbumin-Sensitized Mice. Clin. Dev. Immunol. 2012, 2012, 236564. [Google Scholar] [CrossRef]
- Talaei, M.; Sdona, E.; Calder, P.C.; Jones, L.R.; Emmett, P.M.; Granell, R.; Bergström, A.; Melén, E.; Shaheen, S.O. Intake of N-3 Polyunsaturated Fatty Acids in Childhood, FADS Genotype and Incident Asthma. Eur. Respir. J. 2021, 58, 2003633. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Norman, H.A.; Gillaspy, J.E.; Duke, J.A. Common Purslane: A Source of Omega-3 Fatty Acids and Antioxidants. J. Am. Coll. Nutr. 1992, 11, 374–382. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R.; Ntatsi, G.; Petrotos, K.; Lykas, C.; Khah, E. Chemical Composition and Yield of Six Genotypes of Common Purslane (Portulaca Oleracea L.): An Alternative Source of Omega-3 Fatty Acids. Plant Foods Hum. Nutr. 2015, 70, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kasapidou, E.; Basdagianni, Z.; Papadopoulos, V.; Karaiskou, C.; Kesidis, A.; Tsiotsias, A. Effects of Intensive and Semi-Intensive Production on Sheep Milk Chemical Composition, Physicochemical Characteristics, Fatty Acid Profile, and Nutritional Indices. Animals 2021, 11, 2578. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Christopoulou, G.; Stavroulakis, G.; Freidl, R.; Linhart, B.; Zuidmeer, L.; Lakoumentas, J.; van Ree, R.; Valenta, R.; Papadopoulos, N.G. Natural History of IgE-Mediated Fish Allergy in Children. J. Allergy Clin. Immunol. Pract. 2021, 9, 3147–3156.e5. [Google Scholar] [CrossRef]
- Kalbermatter, C.; Fernandez Trigo, N.; Christensen, S.; Ganal-Vonarburg, S.C. Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn. Front. Immunol. 2021, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Paul, E.; McGee, D.; Sinniah, R.; Flom, E.; Jackson-Humbles, D.; Harkema, J.; Racicot, K.E. Chronic, Elevated Maternal Corticosterone During Pregnancy in the Mouse Increases Allergic Airway Inflammation in Offspring. Front. Immunol. 2020, 10, 3134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierau, M.; Arra, A.; Brunner-Weinzierl, M.C. Preventing Atopic Diseases During Childhood—Early Exposure Matters. Front. Immunol. 2021, 12, 231. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.; Kelly, R.S.; Lasky-Su, J.; Kachroo, P.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Laranjo, N.; et al. Dietary and Plasma Polyunsaturated Fatty Acids Are Inversely Associated with Asthma and Atopy in Early Childhood. J. Allergy Clin. Immunol. Pract. 2019, 7, 529–538.e8. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary Fat Intake and Risk of Cardiovascular Disease and All-Cause Mortality in a Population at High Risk of Cardiovascular Disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [Green Version]
- Van de Vijver, L.P.; Kardinaal, A.F.; Couet, C.; Aro, A.; Kafatos, A.; Steingrimsdottir, L.; Amorim Cruz, J.A.; Moreiras, O.; Becker, W.; van Amelsvoort, J.M.; et al. Association between Trans Fatty Acid Intake and Cardiovascular Risk Factors in Europe: The TRANSFAIR Study. Eur. J. Clin. Nutr. 2000, 54, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Psaltopoulou, T.; Naska, A.; Orfanos, P.; Trichopoulos, D.; Mountokalakis, T.; Trichopoulou, A. Olive Oil, the Mediterranean Diet, and Arterial Blood Pressure: The Greek European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Am. J. Clin. Nutr. 2004, 80, 1012–1018. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Toupadaki, N.; Tzonou, A.; Katsouyanni, K.; Manousos, O.; Kada, E.; Trichopoulos, D. The Macronutrient Composition of the Greek Diet: Estimates Derived from Six Case-Control Studies. Eur. J. Clin. Nutr. 1993, 47, 549–558. [Google Scholar] [PubMed]
