Iron Metabolism following Twice a Day Endurance Exercise in Female Long-Distance Runners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
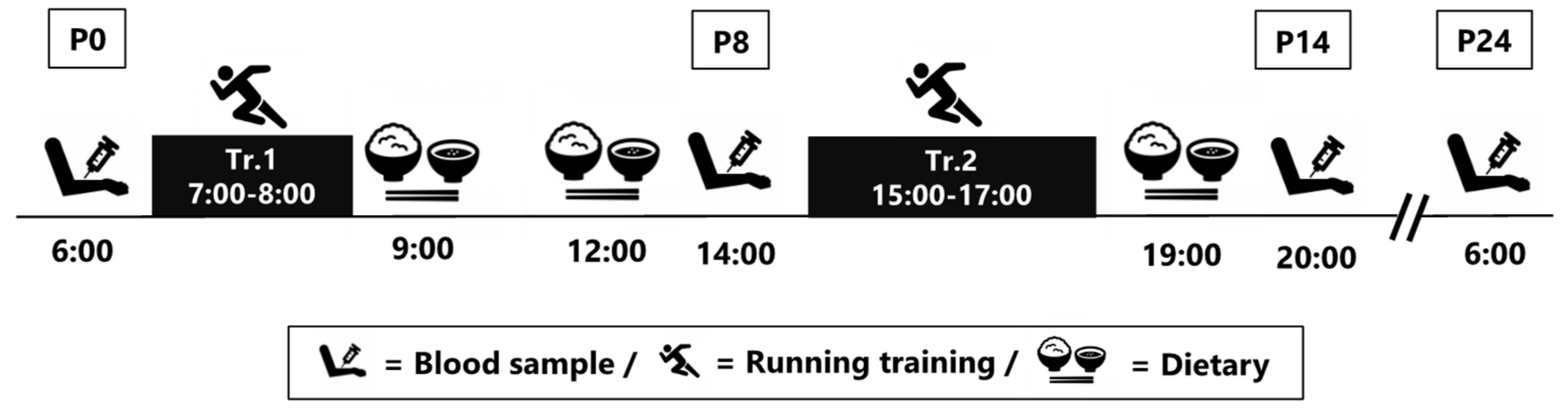
2.2. Experimental Design
2.3. Measurements
2.3.1. Body Composition
2.3.2. Blood Sampling and Analyses
2.3.3. Nutritional Assessment
2.3.4. Statistical Analyses
3. Results
3.1. General Information
3.2. Blood Parameters
3.2.1. Fasting Iron Parameters in the Morning
3.2.2. Haptoglobin, Iron, IL-6, and Hepcidin Level after Exercise
3.3. Nutritional Intake during the Exercise Periods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beard, J.; Tobin, B. Iron status and exercise. Am. J. Clin. Nutr. 2000, 72, 594–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliakim, A.; Nemet, D.; Constantini, N. Screening blood tests in members of the Israeli National Olympic team. J. Sports Med. Phys. Fit. 2002, 42, 250–255. [Google Scholar]
- Dubnov, G.; Constantini, N.W. Prevalence of iron depletion and anemia in top-level basketball players. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 30–37. [Google Scholar] [CrossRef]
- Brownlie, T.; Utermohlen, V.; Hinton, P.S.; Giordano, C.; Haas, J.D. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am. J. Clin. Nutr. 2002, 75, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, D.; Nielsen, P.; Fischer, R.; Engelhardt, R.; Gabbe, E.E. Iron deficiency in distance runners. A reinvestigation using 59Fe-labelling and non-invasive liver iron quantification. Int. J. Sports Med. 1996, 17, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Training surface and intensity: Inflammation, hemolysis, and hepcidin expression. Med. Sci. Sports Exerc. 2009, 41, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Newlin, M.K.; Williams, S.; McNamara, T.; Tjalsma, H.; Swinkels, D.W.; Haymes, E.M. The effects of acute exercise bouts on hepcidin in women. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 79–88. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Effects of exercise on hepcidin response and iron metabolism during recovery. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 583–597. [Google Scholar] [CrossRef] [Green Version]
- Roecker, L.; Meier-Buttermilch, R.; Brechtel, L.; Nemeth, E.; Ganz, T. Iron-regulatory protein hepcidin is increased in female athletes after a marathon. Eur. J. Appl. Physiol. 2005, 95, 569–571. [Google Scholar] [CrossRef]
- Steensberg, A.; Febbraio, M.A.; Osada, T.; Schjerling, P.; Van Hall, G.; Saltin, B.; Pedersen, B.K. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 2001, 537, 633–639. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T.; Nemeth, E. Iron Sequestration and Anemia of Inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronsen, O.; Lea, T.; Bahr, R.; Pedersen, B.K. Enhanced plasma IL-6 and IL-1ra responses to repeated vs. single bouts of prolonged cycling in elite athletes. J. Appl. Physiol. 2002, 92, 2547–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stich, V.; De Glisezinski, I.; Berlan, M.; Bulow, J.; Galitzky, J.; Harant, I.; Crampes, F. Adipose tissue lipolysis is increased during a repeated bout of aerobic exercise. J. Appl. Physiol. 2000, 88, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Nutrition and Athletic Performance. Med. Sci. Sport Exerc. 2016, 48, 543–568. [Google Scholar]
