Bioelectrical Impedance Analysis for the Assessment of Body Composition in Sarcopenia and Type 2 Diabetes
Abstract
:1. Introduction
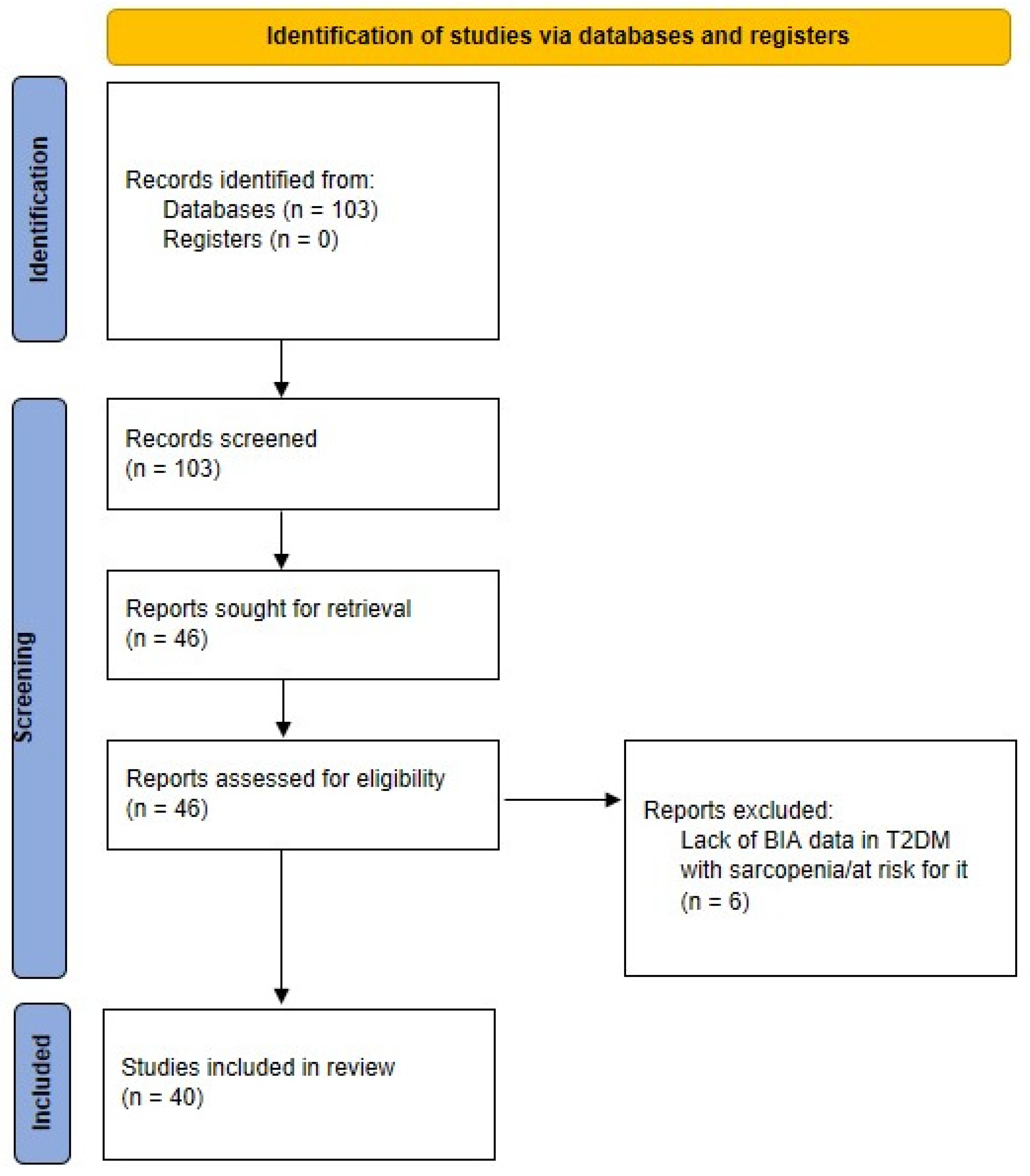
2. Methodology for Scientific Literature Search
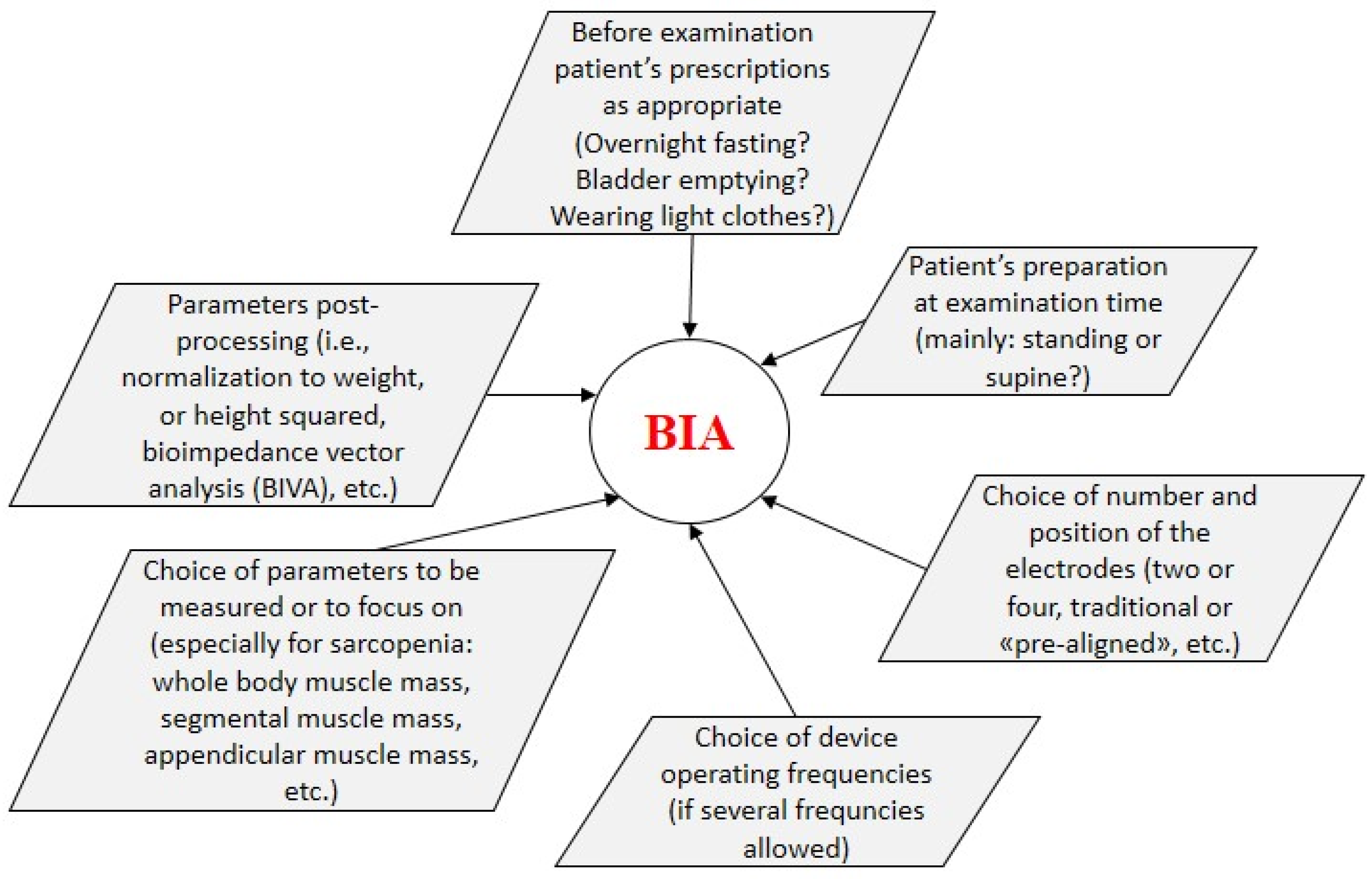
3. BIA in Sarcopenia and T2DM: Cross-Sectional Studies
3.1. “Early” Studies
3.2. Recent Studies
4. Adding Explicit Nutritional Information
5. Longitudinal and Interventional Studies
6. Concluding Comments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawada, T. Mortality Risk of Sarcopenia in Older Subjects. J. Am. Med. Dir. Assoc. 2021, 22, 1883. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wan, C.S.; Ktoris, K.; Reijnierse, E.M.; Maier, A.B. Sarcopenia Is Associated with Mortality in Adults: A Systematic Review and Meta-Analysis. Gerontology 2021, 68, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.K.-Y.; Au, P.C.-M.; Li, G.H.-Y.; Chan, M.; Li, H.-L.; Cheung, B.M.-Y.; Wong, I.C.-K.; Lee, V.H.-F.; Mok, J.; Yip, B.H.-K.; et al. Sarcopenia and Mortality in Different Clinical Conditions: A Meta-Analysis. Osteoporos. Sarcopenia 2021, 7, S19–S27. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; Senmaru, T.; et al. Sarcopenia Is Associated with a Risk of Mortality in People with Type 2 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 783363. [Google Scholar] [CrossRef]
- Wong, E.; Backholer, K.; Gearon, E.; Harding, J.; Freak-Poli, R.; Stevenson, C.; Peeters, A. Diabetes and Risk of Physical Disability in Adults: A Systematic Review and Meta-Analysis. Lancet Diabetes Endocrinol. 2013, 1, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Huang, L.; Zhang, Y.; Hong, X.; Weng, S.; Shen, X.; Zhao, F.; Yan, S. Additive Effect of Sarcopenia and Anemia on the 10-Year Risk of Cardiovascular Disease in Patients with Type 2 Diabetes. J. Diabetes Res. 2022, 2022, 2202511. [Google Scholar] [CrossRef]
- Defronzo, R.A. Banting Lecture. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [Green Version]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and Type 2 Diabetes Mellitus: A Bidirectional Relationship. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [Green Version]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia-What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Ashworth, A. Sarcopenia and Malnutrition: Commonly Occurring Conditions in the Older Population. Br. J. Nurs. 2021, 30, S4–S10. [Google Scholar] [CrossRef]
- Göbl, C.; Tura, A. Focus on Nutritional Aspects of Sarcopenia in Diabetes: Current Evidence and Remarks for Future Research. Nutrients 2022, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della Pepa, G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Hilario Christensen, S.; Grenov, B.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F. Early Nutrition and Its Effect on Growth, Body Composition and Later Obesity. World Rev. Nutr. Diet. 2021, 123, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology Guidelines for Sarcopenia in Liver Disease (1st Edition): Recommendation from the Working Group for Creation of Sarcopenia Assessment Criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Sneed, N.M.; Morrison, S.A. Body Composition Methods in Adults with Type 2 Diabetes or at Risk for T2D: A Clinical Review. Curr. Diabetes Rep. 2021, 21, 14. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Barbosa-Silva, T.G.; Heymsfield, S.B. Bioelectrical Impedance Analysis in the Assessment of Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Di Gregorio, A.; Pasanisi, F.; Scalfi, L. Bioelectrical Impedance Analysis (BIA)-Derived Phase Angle in Sarcopenia: A Systematic Review. Clin. Nutr. 2021, 40, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, Y.; Zhang, H.; Chen, C.; Wang, Y.; Zhang, J.; Xia, F.; Benedict, C.; Tan, X.; Lu, Y. Total and Regional Fat-to-Muscle Mass Ratio Measured by Bioelectrical Impedance and Risk of Incident Type 2 Diabetes. J. Cachexia Sarcopenia Muscle 2021, 12, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Susic, D.; O’Sullivan, A.J.; Henry, A. Reproducibility of Bioelectrical Impedance Analysis in Pregnancy and the Association of Body Composition with the Risk of Gestational Diabetes: A Substudy of MUMS Cohort. J. Obes. 2020, 2020, 3128767. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, P.; Cesaro, U.; Frosolone, M.; Moccaldi, N.; Taglialatela, M. A Micro-Bioimpedance Meter for Monitoring Insulin Bioavailability in Personalized Diabetes Therapy. Sci. Rep. 2020, 10, 13656. [Google Scholar] [CrossRef]
- Tajiri, Y.; Kato, T.; Nakayama, H.; Yamada, K. Reduction of Skeletal Muscle, Especially in Lower Limbs, in Japanese Type 2 Diabetic Patients with Insulin Resistance and Cardiovascular Risk Factors. Metab. Syndr. Relat. Disord. 2010, 8, 137–142. [Google Scholar] [CrossRef]
- Buffa, R.; Saragat, B.; Succa, V.; Ruggiu, R.; Carboni, L.; Putzu, P.F.; Marini, E. Elderly Subjects with Type 2 Diabetes Show Altered Tissue Electrical Properties. Nutrition 2013, 29, 132–137. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Barbieri, M.; Fava, I.; Desiderio, M.; Coppola, C.; Marfella, R.; Paolisso, G. Sarcopenia in Elderly Diabetic Patients: Role of Dipeptidyl Peptidase 4 Inhibitors. J. Am. Med. Dir. Assoc. 2016, 17, 896–901. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Osaka, T.; Fukuda, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. The Relationship between Hepatic Steatosis and Skeletal Muscle Mass Index in Men with Type 2 Diabetes. Endocr. J. 2016, 63, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Tuzun, S.; Oner, C.; Dabak, M.R.; Kasikci, H.O.; Sargin, M. Relation of Muscle Indices with Metabolic Parameters and C-Peptide in Type 2 Diabetes Mellitus. J. Coll. Physicians Surg. Pak. 2017, 27, 673–677. [Google Scholar]
- Osaka, T.; Hamaguchi, M.; Hashimoto, Y.; Ushigome, E.; Tanaka, M.; Yamazaki, M.; Fukui, M. Decreased the Creatinine to Cystatin C Ratio Is a Surrogate Marker of Sarcopenia in Patients with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2018, 139, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Tuzun, S.; Cifcili, S.; Dabak, M.R.; Tamer, I.; Sargin, M. Sarcopenia among Genders in Type 2 Diabetes Mellitus Patients Using Different Formulas of Bioimpedance Analysis. J. Coll. Physicians Surg. Pak. 2018, 28, 586–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murai, J.; Nishizawa, H.; Otsuka, A.; Fukuda, S.; Tanaka, Y.; Nagao, H.; Sakai, Y.; Suzuki, M.; Yokota, S.; Tada, H.; et al. Low Muscle Quality in Japanese Type 2 Diabetic Patients with Visceral Fat Accumulation. Cardiovasc. Diabetol. 2018, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Hamaguchi, M.; Okada, H.; Ushigome, E.; Asano, M.; Yamazaki, M.; Fukui, M. Sarcopenia Is Associated with Blood Pressure Variability in Older Patients with Type 2 Diabetes: A Cross-Sectional Study of the KAMOGAWA-DM Cohort Study. Geriatr. Gerontol. Int. 2018, 18, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, Y.; Narita, T.; Fujita, H.; Morii, T.; Sato, T.; Sassa, M.H.; Yamada, Y. Importance of Physical Evaluation Using Skeletal Muscle Mass Index and Body Fat Percentage to Prevent Sarcopenia in Elderly Japanese Diabetes Patients. J. Diabetes Investig. 2019, 10, 322–330. [Google Scholar] [CrossRef]