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvadó, J.; Schwab, U.; et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitsavos, C.; Panagiotakos, D.B.; Tzima, N.; Chrysohoou, C.; Economou, M.; Zampelas, A.; Stefanadis, C. Adherence to the Mediterranean Diet Is Associated with Total Antioxidant Capacity in Healthy Adults: The ATTICA Study. Am. J. Clin. Nutr. 2005, 82, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Pan, W.H. Dietary Fats and Asthma in Teenagers: Analyses of the First Nutrition and Health Survey in Taiwan (NAHSIT). Clin. Exp. Allergy 2001, 31, 1875–1880. [Google Scholar] [CrossRef]
- Farmaki, A.-E.; Rayner, N.W.; Matchan, A.; Spiliopoulou, P.; Gilly, A.; Kariakli, V.; Kiagiadaki, C.; Tsafantakis, E.; Zeggini, E.; Dedoussis, G. The Mountainous Cretan Dietary Patterns and Their Relationship with Cardiovascular Risk Factors: The Hellenic Isolated Cohorts MANOLIS Study. Public Health Nutr. 2017, 20, 1063–1074. [Google Scholar] [CrossRef] [Green Version]
- Elmadfa, I.; Kornsteiner, M. Dietary Fat Intake–A Global Perspective. Ann. Nutr. Metab. 2009, 54 (Suppl. 1), 8–14. [Google Scholar] [CrossRef]
- Rocha, J.; Borges, N.; Pinho, O. Table Olives and Health: A Review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef]
- Han, M.; Lee, D.; Lee, S.H.; Kim, T.H. Oxidative Stress and Antioxidant Pathway in Allergic Rhinitis. Antioxidants 2021, 10, 1266. [Google Scholar] [CrossRef]
- Brigham, E.P.; Kolahdooz, F.; Hansel, N.; Breysse, P.N.; Davis, M.; Sharma, S.; Matsui, E.C.; Diette, G.; McCormack, M.C. Association between Western Diet Pattern and Adult Asthma: A Focused Review. Ann. Allergy Asthma Immunol. 2015, 114, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Van der Vliet, A.; Janssen-Heininger, Y.M.W.; Anathy, V. Oxidative Stress in Chronic Lung Disease: From Mitochondrial Dysfunction to Dysregulated Redox Signaling. Mol. Asp. Med. 2018, 63, 59–69. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Jones, D.P.; Brown, L.A.S. Glutathione Redox Control of Asthma: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2012, 17, 375–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bédard, A.; Northstone, K.; John Henderson, A.; Shaheen, S.O. Mediterranean Diet during Pregnancy and Childhood Respiratory and Atopic Outcomes: Birth Cohort Study. Eur. Respir. J. 2020, 55, 1901215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzi, L.; Apostolaki, G.; Bibakis, I.; Skypala, I.; Bibaki-Liakou, V.; Tzanakis, N.; Kogevinas, M.; Cullinan, P. Protective Effect of Fruits, Vegetables and the Mediterranean Diet on Asthma and Allergies among Children in Crete. Thorax 2007, 62, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, R.; Moreira, A.; Fonseca, J.; de Oliveira, J.F.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Adherence to the Mediterranean Diet and Fresh Fruit Intake Are Associated with Improved Asthma Control. Allergy 2008, 63, 917–923. [Google Scholar] [CrossRef]
- Arteaga-Badillo, D.A.; Portillo-Reyes, J.; Vargas-Mendoza, N.; Morales-González, J.A.; Izquierdo-Vega, J.A.; Sánchez-Gutiérrez, M.; Álvarez-González, I.; Morales-González, Á.; Madrigal-Bujaidar, E.; Madrigal-Santillán, E. Asthma: New Integrative Treatment Strategies for the Next Decades. Medicina 2020, 56, 438. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Shorey-Kendrick, L.E.; Milner, K.; Schilling, D.; Tiller, C.; Vuylsteke, B.; Scherman, A.; Jackson, K.; Haas, D.M.; Harris, J.; et al. Oral Vitamin C (500 Mg/d) to Pregnant Smokers Improves Infant Airway Function at 3 Months (VCSIP). A Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1139–1147. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free. Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an Essential Micronutrient for Human Health: Common, Novel, and Unexplored Dietary Sources. Free Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef]
