Variability in the Beneficial Effects of Phenolic Compounds: A Review
Abstract
:1. Introduction
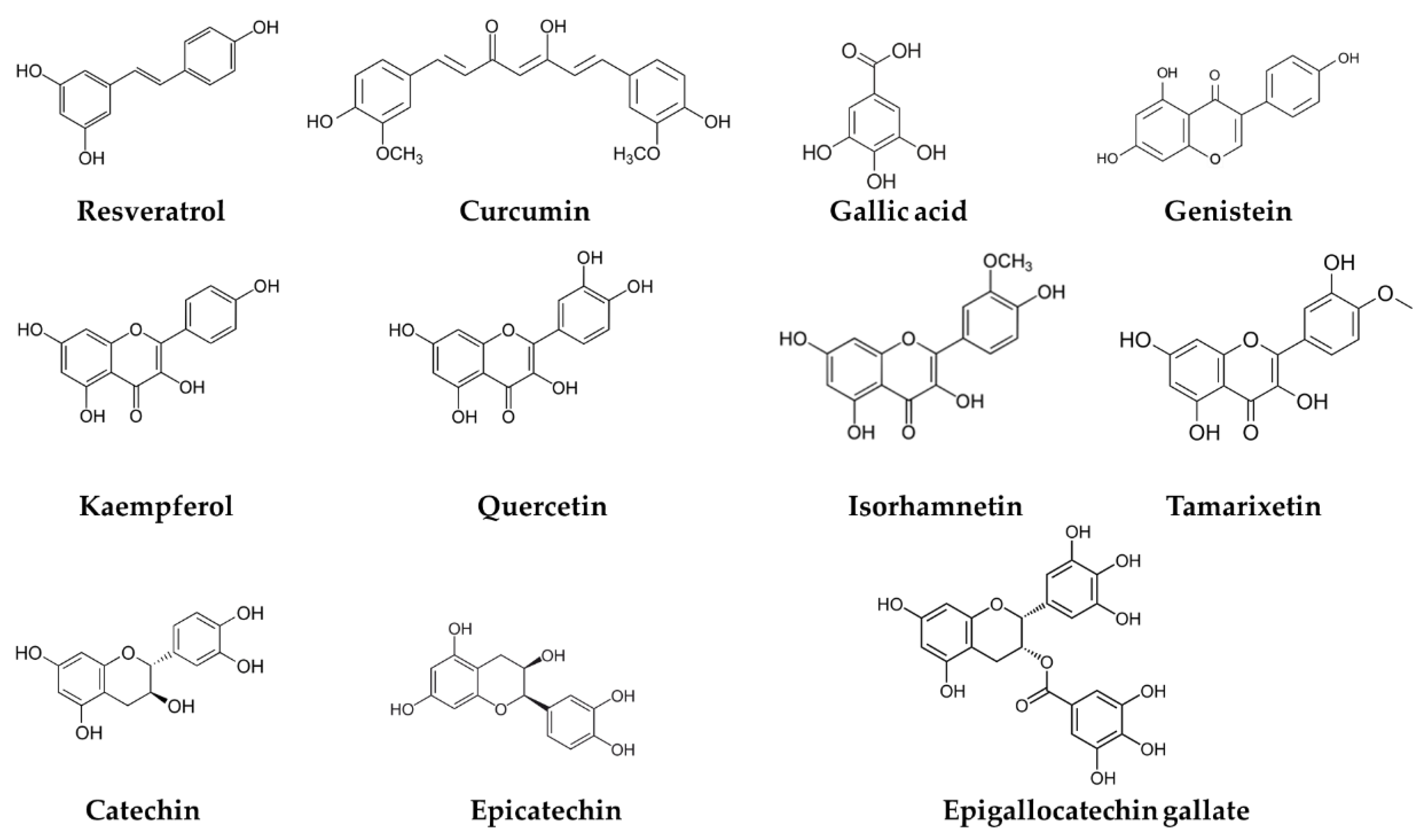
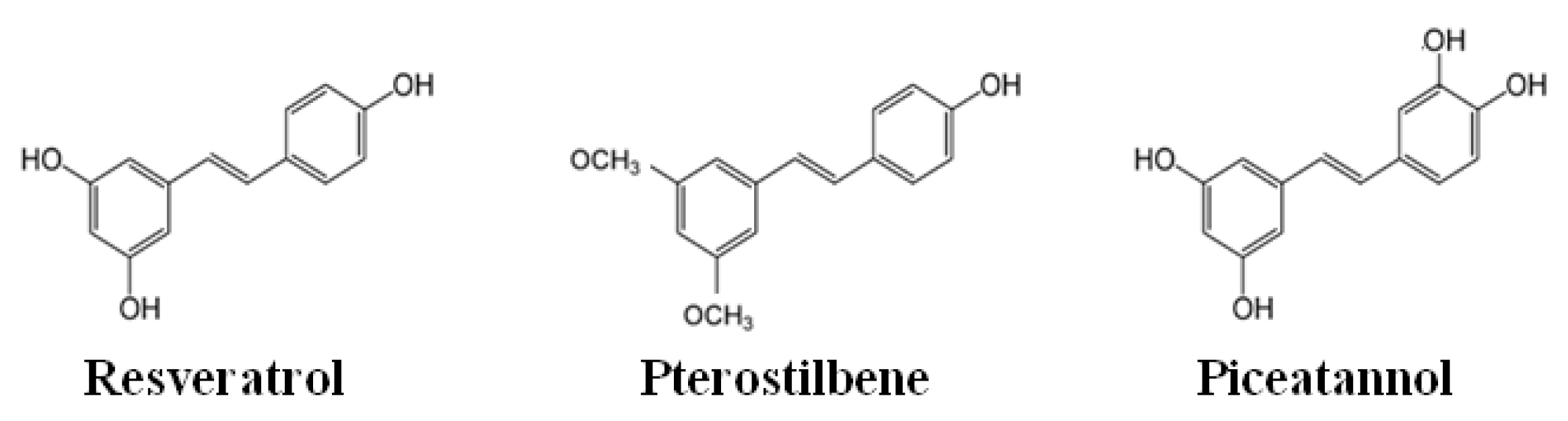
2. Chemistry of Phenolic Compounds
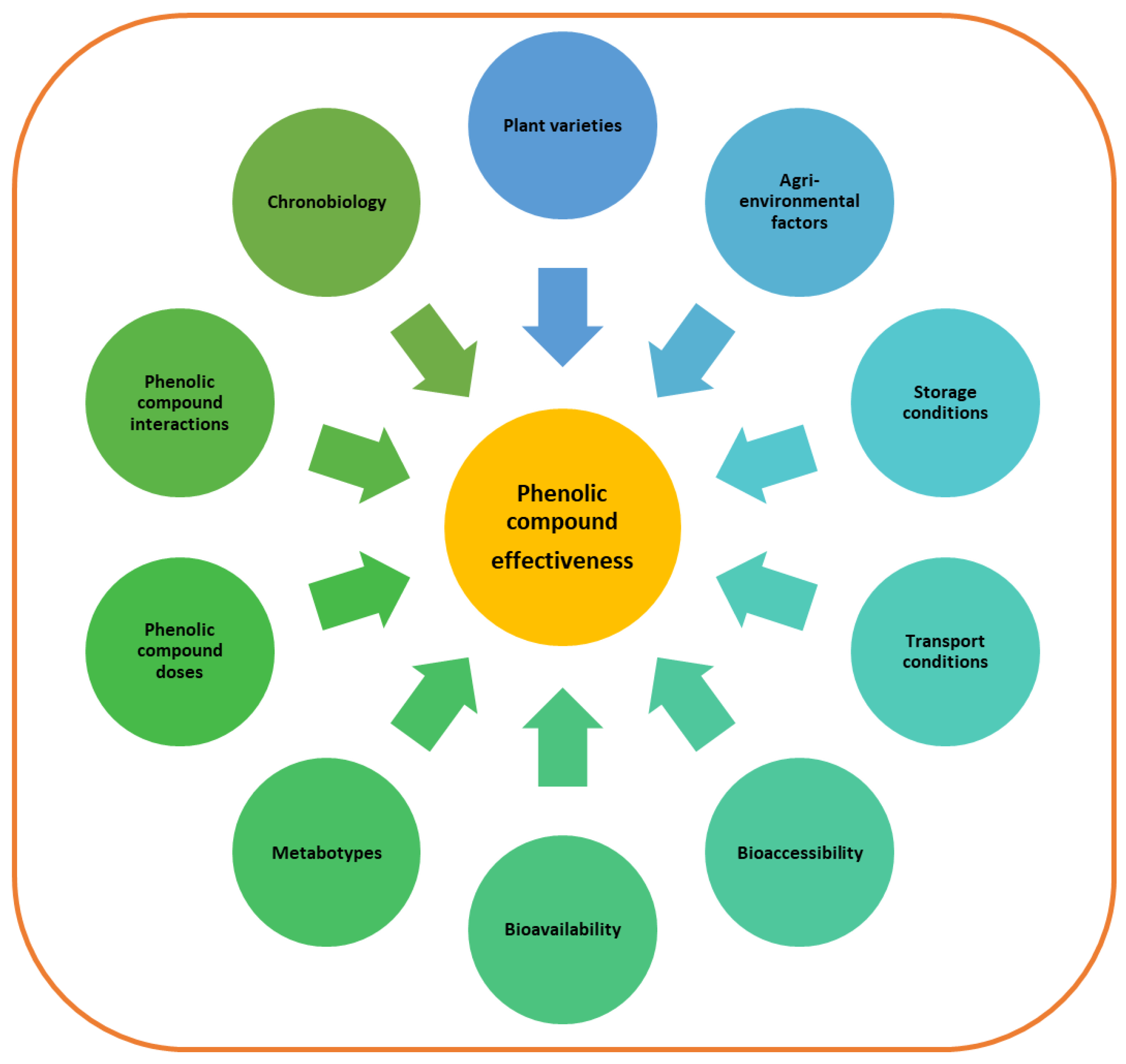
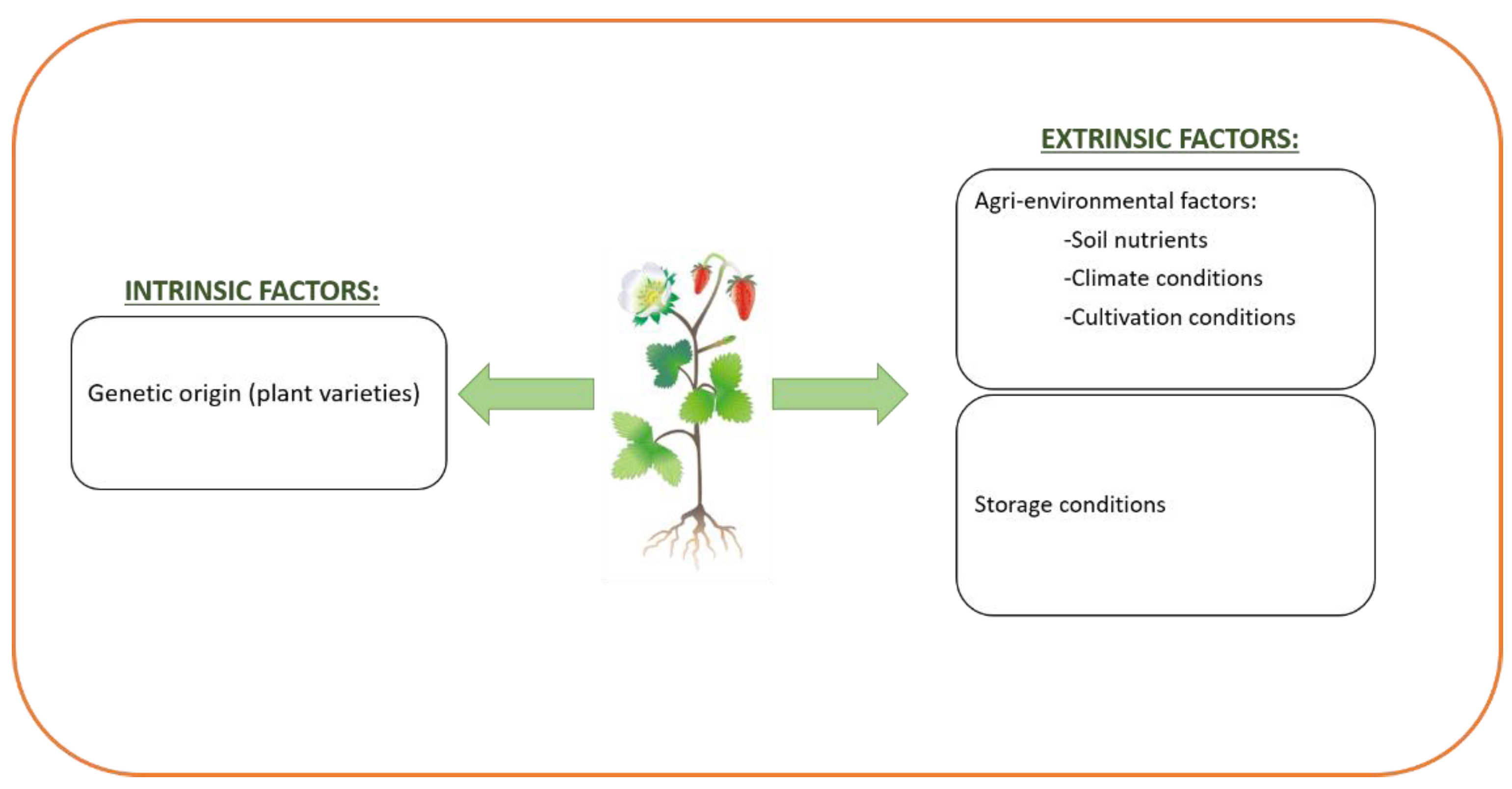
3. Factors That Affect Phenolic Content and Composition of Foods
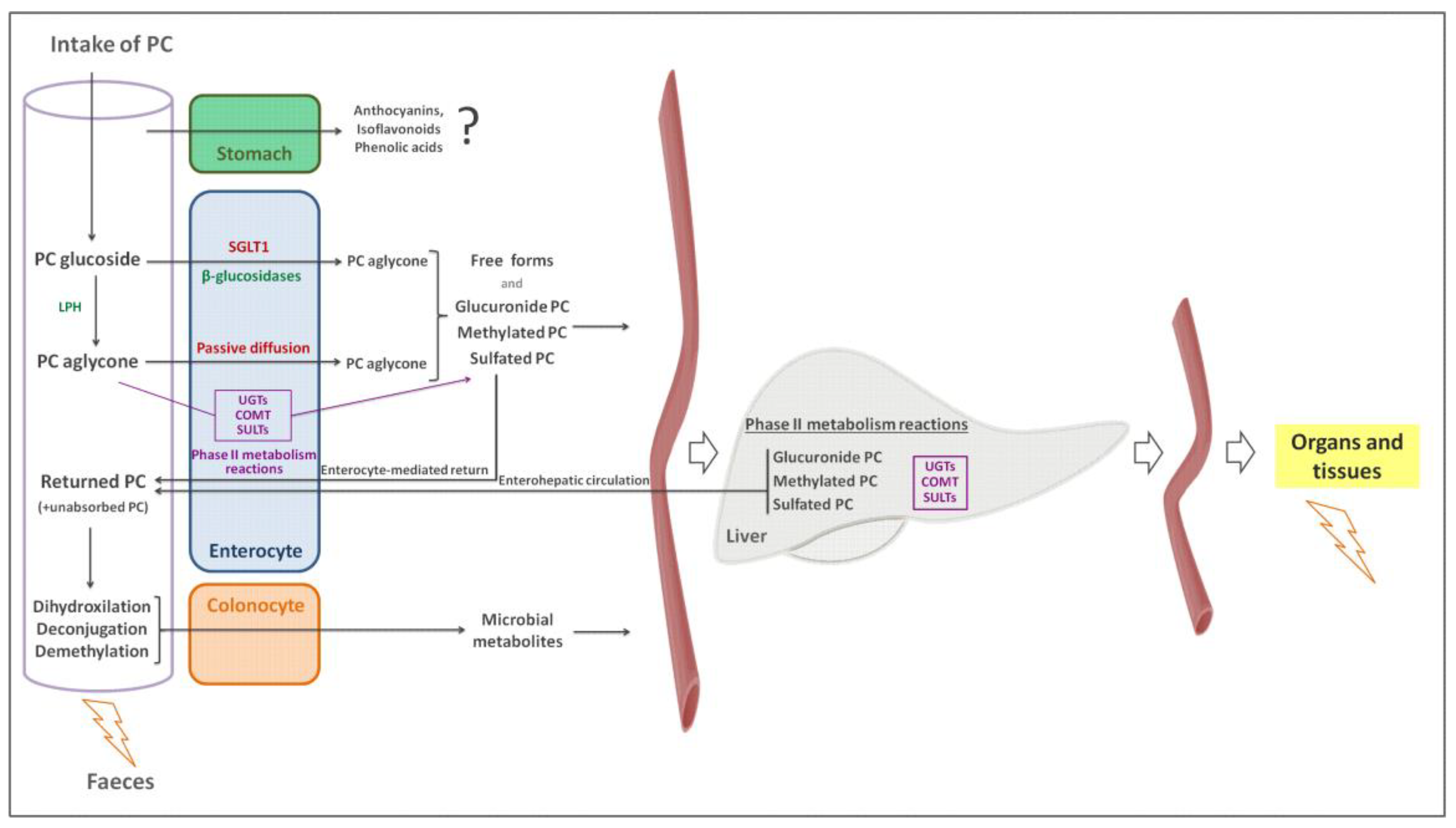
4. Factors Affecting Phenolic Compound Bioaccessibility
| Food Compound | Type of Interaction | Effect | References |
|---|---|---|---|
| Dietary fibre | Prolongation of gastric emptying (soluble fibres) | ↑ absorption time | [42] |
| Increase in viscosity (soluble fibres) | ↓ % absorption | [9] | |
| Physical trapping | |||
| Lipids | Micellization of polar phenolic compounds | ↑ % absorption | [43,44] |
| = % absorption | [45] | ||
| ↓ % absorption | [46] | ||
| Proteins | Protein-phenolic compound complex formation | ↓ % absorption | [53] |
| ↑ % absorption | [49] | ||
| = % absorption | [50,51,52] | ||
| Digestible carbohydrates | Absorption facilitation of phenolic compound glycosides by sugars | ↑ % absorption | [54] |
5. Factors Affecting Phenolic Compound Bioavailability
6. Dosage Considerations
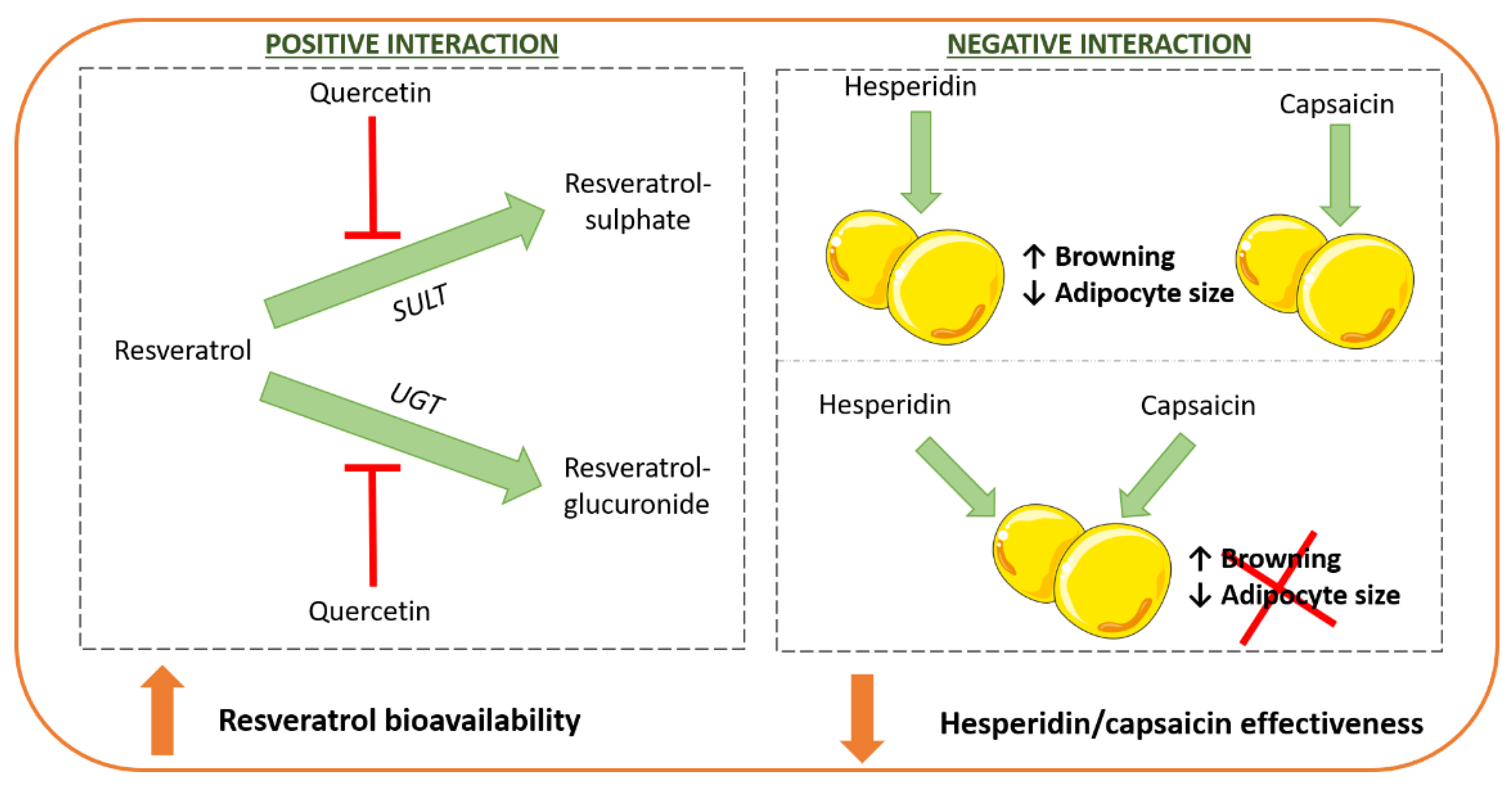
7. Interactions among Phenolic Compounds and other Bioactive Molecules
8. Phenolic Compounds and Chronobiology
9. Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Metere, A.; Giacomelli, L. Absorption, metabolism and protective role of fruits and vegetables polyphenols against gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5850–5858. [Google Scholar] [PubMed]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids—Food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar] [PubMed]
- Qu, G.; Chen, J.; Guo, X. The beneficial and deleterious role of dietary polyphenols on chronic degenerative diseases by regulating gene expression. Biosci. Trends 2019, 12, 526–536. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G. Effects of Polyphenol-Rich Foods on Human Health. Nutrients 2018, 10, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; Selma, M.V.; González-Sarrías, A.; Espín, J.C. Main drivers of (poly)phenol effects on human health: Metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021, 12, 10324–10355. [Google Scholar] [CrossRef]
- Gudžinskaitė, I.; Stackevičienė, E.; Liaudanskas, M.; Zymonė, K.; Žvikas, V.; Viškelis, J.; Urbštaitė, R.; Janulis, V. Variability in the Qualitative and Quantitative Composition and Content of Phenolic Compounds in the Fruit of Introduced American Cranberry. Plants 2020, 9, 1379. [Google Scholar] [CrossRef]
- Kabtni, S.; Sdouga, D.; Bettaib Rebey, I.; Save, M.; Trifi-Farah, N.; Fauconnier, M.L.; Marghali, S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020, 10, 8293. [Google Scholar] [CrossRef]