- Ishibashi, A.; Kojima, C.; Tanabe, Y.; Iwayama, K.; Hiroyama, T.; Tsuji, T.; Takahashi, H. Effect of low energy availability during three consecutive days of endurance training on iron metabolism in male long distance runners. Physiol. Rep. 2020, 8, e14494. [Google Scholar] [CrossRef]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Walberg-Rankin, J.; Shute, M.; Dumke, C.L.; McAnulty, L.S. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J. Appl. Physiol. 2003, 94, 1917–1925. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2018, 31, 3719–3728. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, A.; Maeda, N.; Sumi, D.; Goto, K. Elevated serum hepcidin levels during an intensified training period in well-trained female long-distance runners. Nutrients 2017, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, A.; Maeda, N.; Kamei, A.; Goto, K. Iron supplementation during three consecutive days of endurance training augmented hepcidin levels. Nutrients 2017, 9, 820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeling, P.; McKay, A.K.; Pyne, D.B.; Guelfi, K.J.; McCormick, R.H.; Laarakkers, C.M.; Burke, L.M. Factors influencing the post-exercise hepcidin-25 response in elite athletes. Eur. J. Appl. Physiol. 2017, 117, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Dawson, B.; Landers, G. A seven day running training period increases basal urinary hepcidin levels as compared to cycling. J. Int. Soc. Sports Nutr. 2014, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, A.K.; Peeling, P.; Pyne, D.B.; Welvaert, M.; Tee, N.; Leckey, J.J.; Burke, L.M. Chronic adherence to a ketogenic diet modifies iron metabolism in elite athletes. Med. Sci. Sports Exerc. 2019, 51, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Demura, S.; Sato, S.; Kitabayashi, T. Percentage of total body fat as estimated by three automatic bioelectrical impedance analyzers. J. Physiol. Anthropol. Appl. Hum. Sci. 2004, 23, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Acute dietary carbohydrate manipulation and the subsequent inflammatory and hepcidin responses to exercise. Eur. J. Appl. Physiol. 2015, 115, 2521–2530. [Google Scholar] [CrossRef]
- Vecchi, C.; Montosi, G.; Garuti, C.; Corradini, E.; Sabelli, M.; Canali, S.; Pietrangelo, A. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 2014, 146, 1060–1069. [Google Scholar] [CrossRef] [Green Version]
- Pasiakos, S.M.; Margolis, L.M.; Murphy, N.E.; McClung, H.L.; Martini, S.; Gundersen, Y.; McClung, J.P. Effects of exercise mode, energy, and macronutrient interventions on inflammation during military training. Physiol. Rep. 2016, 4, e12820. [Google Scholar] [CrossRef] [Green Version]
- McKay, A.K.; Peeling, P.; Pyne, D.B.; Tee, N.; Whitfield, J.; Sharma, A.P.; Burke, L.M. Six Days of Low Carbohydrate, Not Energy Availability, Alters the Iron and Immune Response to Exercise in Elite Athletes. Med. Sci. Sports Exerc. 2022, 54, 377–387. [Google Scholar] [CrossRef]