- Oh, T.J.; Song, Y.; Moon, J.H.; Choi, S.H.; Jang, H.C. Diabetic Peripheral Neuropathy as a Risk Factor for Sarcopenia. Ann. Geriatr. Med. Res. 2019, 23, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Yasemin, Ö.; Seydahmet, A.; Özcan, K. Relationship between Diabetic Neuropathy and Sarcopenia. Prim. Care Diabetes 2019, 13, 521–528. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Zheng, C. The Relationship between Non-Alcoholic Fatty Liver and Skeletal Muscle Mass to Visceral Fat Area Ratio in Women with Type 2 Diabetes. BMC Endocr. Disord. 2019, 19, 76. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, M.C.; Rocha, N.; Bandeira, E.; Dantas, I.; Chaves, C.; Oliveira, M.; Bandeira, F. Serum Sclerostin, Body Composition, and Sarcopenia in Hemodialysis Patients with Diabetes. Int. J. Nephrol. 2020, 2020, 4596920. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.H.; Lee, Y.-H.; Suh, Y.J.; Ahn, S.H.; Hong, S.; Choi, Y.J.; Huh, B.W.; Park, S.W.; Lee, E.; Kim, S.H. Low Muscle Mass Is Associated with Carotid Atherosclerosis in Patients with Type 2 Diabetes. Atherosclerosis 2020, 305, 19–25. [Google Scholar] [CrossRef]
- Jung, C.-H.; Cho, Y.Y.; Choi, D.; Kim, B.-Y.; Kim, C.-H.; Mok, J.-O. Relationship of Sarcopenia with Microcirculation Measured by Skin Perfusion Pressure in Patients with Type 2 Diabetes. Endocrinol. Metab. 2020, 35, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Lee, Y.-H.; Park, S.W.; Choi, Y.J.; Huh, B.W.; Lee, E.; Huh, K.B.; Kim, S.H.; Cha, B.-S. Sarcopenia Is Associated with Non-Alcoholic Fatty Liver Disease in Men with Type 2 Diabetes. Diabetes Metab. 2020, 46, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Ng, T.P.; Lim, C.L.; Moh, A.; Ang, S.F.; Wang, J.; Goh, K.S.; Ang, K.; Tang, W.E.; Kwan, P.Y.; et al. Association between Lower Extremity Skeletal Muscle Mass and Impaired Cognitive Function in Type 2 Diabetes. Sci. Rep. 2020, 10, 2956. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Yu, N.-C.; Wu, H.-C.; Lee, Y.-Y.; Lin, W.-C.; Chiu, I.-Y.; Chien, W.-C.; Liu, Y.-C. Association of Body Composition with Type 2 Diabetes: A Retrospective Chart Review Study. Int. J. Environ. Res. Public Health 2021, 18, 4421. [Google Scholar] [CrossRef] [PubMed]
- Minohara, T.; Noso, S.; Babaya, N.; Hiromine, Y.; Taketomo, Y.; Niwano, F.; Makutani, Y.; Yoshida, S.; Yasutake, S.; Imamura, S.; et al. Associations between Genetic Loci Related to Lean Mass and Body Composition in Type 2 Diabetes. Geriatr. Gerontol. Int. 2021, 21, 932–938. [Google Scholar] [CrossRef]
- Jiang, L.-L.; Xu, X.-H.; Luo, M.-H.; Wang, H.-Y.; Ding, B.; Yan, R.-N.; Hu, Y.; Ma, J.-H. Association of Acarbose with Decreased Muscle Mass and Function in Patients with Type 2 Diabetes: A Retrospective, Cross-Sectional Study. Diabetes Ther. 2021, 12, 2955–2969. [Google Scholar] [CrossRef]
- Kis, O.; Buch, A.; Eldor, R.; Rubin, A.; Dunsky, A.; Stern, N.; Moran, D.S. Should Knee Extension Strength Testing Be Implemented as a Screening Test for Identifying Probable and Confirmed Sarcopenia in Older T2DM Patients? Eur. Rev. Aging Phys. Act. 2022, 19, 5. [Google Scholar] [CrossRef]
- Akpinar, T.S.; Tayfur, M.; Tufan, F.; Sahinkaya, T.; Köse, M.; Özşenel, E.B.; Bahat Öztürk, G.; Saka, B.; Erten, N.; Yildiz, S.; et al. Uncomplicated Diabetes Does Not Accelerate Age-Related Sarcopenia. Aging Male 2014, 17, 205–210. [Google Scholar] [CrossRef]
- Hamasaki, H.; Kawashima, Y.; Adachi, H.; Moriyama, S.; Katsuyama, H.; Sako, A.; Yanai, H. Associations between Lower Extremity Muscle Mass and Metabolic Parameters Related to Obesity in Japanese Obese Patients with Type 2 Diabetes. PeerJ 2015, 3, e942. [Google Scholar] [CrossRef] [Green Version]
- Küçükdiler, A.H.E.; Varlı, M.; Yavuz, Ö.; Yalçın, A.; Selvi Öztorun, H.; Devrim, E.; Aras, S. Evaluation of Oxidative Stress Parameters and Antioxidant Status in Plasma and Erythrocytes of Elderly Diabetic Patients with Sarcopenia. J. Nutr. Health Aging 2019, 23, 239–245. [Google Scholar] [CrossRef]