- Hedrén, E.; Diaz, V.; Svanberg, U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Ung, D.; Nagar, S. Variable sulfation of dietary polyphenols by recombinant human sulfotransferase (SULT) 1A1 genetic variants and SULT1E1. Drug Metab. Dispos. 2007, 35, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Zdunczyk, Z.; Frejnagel, S.; Wróblewska, M.; Juśkiewicz, J.; Oszmiański, J.; Estrella, I. Biological activity of polyphenol extracts from different plant sources. Food Res. Int. 2002, 35, 183–186. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds, 1st ed.; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; Intechopen: London, UK, 2019; pp. 1–15. [Google Scholar]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Mosqueda-Solís, A.; Lasa, A.; Gómez-Zorita, S.; Eseberri, I.; Picó, C.; Portillo, M.P. Screening of potential anti-adipogenic effects of phenolic compounds showing different chemical structure in 3T3-L1 preadipocytes. Food Funct. 2017, 8, 3576–3586. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Martínez-Márquez, A.; Morante-Carriel, J.A.; Ramírez-Estrada, K.; Cusidó, R.M.; Palazon, J.; Bru-Martínez, R. Production of highly bioactive resveratrol analogues pterostilbene and piceatannol in metabolically engineered grapevine cell cultures. Plant Biotechnol. J. 2016, 14, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil, M.I.; Castañer, M.; Tomás-Barberán, F.A. Phenolic Metabolites in Red Pigmented Lettuce (Lactuca sativa). Changes with Minimal Processing and Cold Storage. J. Agric. Food Chem. 1997, 45, 4249–4254. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Nagao, A.; Nakazawa, K.; Sato, M. Changes in Anthocyanins in Berry Skins of Merlot and Cabernet Sauvignon Grapes Grown in Two Soils Modified with Limestone or Oyster Shell Versus a Native Soil Over Two Years. Am. J. Enol Vitic. 1999, 50, 1–12. [Google Scholar]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Balda, P.; Martínez de Toda, F. Quantifying the effect of temperature on decoupling anthocyanins and sugars of the grape (Vitis vinifera L. ‘Maturana Tinta de Navarrete’). Vitis 2015, 54, 117–120. [Google Scholar]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Rahmati, M.; Vercambre, G.; Davarynejad, G.; Bannayan, M.; Azizi, M.; Génard, M. Water scarcity conditions affect peach fruit size and polyphenol contents more severely than other fruit quality traits. J. Sci Food Agric. 2015, 95, 1055–1065. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Sayago, A.; Akhatou, I.; Fernández-Recamales, Á. Multi-Chemical Profiling of Strawberry as a Traceability Tool to Investigate the Effect of Cultivar and Cultivation Conditions. Foods 2020, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 30, 125366. [Google Scholar] [CrossRef]