- Dufaux, B.A.I.W.G.; Hoederath, A.; Streitberger, I.; Hollmann, W.; Assmann, G. Serum ferritin, transferrin, haptoglobin, and iron in middle- and long-distance runners, elite rowers, and professional racing cyclists. Int. J. Sports Med. 1981, 2, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, Y.O.; Schmid, A.; Grathwohl, D.; Bültermann, D.I.R.K.; Berg, A. Hematological indices and iron status in athletes of various sports and performances. Med. Sci. Sports Exerc. 2002, 34, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Schaap, C.C.; Hendriks, J.C.; Kortman, G.A.; Klaver, S.M.; Kroot, J.J.; Laarakkers, C.M.; Swinkels, D.W. Diurnal Rhythm rather than Dietary Iron Mediates Daily Hepcidin Variations. Clin. Chem. 2013, 59, 527–535. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.; Moretti, D.; McKay, A.K.; Laarakkers, C.M.; Vanswelm, R.; Trinder, D.; Peeling, P. The Impact of Morning versus Afternoon Exercise on Iron Absorption in Athletes. Med. Sci. Sports Exerc. 2019, 51, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, C.V.; Balakrishnan, S.D. Increased lipoprotein susceptibility to oxidation following long distance running in trained subjects. Clin. Chim. Acta 1998, 271, 97–103. [Google Scholar] [CrossRef]
- Smith, J.A.; Kolbuch-Braddon, M.; Gillam, I.; Telford, R.D.; Weidemann, M.J. Changes in the susceptibility of red blood cells to oxidative and osmotic stress following submaximal exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 427–436. [Google Scholar] [CrossRef]

| On the Day Before Exercise | On the Day of Exercise | p-Value | ||
|---|---|---|---|---|
| Energy | kcal | 2177 ± 55 | 2153 ± 98 | >0.05 |
| Protein | g | 101.2 ± 3.3 | 106.8 ± 5.1 | >0.05 |
| %P | % | 18.6 ± 0.3 | 19.9 ± 0.5 | >0.05 |
| Fat | g | 83.6 ± 2.0 | 61.2 ± 4.0 | <0.001 |
| %F | % | 34.6 ± 0.7 | 25.6 ± 1.3 | <0.001 |
| Carbohydrate | g | 250.5 ± 7.9 | 283.4 ± 17.3 | >0.05 |
| %C | % | 46.8 ± 0.7 | 54.4 ± 1.7 | <0.01 |
| BM | g | 5.3 ± 0.2 | 6.0 ± 0.4 | >0.05 |
| Iron | mg | 15.6 ± 0.4 | 15.7 ± 0.6 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, A.; Maeda, N.; Kojima, C.; Goto, K. Iron Metabolism following Twice a Day Endurance Exercise in Female Long-Distance Runners. Nutrients 2022, 14, 1907. https://doi.org/10.3390/nu14091907
Ishibashi A, Maeda N, Kojima C, Goto K. Iron Metabolism following Twice a Day Endurance Exercise in Female Long-Distance Runners. Nutrients. 2022; 14(9):1907. https://doi.org/10.3390/nu14091907
Chicago/Turabian StyleIshibashi, Aya, Naho Maeda, Chihiro Kojima, and Kazushige Goto. 2022. "Iron Metabolism following Twice a Day Endurance Exercise in Female Long-Distance Runners" Nutrients 14, no. 9: 1907. https://doi.org/10.3390/nu14091907
APA StyleIshibashi, A., Maeda, N., Kojima, C., & Goto, K. (2022). Iron Metabolism following Twice a Day Endurance Exercise in Female Long-Distance Runners. Nutrients, 14(9), 1907. https://doi.org/10.3390/nu14091907






