Global Proteome Profiling of the Temporal Cortex of Female Rats Exposed to Chronic Stress and the Western Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Obesogenic Rodent Diet
2.3. Chronic Social Stress Paradigm
2.4. Glucose Tolerance Test (GTT)
2.5. Tissue Collection
2.6. Biochemical and Hormonal Assays
2.7. Proteomic Analysis
2.8. Data Analysis of Proteins
2.9. Gene Ontology (GO) Term Enrichment Analysis
2.10. Statistical Analysis of Non-Proteome Data
3. Results
3.1. Phenotypic and Biochemical Parameters
3.2. LC-MS/MS Analysis of Differentially Expressed Proteins
3.3. Gene Ontology (GO) Analysis
3.4. KEGG and Protein-Protein Interaction Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bortolin, R.C.; Vargas, A.R.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Martinello, K.B.; Silveira, A.K.; Rabelo, T.K.; Gelain, D.P.; Moreira, J.C.F. A new animal diet based on human Western diet is a robust diet-induced obesity model: Comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. 2018, 42, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.; Beserra-Filho, J.I.; Kubota, M.C.; Cardoso, G.N.; Freitas, F.R.; Gonçalves, B.S.; Vicente-Silva, W.; Silva-Martins, S.; Custódio-Silva, A.C.; Soares-Silva, B.; et al. Palatable high-fat diet intake influences mnemonic and emotional aspects in female rats in an estrous cycle-dependent manner. Metab. Brain Dis. 2021, 36, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav. Brain Res. 2016, 306, 1–7. [Google Scholar] [CrossRef]
- Sharma, S.; Fulton, S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. 2013, 37, 382–389. [Google Scholar] [CrossRef]
- Liśkiewicz, A.D.; Liśkiewicz, D.; Marczak, Ł.; Przybyła, M.; Grabowska, K.; Student, S.; Dębiec, M.; Sługocka, A.; Lewin-Kowalik, J. Obesity-associated deterioration of the hippocampus is partially restored after weight loss. Brain Behav. Immun. 2021, 96, 212–226. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Norman, E.D.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef]
- Blasco, B.V.; García-Jiménez, J.; Bodoano, I.; Gutiérrez-Rojas, L. Obesity and Depression: Its Prevalence and Influence as a Prognostic Factor: A Systematic Review. Psychiatry Investig. 2020, 17, 715–724. [Google Scholar] [CrossRef]
- Becker, J.B.; Monteggia, L.M.; Perrot-Sinal, T.S.; Romeo, R.D.; Taylor, J.R.; Yehuda, R.; Bale, T.L. Stress and disease: Is being female a predisposing factor? J. Neurosci. 2007, 27, 11851–11855. [Google Scholar] [CrossRef]
- Knapman, A.; Heinzmann, J.M.; Hellweg, R.; Holsboer, F.; Landgraf, R.; Touma, C. Increased stress reactivity is associated with cognitive deficits and decreased hippocampal brain-derived neurotrophic factor in a mouse model of affective disorders. J. Psychiatr. Res. 2010, 44, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997, 48, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Phillips, P.J.; Herbert, J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur. J. Neurosci. 1998, 10, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Mansur, R.B.; Brietzke, E.; McIntyre, R.S. Is there a “metabolic-mood syndrome”? A review of the relationship between obesity and mood disorders. Neurosci. Biobehav. Rev. 2015, 52, 89–104. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef]
- Krolow, R.; Noschang, C.G.; Arcego, D.; Andreazza, A.C.; Peres, W.; Gonçalves, C.A.; Dalmaz, C. Consumption of a palatable diet by chronically stressed rats prevents effects on anxiety-like behavior but increases oxidative stress in a sex-specific manner. Appetite 2010, 55, 108–116. [Google Scholar] [CrossRef]
- Santos, C.J.; Ferreira, A.V.; Oliveira, A.L.; Oliveira, M.C.; Gomes, J.S.; Aguiar, D.C. Carbohydrate-enriched diet predispose to anxiety and depression-like behavior after stress in mice. Nutr. Neurosci. 2018, 21, 33–39. [Google Scholar] [CrossRef]
- Fu, J.H.; Xie, S.R.; Kong, S.J.; Wang, Y.; Wei, W.; Shan, Y.; Luo, Y.M. The combination of a high-fat diet and chronic stress aggravates insulin resistance in Wistar male rats. Exp. Clin. Endocrinol. Diabetes 2009, 117, 354–360. [Google Scholar] [CrossRef]
- Chella Krishnan, K.; Mehrabian, M.; Lusis, A.J. Sex differences in metabolism and cardiometabolic disorders. Curr. Opin. Lipidol. 2018, 29, 404–410. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6, 60–75. [Google Scholar] [CrossRef]