- Di Stefano, V.; Scandurra, S.; Pagliaro, A.; Di Martino, V.; Melilli, M.G. Effect of Sunlight Exposure on Anthocyanin and Non-Anthocyanin Phenolic Levels in Pomegranate Juices by High Resolution Mass Spectrometry Approach. Foods 2020, 9, 1161. [Google Scholar] [CrossRef]
- Stracke, B.A.; Rüfer, C.E.; Weibel, F.P.; Bub, A.; Watzl, B. Three-year comparison of the polyphenol contents and antioxidant capacities in organically and conventionally produced apples (Malus domestica Bork. Cultivar ‘Golden Delicious’). J. Agric. Food Chem. 2009, 57, 4598–4605. [Google Scholar] [CrossRef] [PubMed]
- Mulero, J.; Pardo, F.; Zafrlla, P. Antioxidant activity and phenolic composition of organic and conventional grapes and wines. J. Food. Compos. Anal. 2010, 23, 569–574. [Google Scholar] [CrossRef]
- Winter, C.K.; Davis, S.F. Organic Foods. J. Food Sci. 2006, 71, R117–R124. [Google Scholar] [CrossRef]
- Samaniego, I.; Brito, B.; Viera, W.; Cabrera, A.; Llerena, W.; Kannangara, T.; Vilcacundo, R.; Angós, I.; Carrillo, W. Influence of the Maturity Stage on the Phytochemical Composition and the Antioxidant Activity of Four Andean Blackberry Cultivars. Plants 2020, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Bouamar, S.; Di Lorenzo, A.; Temporini, C.; Daglia, M.; Riazi, A. The Influence of Ripeness on the Phenolic Content, Antioxidant and Antimicrobial Activities of Pumpkins. Molecules 2021, 26, 3623. [Google Scholar] [CrossRef] [PubMed]
- Özgüven, A.I.; Tümer, L.Ö.; Yılmaz, C. Changes in the content of phenolic compounds at different maturation stages of three pomegranate cultivars. Acta Hortic. 2019, 1254, 103–108. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.; Ferreres, F.; Gil, M. Antioxidant phenolic metabolites from fruit and vegetables and changes during postharvest storage and processing. Stud. Nat. Prod. Chem. 2000, 23, 739–795. [Google Scholar]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes: A new “functional” fruit? J. Agric. Food Chem. 2001, 49, 5052–5058. [Google Scholar] [CrossRef]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Saura-Calixto, F. Bioavailability of phenolic antioxidants associated with dietary fiber: Plasma antioxidant capacity after acute and long-term intake in humans. Plant Foods Hum. Nutr. 2009, 64, 102–107. [Google Scholar] [CrossRef]
- Tew, B.Y.; Xu, X.; Wang, H.J.; Murphy, P.A.; Hendrich, S. A diet high in wheat fiber decreases the bioavailability of soybean isoflavones in a single meal fed to women. J. Nutr. 1996, 126, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Mah, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Lesser, S.; Cermak, R.; Wolffram, S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. J. Nutr. 2004, 134, 1508–1511. [Google Scholar] [CrossRef]