- Okamura, T.; Miki, A.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Shortage of Energy Intake Rather than Protein Intake Is Associated with Sarcopenia in Elderly Patients with Type 2 Diabetes: A Cross-Sectional Study of the KAMOGAWA-DM Cohort. J. Diabetes 2019, 11, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hashimoto, Y.; Miki, A.; Kaji, A.; Sakai, R.; Iwai, K.; Osaka, T.; Kitagawa, N.; Ushigome, E.; Hamaguchi, M.; et al. High Brain Natriuretic Peptide Is Associated with Sarcopenia in Patients with Type 2 Diabetes: A Cross-Sectional Study of KAMOGAWA-DM Cohort Study. Endocr. J. 2019, 66, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Freitas, M.M.; de Oliveira, V.L.P.; Grassi, T.; Valduga, K.; Miller, M.E.P.; Schuchmann, R.A.; Souza, K.L.A.; de Azevedo, M.J.; Viana, L.V.; de Paula, T.P. Difference in Sarcopenia Prevalence and Associated Factors According to 2010 and 2018 European Consensus (EWGSOP) in Elderly Patients with Type 2 Diabetes Mellitus. Exp. Gerontol. 2020, 132, 110835. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hashimoto, Y.; Miki, A.; Kaji, A.; Sakai, R.; Iwai, K.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Reduced Dietary Omega-3 Fatty Acids Intake Is Associated with Sarcopenia in Elderly Patients with Type 2 Diabetes: A Cross-Sectional Study of KAMOGAWA-DM Cohort Study. J. Clin. Biochem. Nutr. 2020, 66, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Oguz, A.; Sahin, M.; Tuzun, D.; Kurutas, E.B.; Ulgen, C.; Bozkus, O.; Gul, K. Irisin Is a Predictor of Sarcopenic Obesity in Type 2 Diabetes Mellitus: A Cross-Sectional Study. Medicine 2021, 100, e26529. [Google Scholar] [CrossRef]
- Sugiyama, S.; Jinnouchi, H.; Kurinami, N.; Hieshima, K.; Yoshida, A.; Jinnouchi, K.; Nishimura, H.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. Dapagliflozin Reduces Fat Mass without Affecting Muscle Mass in Type 2 Diabetes. J. Atheroscler. Thromb. 2018, 25, 467–476. [Google Scholar] [CrossRef] [Green Version]
- LeCroy, M.N.; Hua, S.; Kaplan, R.C.; Sotres-Alvarez, D.; Qi, Q.; Thyagarajan, B.; Gallo, L.C.; Pirzada, A.; Daviglus, M.L.; Schneiderman, N.; et al. Associations of Changes in Fat Free Mass with Risk for Type 2 Diabetes: Hispanic Community Health Study/Study of Latinos. Diabetes Res. Clin. Pract. 2021, 171, 108557. [Google Scholar] [CrossRef]
- Low, S.; Pek, S.; Moh, A.; Khin, C.Y.A.; Lim, C.L.; Ang, S.F.; Wang, J.; Ang, K.; Tang, W.E.; Lim, Z.; et al. Low Muscle Mass Is Associated with Progression of Chronic Kidney Disease and Albuminuria—An 8-Year Longitudinal Study in Asians with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2021, 174, 108777. [Google Scholar] [CrossRef]
- Buscemi, C.; Ferro, Y.; Pujia, R.; Mazza, E.; Boragina, G.; Sciacqua, A.; Piro, S.; Pujia, A.; Sesti, G.; Buscemi, S.; et al. Sarcopenia and Appendicular Muscle Mass as Predictors of Impaired Fasting Glucose/Type 2 Diabetes in Elderly Women. Nutrients 2021, 13, 1909. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.J.; Chang, T.I.; Kang, E.W.; Joo, Y.S.; Kim, H.W.; Park, J.T.; Yoo, T.-H.; Kang, S.-W.; Han, S.H. Synergic Association of Diabetes Mellitus and Chronic Kidney Disease with Muscle Loss and Cachexia: Results of a 16-Year Longitudinal Follow-up of a Community-Based Prospective Cohort Study. Aging 2021, 13, 21941–21961. [Google Scholar] [CrossRef]