- Tronieri, J.S.; Wurst, C.M.; Pearl, R.L.; Allison, K.C. Sex Differences in Obesity and Mental Health. Curr. Psychiatry Rep. 2017, 19, 29. [Google Scholar] [CrossRef]
- Pitkänen, H.T.; Oja, S.S.; Kemppainen, K.; Seppä, J.M.; Mero, A.A. Serum amino acid concentrations in aging men and women. Amino Acids 2003, 24, 413–421. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Nowacka-Chmielewska, M.; Liśkiewicz, D.; Liśkiewicz, A.; Marczak, Ł.; Wojakowska, A.; Barski, J.; Małecki, A. Cerebrocortical proteome profile of female rats subjected to the Western diet and chronic social stress. Nutr. Neurosci. 2022, 25, 567–580. [Google Scholar] [CrossRef]
- Nowacka-Chmielewska, M.; Kasprowska-Liśkiewicz, D.; Barski, J.J.; Obuchowicz, E.; Małecki, A. The behavioral and molecular evaluation of effects of social instability stress as a model of stress-related disorders in adult female rats. Stress 2017, 20, 549–561. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Thomas, P.D.; Kejariwal, A.; Guo, N.; Mi, H.; Campbell, M.J.; Muruganujan, A.; Lazareva-Ulitsky, B. Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res. 2006, 34, 645–650. [Google Scholar] [CrossRef]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.P.; Mushayamaha, T.; Thomas, P.D. PANTHER version 16: A revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2021, 49, 394–403. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Adam, T.C.; Epel, E.S. Stress, eating and the reward system. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef]
- Dallman, M.F.; la Fleur, S.E.; Pecoraro, N.C.; Gomez, F.; Houshyar, H.; Akana, S.F. Minireview: Glucocorticoids—food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology 2004, 145, 2633–2638. [Google Scholar] [CrossRef]
- Michel, C.; Duclos, M.; Cabanac, M.; Richard, D. Chronic stress reduces body fat content in both obesity-prone and obesity-resistant strains of mice. Horm. Behav. 2005, 48, 172–179. [Google Scholar] [CrossRef]
- Egan, A.E.; Seemiller, L.R.; Packard, A.E.B.; Solomon, M.B.; Ulrich-Lai, Y.M. Palatable food reduces anxiety-like behaviors and HPA axis responses to stress in female rats in an estrous-cycle specific manner. Horm. Behav. 2019, 115, 104557. [Google Scholar] [CrossRef]
- de Sousa Rodrigues, M.E.; Bekhbat, M.; Houser, M.C.; Chang, J.; Walker, D.I.; Jones, D.P.; Oller do Nascimento, C.M.; Barnum, C.J.; Tansey, M.G. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain Behav. Immun. 2017, 59, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Macedo, I.C.; Medeiros, L.F.; Oliveira, C.; Oliveira, C.M.; Rozisky, J.R.; Scarabelot, V.L.; Souza, A.; Silva, F.R.; Santos, V.S.; Cioato, S.G.; et al. Cafeteria diet-induced obesity plus chronic stress alter serum leptin levels. Peptides 2012, 38, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Finger, B.C.; Dinan, T.G.; Cryan, J.F. The temporal impact of chronic intermittent psychosocial stress on high-fat diet-induced alterations in body weight. Psychoneuroendocrinology 2012, 37, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.C.; Krishnan, V.; Yu, H.G.; Mason, B.; Cui, H.; Wilkinson, M.B.; Zigman, J.M.; Elmquist, J.K.; Nestler, E.J.; Lutter, M. A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol. Psychiatry 2010, 67, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, E.F.; Tai, W.S.; Cox, A.; O’Connor, D.; Griffith, T.A.; Helman, T.; Wendt, L.; Peart, J.N.; Stapelberg, N.J.; Headrick, J.P. Synergistic effects of low-level stress and a Western diet on metabolic homeostasis, mood, and myocardial ischemic tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, 347–357. [Google Scholar] [CrossRef]
- Katsu, M.; Niizuma, K.; Yoshioka, H.; Okami, N.; Sakata, H.; Chan, P.H. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood-brain barrier dysfunction in vivo. J. Cereb. Blood Flow Metab. 2010, 30, 1939–1950. [Google Scholar] [CrossRef]