- Vaz-da-Silva, M.; Loureiro, A.I.; Falcao, A.; Nunes, T.; Rocha, J.F.; Fernandes-Lopes, C.; Soares, E.; Wright, L.; Almeida, L.; Soares-da-Silva, P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int. J. Clin. Pharmacol. Ther. 2008, 46, 564–570. [Google Scholar] [CrossRef] [PubMed]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State Pharmacokinetics and Tolerability of Trans-Resveratrol 2000mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Jenner, A.; Butler, J.; Aruoma, O.I.; Dexter, D.T.; Jenner, P.; Halliwell, B. Evaluation of the Pro-Oxidant and Antioxidant Actions of L-DOPA and Dopamine in Vitro: Implications for Parkinson’s Disease. Free Radic. Res. 1996, 24, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Ghiselli, A.; Ferro-Luzzi, A. In vivo antioxidant effect of green and black tea in man. Eur. J. Clin. Nutr. 1996, 50, 28–32. [Google Scholar]
- Lang, Y.; Tian, J.; Meng, X.; Si, X.; Tan, H.; Wang, Y.; Shu, C.; Chen, Y.; Zang, Z.; Zhang, Y.; et al. Effects of α-Casein on the Absorption of Blueberry Anthocyanins and Metabolites in Rat Plasma Based on Pharmacokinetic Analysis. J. Agric. Food Chem. 2021, 69, 6200–6213. [Google Scholar] [CrossRef]
- Draijer, R.; Van Dorsten, F.A.; Zebregs, Y.E.; Hollebrands, B.; Peters, S.; Duchateau, G.S.; Grün, C.H. Impact of Proteins on the Uptake, Distribution, and Excretion of Phenolics in the Human Body. Nutrients 2016, 8, 814. [Google Scholar] [CrossRef] [Green Version]
- Keogh, J.B.; McInerney, J.; Clifton, P.M. The effect of milk protein on the bioavailability of cocoa polyphenols. J. Food Sci. 2007, 72, S230–S233. [Google Scholar] [CrossRef]
- Van der Burg-Koorevaar, M.C.; Miret, S.; Duchateau, G.S. Effect of milk and brewing method on black tea catechin bioaccessibility. J. Agric. Food Chem. 2011, 59, 7752–7758. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Testa, M.F.; Villaño, D.; Pecorari, M.; Van Wieren, K.; Azzini, E.; Brambilla, A.; Maiani, G. Antioxidant activity of blueberry fruit is impaired by association with milk. Free Radic. Biol. Med. 2009, 46, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Kirkpatrick, N.J.; Polagruto, J.A.; Ensunsa, J.L.; Schmitz, H.H.; Keen, C.L. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003, 73, 857–869. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cardinali, A.; Linsalata, V. Plant Phenolics: A Biochemical and Physiological Perspective. In Recent Advances in Polyphenol Research; Cheyner, V., Sarni-Manchado, P., Quideau, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; Volume 3, pp. 1–39. [Google Scholar]