- Sundar, V.V.; Ong, S.H.; Easaw, M.E.P.M.; Chee, W.S.S. Sarcopenia with Co-Existent Type 2 Diabetes Mellitus Is Associated with Worse Clinical Outcomes among Hospitalised Cardiac Patients. Clin. Nutr. ESPEN 2021, 46, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Moon, S.; Moon, M.K. Muscle Strength, an Independent Determinant of Glycemic Control in Older Adults with Long-Standing Type 2 Diabetes: A Prospective Cohort Study. BMC Geriatr. 2021, 21, 684. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Takahashi, F.; Hashimoto, Y.; Munekawa, C.; Hosomi, Y.; Okamura, T.; Okada, H.; Senmaru, T.; Nakanishi, N.; Majima, S.; et al. Effect of COVID-19 Pandemic on the Change in Skeletal Muscle Mass in Older Patients with Type 2 Diabetes: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 4188. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Wang, J.; Moh, A.; Fen Ang, S.; Ang, K.; Shao, Y.-M.; Ching, J.; Ning Wee, H.; Siang Lee, L.; Kovalik, J.-P.; et al. Amino Acid Profile of Skeletal Muscle Loss in Type 2 Diabetes: Results from a 7-Year Longitudinal Study in Asians. Diabetes Res. Clin. Pract. 2022, 186, 109803. [Google Scholar] [CrossRef]
- Hoppe, K.; Schwermer, K.; Dopierała, M.; Kałużna, M.; Hoppe, A.; Chou, J.T.-T.; Oko, A.; Pawlaczyk, K. Can Overnutrition Lead to Wasting?—The Paradox of Diabetes Mellitus in End-Stage Renal Disease Treated with Maintenance Hemodialysis. Nutrients 2022, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, J.R.; Johnson, B.A.; Benavides, M.L.; Moore, M.L.; Stratton, M.T.; Harty, P.S.; Siedler, M.R.; Tinsley, G.M. Agreement of Bioelectrical Resistance, Reactance, and Phase Angle Values from Supine and Standing Bioimpedance Analyzers. Physiol. Meas. 2021, 42, 035003. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.T.; Smith, R.W.; Harty, P.S.; Rodriguez, C.; Johnson, B.A.; Dellinger, J.R.; Williams, A.D.; White, S.J.; Benavides, M.L.; Tinsley, G.M. Longitudinal Agreement of Four Bioimpedance Analyzers for Detecting Changes in Raw Bioimpedance during Purposeful Weight Gain with Resistance Training. Eur. J. Clin. Nutr. 2021, 75, 1060–1068. [Google Scholar] [CrossRef]
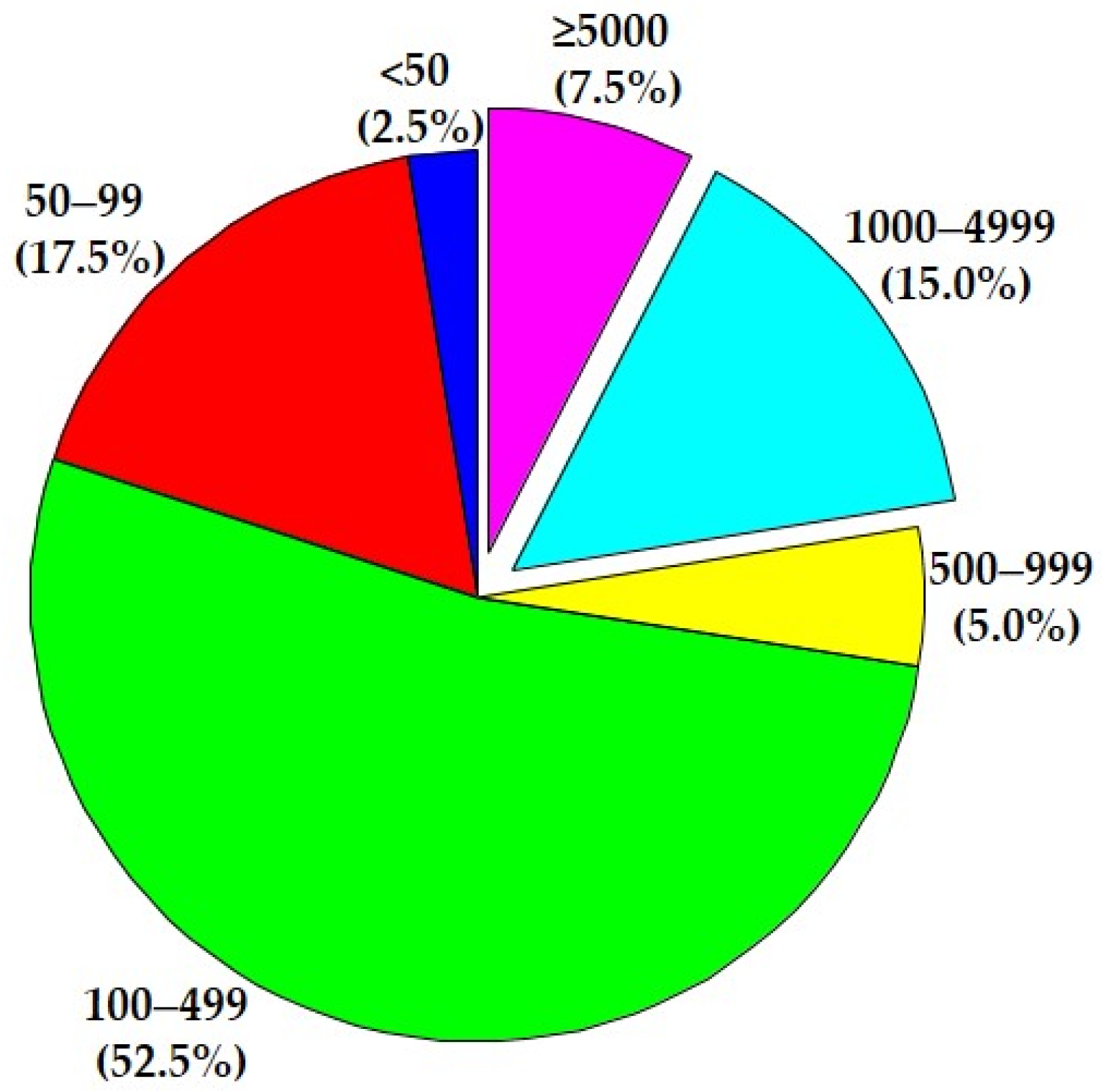
| Ref. No. | “Tweet” on Study Characteristics/Outcomes | BIA Estimated/Calculated Parameters | No. of Subjects | Publication Year | No. of Citations |
|---|---|---|---|---|---|
| Cross-sectional studies | |||||
| “Early” studies | |||||
| [26] | Regional body composition analysis in T2DM patients shows decreased leg muscle mass, leg sarcopenia, and increased risk for cardiovascular diseases | Whole body and isolated (arms and legs) muscle mass (absolute and normalized to body weight), whole body fat mass (absolute and normalized) | 198 T2DM, 198 healthy | 2010 | 35 (2.9) |
| [27] | T2DM patients under bioelectrical impedance vector analysis show bioelectrical abnormalities, such as smaller appendicular muscular area, which can be risk factor for sarcopenia | Body resistance (R) and reactance (Xc), phase angle as arctan(Xc/R), and impedance vector as (R2 + Xc2)0.5 | 144 T2DM, 209 healthy | 2013 | 10 (1.1) |
| [28] | In T2DM, DPP4 inhibitors treatment improves sarcopenic parameters as compared to sulphonylurea treatment | Fat-free mass (FFM), fat mass (FM), FFM/FM ratio, total, extracellular and intracellular water, skeletal muscle mass (SMM), SMM index | 80 T2DM | 2016 | 30 (5) |
| [29] | The skeletal muscle mass index is inversely associated with hepatic steatosis in T2DM men, likely due to factors such as insulin resistance, and abnormal levels of insulin-like growth factor 1 | Skeletal muscle mass (normalized to total body weight), visceral fat area | 145 T2DM | 2016 | 47 (7.8) |
| [30] | In T2DM, there is direct correlation with BIA-derived parameters and plasma C-peptide, as well as inverse correlation with HDL-cholesterol, whereas no correlation is observed with glycemia and LDL | Body fat mass, total muscle mass, appendicular muscle mass, skeletal muscle index and percentage, total muscle index and percentage | 359 T2DM | 2017 | 1 (0.2) |
| [31] | The serum creatinine to cystatin C ratio (Cre/CysC) is usable as a simple screening tool to identify T2DM patients at high risk for sarcopenia, with an optimal cut-off value for Cre/CysC equal to 0.90 | Appendicular skeletal muscle mass, skeletal muscle index | 285 T2DM | 2018 | 49 (12.3) |