- Shams, M.-H.; Jafari, R.; Eskandari, N.; Masjedi, M.; Kheirandish, F.; Ganjalikhani Hakemi, M.; Ghasemi, R.; Varzi, A.-M.; Sohrabi, S.-M.; Baharvand, P.A.; et al. Anti-Allergic Effects of Vitamin E in Allergic Diseases: An Updated Review. Int. Immunopharmacol. 2021, 90, 107196. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory Role of Vitamin E in the Immune System and Inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef]
- Sommer, A.; Vyas, K.S. A Global Clinical View on Vitamin A and Carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204S–1206S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lips, P.; van Schoor, N.M.; de Jongh, R.T. Diet, Sun, and Lifestyle as Determinants of Vitamin D Status. Ann. N. Y. Acad. Sci. 2014, 1317, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current Vitamin D Status in European and Middle East Countries and Strategies to Prevent Vitamin D Deficiency: A Position Statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolsk, H.M.; Chawes, B.L.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal Vitamin D Supplementation Reduces Risk of Asthma/Recurrent Wheeze in Early Childhood: A Combined Analysis of Two Randomized Controlled Trials. PLoS ONE 2017, 12, e0186657. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef]
- Shen, S.-Y.; Xiao, W.-Q.; Lu, J.-H.; Yuan, M.-Y.; He, J.-R.; Xia, H.-M.; Qiu, X.; Cheng, K.K.; Lam, K.B.H. Early Life Vitamin D Status and Asthma and Wheeze: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2018, 18, 120. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory Epithelial Cells Convert Inactive Vitamin D to Its Active Form: Potential Effects on Host Defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef] [Green Version]
- Gayan-Ramirez, G.; Janssens, W. Vitamin D Actions: The Lung Is a Major Target for Vitamin D, FGF23, and Klotho. JBMR Plus 2021, 5, e10569. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary Polyphenol Intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
- Bellik, Y.; Boukraâ, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular Mechanism Underlying Anti-Inflammatory and Anti-Allergic Activities of Phytochemicals: An Update. Molecules 2013, 18, 322–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Shytle, R.D. Apigenin and Luteolin Modulate Microglial Activation via Inhibition of STAT1-Induced CD40 Expression. J. Neuroinflammation 2008, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid Apigenin Inhibits Lipopolysaccharide-Induced Inflammatory Response through Multiple Mechanisms in Macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Patil, S.; Zhuge, J.; Wen, M.-C.; Bolleddula, J.; Doddaga, S.; Goldfarb, J.; Sampson, H.A.; Li, X.-M. Glycyrrhiza Uralensis Flavonoids Present in Anti-Asthma Formula, ASHMITM, Inhibit Memory Th2 Responses In Vitro and In Vivo. Phytother. Res. 2013, 27, 1381–1391. [Google Scholar] [CrossRef] [Green Version]
- Toledo, A.C.; Sakoda, C.P.P.; Perini, A.; Pinheiro, N.M.; Magalhães, R.M.; Grecco, S.; Tibério, I.F.L.C.; Câmara, N.O.; Martins, M.A.; Lago, J.H.G.; et al. Flavonone Treatment Reverses Airway Inflammation and Remodelling in an Asthma Murine Model. Br. J. Pharmacol. 2013, 168, 1736–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Takahashi, R. Flavonoids and Asthma. Nutrients 2013, 5, 2128–2143. [Google Scholar] [CrossRef]
- Saeed, M.A.; Gribben, K.C.; Alam, M.; Lyden, E.R.; Hanson, C.K.; LeVan, T.D. Association of Dietary Fiber on Asthma, Respiratory Symptoms, and Inflammation in the Adult National Health and Nutrition Examination Survey Population. Ann. Am. Thorac. Soc. 2020, 17, 1062–1068. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.; Greenhawt, M.; Pali-Schöll, I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.; et al. Role of Dietary Fiber in Promoting Immune Health-An EAACI Position Paper. Allergy 2021. Available online: https://d197for5662m48.cloudfront.net/documents/publicationstatus/72991/preprint_pdf/4a1577b89473c7b24df8429099743173.pdf (accessed on 24 March 2022). submitted.