- Gee, J.M.; DuPont, M.S.; Day, A.J.; Plumb, G.W.; Williamson, G.; Johnson, I.T. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J. Nutr. 2000, 130, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Hussain, M.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.; Tahir, A. Bioavailability and Metabolic Pathway of Phenolic Compounds. In Plant. Physiological Aspects of Phenolic Compounds, 1st ed.; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; Intechopen: London, UK, 2019; pp. 1–15. [Google Scholar]
- Liu, Z.; Hu, M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin. Drug Metab. Toxicol. 2007, 3, 389–406. [Google Scholar] [CrossRef]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Guerrero, L.; Margalef, M.; Pons, Z.; Quiñones, M.; Arola, L.; Arola-Arnal, A.; Muguerza, B. Serum metabolites of proanthocyanidin-administered rats decrease lipid synthesis in HepG2 cells. J. Nutr. Biochem. 2013, 24, 2092–2099. [Google Scholar] [CrossRef]
- Lasa, A.; Churruca, I.; Eseberri, I.; Andrés-Lacueva, C.; Portillo, M.P. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2012, 56, 1559–1568. [Google Scholar] [CrossRef]
- Eseberri, I.; Lasa, A.; Churruca, I.; Portillo, M.P. Resveratrol metabolites modify adipokine expression and secretion in 3T3-L1 pre-adipocytes and mature adipocytes. PLoS ONE 2013, 8, e63918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eseberri, I.; Lasa, A.; Miranda, J.; Gracia, A.; Portillo, M.P. Potential miRNA involvement in the anti-adipogenic effect of resveratrol and its metabolites. PLoS ONE 2017, 12, e0184875. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.F.; Zhang, Q.; Raheem, K.S.; O’Hagan, D.; O’Connell, M.A.; Kay, C.D. Common Phenolic Metabolites of Flavonoids, but Not Their Unmetabolized Precursors, Reduce the Secretion of Vascular Cellular Adhesion Molecules by Human Endothelial Cells. J. Nutr. 2016, 146, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Miranda, J.; Lasa, A.; Mosqueda-Solís, A.; González-Manzano, S.; Santos-Buelga, C.; Portillo, M.P. Effects of Quercetin Metabolites on Triglyceride Metabolism of 3T3-L1 Preadipocytes and Mature Adipocytes. Int. J. Mol. Sci. 2019, 20, 264. [Google Scholar] [CrossRef] [Green Version]
- Trepiana, J.; Krisa, S.; Renouf, E.; Portillo, M.P. Resveratrol Metabolites Are Able to Reduce Steatosis in Cultured Hepatocytes. Pharmaceuticals 2020, 13, 285. [Google Scholar] [CrossRef]
- Trepiana, J.; Krisa, S.; Portillo, M.P. Activity of Pterostilbene Metabolites against Liver Steatosis in Cultured Hepatocytes. Molecules 2020, 25, 5444. [Google Scholar] [CrossRef]
- Ireson, C.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.K.; Luo, J.L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar]