| [32] | In obese T2DM, the prevalence of sarcopenia is low when diagnosed by the skeletal muscle index or the appendicular muscle mass/BMI ratio, and is much higher when using the body muscle ratio | Total fat mass, total muscle mass, sum of the appendicular muscle masses of the four limbs, skeletal muscle index, body muscle ratio | 295 T2DM | 2018 | 2 (0.5) |
| [33] | T2DM patients with visceral fat accumulation have low muscle quality, and patients with low muscle quality are more affected with cardiovascular diseases | Trunk, muscle masses of arms and legs, muscle quality (ratio of grip strength to arm muscle mass), skeletal muscle index | 183 T2DM | 2018 | 26 (6.5) |
| [34] | In elderly T2DM patients, sarcopenia is associated with blood pressure variability, but not with its absolute values | Body fat mass, skeletal muscle mass, appendicular muscle mass, skeletal muscle mass index (appendicular muscle mass /height squared) | 146 T2DM | 2018 | 20 (5) |
| [35] | Elderly T2DM patients are at higher risk for sarcopenia when having high body fat percentage but low BMI | Limb skeletal muscle mass, skeletal muscle mass index | 267 T2DM | 2019 | 35 (11.7) |
| [36] | Neuropathy screening questionnaire scores are higher in T2DM sarcopenic than in non-sarcopenic patients, thus a questionnaire may be used for screening for sarcopenia in subjects with diabetic neuropathy | Appendicular skeletal muscle mass (divided by height squared) | 170 T2DM | 2019 | 1 (0.3) |
| [37] | In sarcopenic obese patients, diabetic neuropathy prevalence reaches 96%, indicating a clear relationship between sarcopenia and diabetic neuropathy | Absolute skeletal muscle mass, skeletal muscle mass index | 602 T2DM | 2019 | 6 (2) |
| [38] | In T2DM, lower values of skeletal muscle mass normalized to visceral fat area (skeletal-to-visceral ratio) are associated with higher risks of developing non-alcoholic fatty liver disease | Lean body mass of arms and legs, appendicular skeletal muscle mass (sum of arms and legs lean masses), visceral fat area | 445 T2DM | 2019 | 5 (1.7) |
| Recent studies | |||||
| [39] | In hemodialysis patients, serum sclerostin is directly related to diabetes and inversely related to muscle mass | Fat-free mass, skeletal muscle mass index (fat-free mass divided by height squared) | 41 T2DM, 51 non-diabetic | 2020 | 8 (4) |
| [40] | In T2DM, low skeletal muscle mass, which is typical trait of sarcopenia, is independently associated with presence of carotid atherosclerosis | Skeletal muscle mass (SMM), skeletal muscle mass index (SMM divided by total body weight) | 8202 T2DM | 2020 | 8 (4) |
| [41] | In T2DM, sarcopenia appears significantly associated with impaired microcirculation, defined as low skin perfusion pressure | Appendicular skeletal muscle mass (as a sum of lean mass in the arms and legs) normalized to height squared | 102 T2DM | 2020 | _ 1 |
| [42] | In men with T2DM, sarcopenia appears independently associated with non-alcoholic fatty liver disease (NAFLD), suggesting sarcopenia as risk factor for NAFLD in that population | Appendicular skeletal muscle mass (ASM, as a sum of lean mass in the arms and legs), skeletal muscle mass index (ASM normalized to body weight) | 4210 T2DM | 2020 | 6 (3) |
| [43] | In T2DM, low extremity skeletal muscle mass may be a marker of possible co-occurring cognitive dysfunction | Skeletal muscle mass in legs and arms, appendicular lean mass (ASM, mass of four limbs), skeletal muscle mass index as ASM / height squared | 1235 T2DM | 2020 | 5 (2.5) |