- Durban, R.; Groetch, M.; Meyer, R.; Coleman Collins, S.; Elverson, W.; Friebert, A.; Kabourek, J.; Marchand, S.M.; McWilliam, V.; Netting, M.; et al. Dietary Management of Food Allergy. Immunol. Allergy Clin. N. Am. 2021, 41, 233–270. [Google Scholar] [CrossRef]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The Abundance and Variety of Carbohydrate-Active Enzymes in the Human Gut Microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Jo, A.; Casale, T.; Jeong, S.J.; Hong, S.-J.; Cho, J.K.; Holbrook, J.T.; Kumar, R.; Smith, L.J. Soy Isoflavones Reduce Asthma Exacerbation in Asthmatic Patients with High PAI-1-Producing Genotypes. J. Allergy Clin. Immunol. 2019, 144, 109–117.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.J.; Kalhan, R.; Wise, R.A.; Sugar, E.A.; Lima, J.J.; Irvin, C.G.; Dozor, A.J.; Holbrook, J.T. Effect of a Soy Isoflavone Supplement on Lung Function and Clinical Outcomes in Patients with Poorly Controlled Asthma: A Randomized Clinical Trial. JAMA 2015, 313, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Kamholz, S.L. Wine, Spirits and the Lung: Good, Bad or Indifferent? Trans. Am. Clin. Climatol. Assoc. 2006, 117, 129–145, discussion 145. [Google Scholar]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Marval-León, J.R.; Cámara-Martos, F.; Amaro-López, M.A.; Moreno-Rojas, R. Bioaccessibility and Content of Se in Fish and Shellfish Widely Consumed in Mediterranean Countries: Influence of Proteins, Fat and Heavy Metals. Int. J. Food Sci. Nutr. 2014, 65, 678–685. [Google Scholar] [CrossRef]
- Ariaee, N.; Farid, R.; Shabestari, F.; Shabestari, M.; Jabbari Azad, F. Trace Elements Status in Sera of Patients with Allergic Asthma. Reports Biochem. Mol. Biol. 2016, 5, 20–25. [Google Scholar]
- Hoffmann, P.R.; Jourdan-Le Saux, C.; Hoffmann, F.W.; Chang, P.S.; Bollt, O.; He, Q.; Tam, E.K.; Berry, M.J. A Role for Dietary Selenium and Selenoproteins in Allergic Airway Inflammation. J. Immunol. 2007, 179, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Hasselmark, L.; Malmgren, R.; Zetterström, O.; Unge, G. Selenium Supplementation in Intrinsic Asthma. Allergy 1993, 48, 30–36. [Google Scholar] [PubMed]
- Burney, P.; Potts, J.; Makowska, J.; Kowalski, M.; Phillips, J.; Gnatiuc, L.; Shaheen, S.; Joos, G.; Van Cauwenberge, P.; van Zele, T.; et al. A Case-Control Study of the Relation between Plasma Selenium and Asthma in European Populations: A GAL2EN Project. Allergy 2008, 63, 865–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Marcos, L. MEDITERRANEAN DIET AND ASTHMA: TIME FOR CLINICAL TRIALS IN CHILDREN. Allergol. Immunopathol. 2019, 47, 207–208. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Jones, M.; Potts, J.F.; Newson, R.B.; Obaseki, D.; Burney, P.G.J. Low Grade Systemic Inflammation and Lung Function Outcomes in European Adults from the Global Asthma and Allergy Network of Excellence (GA2LEN) Follow-up Survey. Eur. Respir. J. 2014, 44 (Suppl. 58), 4441. [Google Scholar]
- Crespo, A.; Giner, J.; Torrejón, M.; Belda, A.; Mateus, E.; Granel, C.; Torrego, A.; Ramos-Barbón, D.; Plaza, V. Clinical and Inflammatory Features of Asthma with Dissociation between Fractional Exhaled Nitric Oxide and Eosinophils in Induced Sputum. J. Asthma 2016, 53, 459–464. [Google Scholar] [CrossRef]
- Wood, L.G.; Gibson, P.G. Dietary Factors Lead to Innate Immune Activation in Asthma. Pharmacol. Ther. 2009, 123, 37–53. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of Plasma Polyunsaturated Fatty Acids to Circulating Inflammatory Markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Woolson, R.F. Dietary Magnesium and C-Reactive Protein Levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef]