- Brown, N.M.; Belles, C.A.; Lindley, S.L.; Zimmer-Nechemias, L.D.; Zhao, X.; Witte, D.P.; Kim, M.O.; Setchell, K.D. The chemopreventive action of equol enantiomers in a chemically induced animal model of breast cancer. Carcinogenesis 2010, 31, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wu, B.; Pan, G.; He, L.; Li, Z.; Fan, M.; Jian, L.; Chen, M.; Wang, K.; Huang, C. Metabolism and pharmacokinetics of mangiferin in conventional rats, pseudo-germ-free rats, and streptozotocin-induced diabetic rats. Drug Metab. Dispos. 2012, 40, 2109–2118. [Google Scholar] [CrossRef] [Green Version]
- Dostalek, M.; Court, M.H.; Hazarika, S.; Akhlaghi, F. Diabetes mellitus reduces activity of human UDP-glucuronosyltransferase 2B7 in liver and kidney leading to decreased formation of mycophenolic acid acyl-glucuronide metabolite. Drug Metab. Dispos. 2011, 39, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yalcin, E.B.; More, V.; Neira, K.L.; Lu, Z.J.; Cherrington, N.J.; Slitt, A.L.; King, R.S. Downregulation of sulfotransferase expression and activity in diseased human livers. Drug Metab. Dispos. 2013, 41, 1642–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L. Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Örem, A.; Zafrilla, P.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1500900. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; García-Villalba, R.; González-Sarrías, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramírez-de-Molina, A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef]
- Macarulla, M.T.; Alberdi, G.; Gómez, S.; Tueros, I.; Bald, C.; Rodríguez, V.M.; Martínez, J.A.; Portillo, M.P. Effects of different doses of resveratrol on body fat and serum parameters in rats fed a hypercaloric diet. J. Physiol. Biochem. 2009, 65, 369–376. [Google Scholar] [CrossRef]
- Cho, S.J.; Jung, U.J.; Choi, M.S. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br. J. Nutr. 2012, 108, 2166–2175. [Google Scholar] [CrossRef] [Green Version]
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Ke, P.Y.; Wu, C.Y.; Peng, H.H.; Young, J.D. Hormetic Effects of Phytochemicals on Health and Longevity. Trends Endocrinol. Metab. 2019, 30, 335–346. [Google Scholar] [CrossRef]
- Calabrese, E.J. Overcompensation stimulation: A mechanism for hormetic effects. Crit. Rev. Toxicol. 2001, 31, 425–470. [Google Scholar] [CrossRef]
- Dudley, J.; Das, S.; Mukherjee, S.; Das, D.K. Resveratrol, a unique phytoalexin present in red wine, delivers either survival signal or death signal to the ischemic myocardium depending on dose. J. Nutr. Biochem. 2009, 20, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Knight, T.J.; Beitz, D.C.; Lewis, D.S.; Engen, R.L. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life. Sci. 1996, 59, 15–21. [Google Scholar] [CrossRef]