| [44] | In T2DM, the prevalence of low muscle mass and sarcopenia may be found higher in older people and in people with normal BMI | Fat-free mass, body fat mass, percent body fat, visceral fat area, appendicular skeletal muscle mass (ASM), skeletal muscle index (ASM divided by height squared) | 2404 T2DM | 2021 | 1 (1) |
| [45] | In T2DM, some genetic factors (IRS1 and ADAMTSL3) contribute to interindividual variability in body composition, and this can help to establish effective methods for the prediction and prevention of sarcopenia | Total lean mass, appendicular lean mass, body fat mass, body resistance, skeletal muscle mass | 176 T2DM | 2021 | 0 (0) |
| [46] | Acarbose may contribute to decreased muscle mass and strength, thus muscle condition assessment and proper exercise may be important in T2DM patients using acarbose | Skeletal muscle mass (SMM), skeletal muscle (SMM divided by height squared) | 1042 T2DM | 2021 | 0 (0) |
| [47] | In T2DM elderly patients, the knee extension strength test can assist in the identification of probable and confirmed sarcopenia, as diagnosed by EWGSOP2 criteria | Fat mass, % body fat, total and segmental skeletal muscle mass (both legs, trunk, and both arms), appendicular skeletal mass index (sum of arms and legs masses/height squared) | 100 T2DM | 2022 | _ 1 |
| Cross-sectional studied with nutritional data | |||||
| [48] | Uncomplicated T2DM does not seem to accelerate age-related muscle mass or strength loss, thus aging may be more relevant than diabetes for sarcopenia risk | Fat-free mass (normalized to height squared) | 32 T2DM, 34 non-diabetic | 2014 | 11 (1.4) |
| [49] | In obese people with T2DM, preserved muscle fitness, especially of the lower extremities, may prevent sarcopenic obesity | Fat mass and lean mass, at five body segments (right and left upper extremities, trunk, right and left lower extremities) | 26 T2DM | 2015 | 15 (2.1) |
| [50] | Oxidative stress and antioxidant status may be associated with sarcopenia in T2DM elderly individuals; however, the association is likely mediated by other factors | Skeletal muscle mass, absolute skeletal muscle mass (normalizing to height squared) | 60 T2DM | 2019 | 4 (1.3) |
| [51] | In T2DM elderly patients, low energy intake is associated with sarcopenia | Skeletal muscle mass, appendicular muscle mass, body fat mass, skeletal muscle mass index (appendicular muscle mass/height squared) | 391 | 2019 | 31 (10.3) |
| [52] | In T2DM non-obese patients without heart failure, brain natriuretic peptide levels are associated with sarcopenia | Skeletal muscle mass, appendicular muscle mass, body fat mass, skeletal muscle mass index (appendicular muscle mass/height squared) | 433 T2DM | 2019 | 8 (2.7) |
| [53] | In T2DM elderly patients, prevalence of sarcopenia is more than double when referring to the 2010 EWGSOP criteria, compared to revised 2019 criteria | Appendicular skeletal muscle mass (ASM, sum of arms and legs muscle mass), skeletal muscle mass index (ASM divided by height squared) | 242 T2DM | 2020 | 17 (8.5) |
| [54] | In T2DM elderly patients, omega-3 fatty acids contribute to increase muscle mass and improve skeletal muscle strength, thus decreasing sarcopenia risk | Skeletal muscle mass, appendicular muscle mass, body fat mass, skeletal muscle mass index (appendicular muscle mass/height squared) | 342 T2DM | 2020 | 7 (3.5) |
| [55] | In T2DM, low irisin levels and poor glycemic control are independent risk factors for sarcopenia, and especially for sarcopenic obesity | Fat mass, fat-free mass, appendicular skeletal muscle (ASM), skeletal muscle index (ASM/height squared) | 90 T2DM | 2021 | 4 (4) |
| Longitudinal (interventional) studies | |||||
| [56] | In T2DM, treatment with dapagliflozin for six months improves glycemic control and reduced body weight without reducing muscle mass | Total fat mass, soft lean mass, skeletal muscle mass at five body segments (arms, legs, trunk), skeletal muscle mass index (normalization to height squared) | 50 T2DM | 2018 | 36 (9) |