- Sierksma, A.; van der Gaag, M.S.; Kluft, C.; Hendriks, H.F.J. Moderate Alcohol Consumption Reduces Plasma C-Reactive Protein and Fibrinogen Levels; a Randomized, Diet-Controlled Intervention Study. Eur. J. Clin. Nutr. 2002, 56, 1130–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, L.G.; Shivappa, N.; Berthon, B.S.; Gibson, P.G.; Hebert, J.R. Dietary Inflammatory Index Is Related to Asthma Risk, Lung Function and Systemic Inflammation in Asthma. Clin. Exp. Allergy 2015, 45, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Sexton, P.; Black, P.; Metcalf, P.; Wall, C.R.; Ley, S.; Wu, L.; Sommerville, F.; Brodie, S.; Kolbe, J. Influence of Mediterranean Diet on Asthma Symptoms, Lung Function, and Systemic Inflammation: A Randomized Controlled Trial. J. Asthma 2013, 50, 75–81. [Google Scholar] [CrossRef]
- Douros, K.; Thanopoulou, M.-I.; Boutopoulou, B.; Papadopoulou, A.; Papadimitriou, A.; Fretzayas, A.; Priftis, K.N. Adherence to the Mediterranean Diet and Inflammatory Markers in Children with Asthma. Allergol. Immunopathol. 2019, 47, 209–213. [Google Scholar] [CrossRef]
- Mathioudakis, A.G.; Tsilochristou, O.; Adcock, I.M.; Bikov, A.; Bjermer, L.; Clini, E.; Flood, B.; Herth, F.; Horvath, I.; Kalayci, O.; et al. ERS/EAACI Statement on Adherence to International Adult Asthma Guidelines. Eur. Respir. Rev. 2021, 30, 161. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Feketea, G.; Konstantinou, G.N.; Zekakos, X.D.; Valianatou, M.; Petrodimopoulou, M.; Vourga, V.; Tasios, I.; Papadopoulos, N.G. Food Protein-Induced Allergic Proctocolitis: The Effect of Maternal Diet During Pregnancy and Breastfeeding in a Mediterranean Population. Front Nutr. 2022, 9, 843437. [Google Scholar] [CrossRef]
| Type of Lipids | Asthma/Atopy Outcomes | Reference |
|---|---|---|
| MUFAs | • Reduced risk of asthma/atopy | [70,92] |
| • Cell membranes support-reduced AD | [93] | |
| n-3 PUFAs (fish oils) | Prenatal | |
| • reduced risk of wheeze/asthma | [62,63,66,67,87,94] | |
| • respiratory infections | [65] | |
| • atopy | [90,91,95] | |
| • AD | [96] | |
| Postnatal | ||
| observational: protection on asthma, atopy | [26,69,97,98,99,100,101,102,103] | |
| interventional: inconclusive on | ||
| • AD | [104,105] | |
| • asthma/wheeze | [66,106,107,108,109,110,111,112,113] | |
| • worsening on asthma in aspirin-intolerant patients | [114,115] | |
| n-6 PUFAs | Prenatal | |
| no effect or increased risk of | ||
| • AD | [43,116,117] | |
| • atopy | [118] | |
| Postnatal | ||
| • contradictory results on AD and asthma | [119,120,121,122] | |
| Trans-fat | Increased risk of asthma/atopy | [123,124,125,126] |
| Saturated FAs | Increased | |
| • bronchial hyperresponsiveness | [127] | |
| • asthma | [128] | |
| • atopy | [129] | |
| Vitamins C, E, A (fruits and vegetables) | Prenatal | |
| • observational: conflicting evidence on asthma/atopy | [130] | |
| • interventional: lack of evidence | ||
| Postnatal | ||
| • observational: generally favorable on asthma/wheeze, atopy | [43,50,131,132,133,134,135,136,137,138,139,140] | |
| • interventional: conflicting evidence, nutrient specific | [12,141,142,143,144,145,146,147,148] | |
| Vitamin D | Prenatal | |
| • observational: generally favorable on bronchial outcomes | [12,13,149,150,151,152,153] | |
| • interventional: favorable when mother has asthma | [154,155,156] | |
| Postnatal | ||
| • observational: favorable on asthma/atopy | [157,158,159] | |
| • interventional: favorable on asthma/atopy | [160,161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassilopoulou, E.; Guibas, G.V.; Papadopoulos, N.G. Mediterranean-Type Diets as a Protective Factor for Asthma and Atopy. Nutrients 2022, 14, 1825. https://doi.org/10.3390/nu14091825
Vassilopoulou E, Guibas GV, Papadopoulos NG. Mediterranean-Type Diets as a Protective Factor for Asthma and Atopy. Nutrients. 2022; 14(9):1825. https://doi.org/10.3390/nu14091825
Chicago/Turabian StyleVassilopoulou, Emilia, George V. Guibas, and Nikolaos G. Papadopoulos. 2022. "Mediterranean-Type Diets as a Protective Factor for Asthma and Atopy" Nutrients 14, no. 9: 1825. https://doi.org/10.3390/nu14091825