- Cao, J.; Jia, L.; Zhou, H.M.; Liu, Y.; Zhong, L.F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol. Sci. 2006, 91, 476–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.M.; Kim, Y.P.; Chung, K.H. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch. Pharm. Res. 2006, 29, 354–362. [Google Scholar] [CrossRef]
- El Touny, L.H.; Banerjee, P.P. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009, 69, 3695–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, H.S.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Rangel-Ordóñez, L.; Nöldner, M.; Schubert-Zsilavecz, M.; Wurglics, M. Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba extract EGb 761®. Plant. Med. 2010, 76, 1683–1690. [Google Scholar] [CrossRef] [Green Version]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Cooper, B.; Simon, J.E.; Wu, Q.L.; Welch, C.; Ho, L.; Weaver, C.; Pasinetti, G.M. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: Implications for treatment in Alzheimer’s disease. J. Alzheimers Dis. 2009, 18, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Silberberg, M.; Morand, C.; Mathevon, T.; Besson, C.; Manach, C.; Scalbert, A.; Remesy, C. The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur. J. Nutr. 2006, 45, 88–96. [Google Scholar] [CrossRef]
- Orrego-Lagarón, N.; Martínez-Huélamo, M.; Quifer-Rada, P.; Lamuela-Raventos, R.M.; Escribano-Ferrer, E. Absorption and disposition of naringenin and quercetin after simultaneous administration via intestinal perfusion in mice. Food Funct. 2016, 7, 3880–3889. [Google Scholar] [CrossRef]
- De Santi, C.; Pietrabissa, A.; Spisni, R.; Mosca, F.; Pacifici, G.M. Sulphation of resveratrol, a natural compound present in wine, and its inhibition by natural flavonoids. Xenobiotica 2000, 30, 857–866. [Google Scholar] [CrossRef] [PubMed]
- De Santi, C.; Pietrabissa, A.; Mosca, F.; Pacifici, G.M. Glucuronidation of resveratrol, a natural product present in grape and wine, in the human liver. Xenobiotica 2000, 30, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Milton, I.; Portillo, M.P. The combination of resveratrol and quercetin enhances the individual effects of these molecules on triacylglycerol metabolism in white adipose tissue. Eur. J. Nutr. 2016, 55, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Percival, S.S. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005, 218, 141–151. [Google Scholar] [CrossRef]
- Mosqueda-Solís, A.; Sánchez, J.; Portillo, M.P.; Palou, A.; Picó, C. Combination of Capsaicin and Hesperidin Reduces the Effectiveness of Each Compound to Decrease the Adipocyte Size and To Induce Browning Features in Adipose Tissue of Western Diet Fed Rats. J. Agric. Food Chem. 2018, 66, 9679–9689. [Google Scholar] [CrossRef]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Martínez-Castaño, M.G.; Gómez-Zorita, S.; Miranda, J.; Martínez, J.A.; Portillo, M.P. The combination of resveratrol and conjugated linoleic acid is not useful in preventing obesity. J. Physiol. Biochem. 2011, 67, 471–477. [Google Scholar] [CrossRef]