| [57] | In T2DM, reducing loss of fat-free mass over time may reduce insulin resistance and prediabetes risk, particularly among individuals with overweight/obesity | Fat mass, fat-free mass (FFM), relative FFM (normalization to body weight), relative FFM percent change between baseline and follow-up | 6264 T2DM | 2021 | 3 (3) |
| [58] | In T2DM patients, low baseline skeletal muscle mass and its reduction over time is associated with increased risk for progression of chronic kidney disease | Total skeletal muscle mass, skeletal muscle mass index (normalization to weight) | 1272 T2DM | 2021 | 2 (2) |
| [59] | Elderly women with low skeletal muscle or overt sarcopenia have higher probability of developing glucose intolerance or even diabetes | Body resistance, reactance, phase angle, fat mass appendicular skeletal muscle mass | 159 non-T2DM (at baseline) | 2021 | 3 (3) |
| [60] | T2DM and chronic kidney disease are synergically associated with muscle mass loss over time, and mortality is higher in individuals with muscle loss | Fat-free mass index (details not provided) | 6247 subjects (some with T2DM) | 2021 | 0 (0) |
| [61] | Sarcopenia with co-existent T2DM was associated with increased risk for readmission and infections among hospitalized cardiac patients | Fat mass, appendicular skeletal muscle mass, appendicular skeletal muscle mass index (normalization to height squared) | 50 T2DM, 50 non-T2DM | 2021 | 0 (0) |
| [62] | In T2DM elderly people with long diabetes duration, low muscle strength and insulin resistance are the main risk factors for aggravated glycemic control | Muscle mass, fat mass (both normalized to weight, and stratified in quartiles) | 100 T2DM | 2021 | 0 (0) |
| [63] | COVID-19 pandemic restrictions cause muscle mass loss in older patents with T2DM; thus, exercise and adequate diet intake are needed to prevent sarcopenia | Appendicular muscle mass, fat mass, skeletal muscle mass index (SMI, as appendicular muscle mass/height squared), percent fat mass (fat mass/body weight), change in SMI per year | 56 T2DM | 2021 | 2 (1) |
| [64] | In T2DM, branched-chain amino acids (valine, leucine and isoleucine) appear to have preventive role in muscle mass loss | Skeletal muscle mass, skeletal muscle mass index (normalization to weight) | 1140 T2DM | 2022 | 0 (0) |
| [65] | Hemodialysis patients with T2DM show overnutrition, but also paradoxically higher predisposition to protein–energy wasting (possible traits of obese sarcopenia) | Lean tissue mass and fat tissue mass, lean tissue index and fat tissue index (normalization to height squared), overhydration and relative overhydration | 198 T2DM, 317 non-T2DM | 2022 | _ 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbrignadello, S.; Göbl, C.; Tura, A. Bioelectrical Impedance Analysis for the Assessment of Body Composition in Sarcopenia and Type 2 Diabetes. Nutrients 2022, 14, 1864. https://doi.org/10.3390/nu14091864
Sbrignadello S, Göbl C, Tura A. Bioelectrical Impedance Analysis for the Assessment of Body Composition in Sarcopenia and Type 2 Diabetes. Nutrients. 2022; 14(9):1864. https://doi.org/10.3390/nu14091864
Chicago/Turabian StyleSbrignadello, Stefano, Christian Göbl, and Andrea Tura. 2022. "Bioelectrical Impedance Analysis for the Assessment of Body Composition in Sarcopenia and Type 2 Diabetes" Nutrients 14, no. 9: 1864. https://doi.org/10.3390/nu14091864
APA StyleSbrignadello, S., Göbl, C., & Tura, A. (2022). Bioelectrical Impedance Analysis for the Assessment of Body Composition in Sarcopenia and Type 2 Diabetes. Nutrients, 14(9), 1864. https://doi.org/10.3390/nu14091864