- Lasa, A.; Miranda, J.; Churruca, I.; Simon, E.; Arias, N.; Milagro, F.; Martinez, J.A.; del Puy Portillo, M. The combination of resveratrol and CLA does not increase the delipidating effect of each molecule in 3T3-L1 adipocytes. Nutr. Hosp. 2011, 26, 997–1003. [Google Scholar]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Martínez-Castaño, M.G.; Portillo, M.P. Quercetin can reduce insulin resistance without decreasing adipose tissue and skeletal muscle fat accumulation. Genes Nutr. 2014, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Bruckbauer, A.; Zemel, M.B.; Thorpe, T.; Akula, M.R.; Stuckey, A.C.; Osborne, D.; Martin, E.B.; Kennel, S.; Wall, J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. 2012, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Bruckbauer, A.; Zemel, M.B. Synergistic effects of polyphenols and methylxanthines with Leucine on AMPK/Sirtuin-mediated metabolism in muscle cells and adipocytes. PLoS ONE 2014, 9, e89166. [Google Scholar] [CrossRef] [Green Version]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and Polyphenols: Roles and Diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar-Martínez, I.; Arreaza-Gil, V.; Muguerza, B.; Arola-Arnal, A.; Bravo, F.I.; Torres-Fuentes, C.; Suárez, M. Administration Time Significantly Affects Plasma Bioavailability of Grape Seed Proanthocyanidins Extract in Healthy and Obese Fischer 344 Rats. Mol. Nutr. Food Res. 2022, 66, e2100552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Yeager, R.L.; Klaassen, C.D. Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab. Dispos. 2009, 37, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmrzljak, U.P.; Rozman, D. Circadian regulation of the hepatic endobiotic and xenobitoic detoxification pathways: The time matters. Chem. Res. Toxicol. 2012, 25, 811–824. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. https://doi.org/10.3390/nu14091925
Eseberri I, Trepiana J, Léniz A, Gómez-García I, Carr-Ugarte H, González M, Portillo MP. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients. 2022; 14(9):1925. https://doi.org/10.3390/nu14091925
Chicago/Turabian StyleEseberri, Itziar, Jenifer Trepiana, Asier Léniz, Iker Gómez-García, Helen Carr-Ugarte, Marcela González, and María P. Portillo. 2022. "Variability in the Beneficial Effects of Phenolic Compounds: A Review" Nutrients 14, no. 9: 1925. https://doi.org/10.3390/nu14091925
APA StyleEseberri, I., Trepiana, J., Léniz, A., Gómez-García, I., Carr-Ugarte, H., González, M., & Portillo, M. P. (2022). Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients, 14(9), 1925. https://doi.org/10.3390/nu14091925