- Morrison, C.D.; Pistell, P.J.; Ingram, D.K.; Johnson, W.D.; Liu, Y.; Fernandez-Kim, S.O.; White, C.L.; Purpera, M.N.; Uranga, R.M.; Bruce-Keller, A.J.; et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: Implications for decreased Nrf2 signaling. J. Neurochem. 2010, 114, 1581–1589. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Cutler, R.G.; Button, C.; Telljohann, R.; Mattson, M.P. Diet-induced elevations in serum cholesterol are associated with alterations in hippocampal lipid metabolism and increased oxidative stress. J. Neurochem. 2011, 118, 611–615. [Google Scholar] [CrossRef]
- Jain, S.S.; Paglialunga, S.; Vigna, C.; Ludzki, A.; Herbst, E.A.; Lally, J.S.; Schrauwen, P.; Hoeks, J.; Tupling, A.R.; Bonen, A.; et al. High-fat diet-induced mitochondrial biogenesis is regulated by mitochondrial-derived reactive oxygen species activation of CaMKII. Diabetes 2014, 63, 1907–1913. [Google Scholar] [CrossRef]
- Ozcan, L.; Cristina de Souza, J.; Harari, A.A.; Backs, J.; Olson, E.N.; Tabas, I. Activation of calcium/calmodulin-dependent protein kinase II in obesity mediates suppression of hepatic insulin signaling. Cell Metab. 2013, 18, 803–815. [Google Scholar] [CrossRef]
- Kim, H.; Kang, H.; Heo, R.W.; Jeon, B.T.; Yi, C.O.; Shin, H.J.; Kim, J.; Jeong, S.Y.; Kwak, W.; Kim, W.H.; et al. Caloric restriction improves diabetes-induced cognitive deficits by attenuating neurogranin-associated calcium signaling in high-fat diet-fed mice. J. Cereb. Blood Flow Metab. 2016, 6, 1098–1110. [Google Scholar] [CrossRef]
- Oliveira, C.D.; Oliveira, C.M.; de Macedo, I.C.; Quevedo, A.S.; Filho, P.R.; Silva, F.R.; Vercelino, R.; de Souza, I.C.; Caumo, W.; Torres, I.L. Hypercaloric diet modulates effects of chronic stress: A behavioral and biometric study on rats. Stress 2015, 18, 514–523. [Google Scholar] [CrossRef]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.N.; Pu, J.C.; Liu, L.X.; Wang, G.W.; Zhou, X.Y.; Zhang, Y.Q.; Liu, Y.Y.; Xie, P. Integrated Metabolomics and Proteomics Analysis Revealed Second Messenger System Disturbance in Hippocampus of Chronic Social Defeat Stress Rat. Front. Neurosci. 2019, 13, 247. [Google Scholar] [CrossRef]
- Tang, M.; Huang, H.; Li, S.; Zhou, M.; Liu, Z.; Huang, R.; Liao, W.; Xie, P.; Zhou, J. Hippocampal proteomic changes of susceptibility and resilience to depression or anxiety in a rat model of chronic mild stress. Transl. Psychiatry 2019, 9, 260. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Pu, J.; Yang, L.; Zhou, X.; Liu, L.; Jiang, X.; Zhang, H.; Teng, T.; Tian, L.; et al. Integrated Metabolomics and Proteomics Analysis of Hippocampus in a Rat Model of Depression. Neuroscience 2018, 10, 207–220. [Google Scholar] [CrossRef]
- McKlveen, J.M.; Morano, R.L.; Fitzgerald, M.; Zoubovsky, S.; Cassella, S.N.; Scheimann, J.R.; Ghosal, S.; Mahbod, P.; Packard, B.A.; Myers, B.; et al. Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biol. Psychiatry 2016, 80, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Treccani, G.; Musazzi, L.; Perego, C.; Milanese, M.; Nava, N.; Bonifacino, T.; Lamanna, J.; Malgaroli, A.; Drago, F.; Racagni, G.; et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol. Psychiatry 2014, 19, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.M.; Benway, T.A.; Ho, H.; Tedoldi, A.; Fernandes Freitas, M.M.; Shahab, L.; Murray, C.E.; Richard-Loendt, A.; Brandner, S.; Lashley, T.; et al. Neuronal and Peripheral Pentraxins Modify Glutamate Release and may Interact in Blood-Brain Barrier Failure. Cereb. Cortex 2017, 27, 3437–3448. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.A.; Enguita, M.; DeGregorio-Rocasolano, N.; Ferrer, I.; Trullas, R. Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-beta and is overexpressed in dystrophic neurites in Alzheimer’s brain. J. Neurosci. 2006, 26, 12735–12747. [Google Scholar] [CrossRef] [PubMed]
- Clayton, K.B.; Podlesniy, P.; Figueiro-Silva, J.; López-Doménech, G.; Benitez, L.; Enguita, M.; Abad, M.A.; Soriano, E.; Trullas, R. NP1 regulates neuronal activity-dependent accumulation of BAX in mitochondria and mitochondrial dynamics. J. Neurosci. 2012, 32, 1453–1466. [Google Scholar] [CrossRef]
- Wainwright, S.R.; Galea, L.A. The neural plasticity theory of depression: Assessing the roles of adult neurogenesis and PSA-NCAM within the hippocampus. Neural Plast. 2013, 2013, 805497. [Google Scholar] [CrossRef]
- Tochigi, M.; Iwamoto, K.; Bundo, M.; Sasaki, T.; Kato, N.; Kato, T. Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neurosci. Res. 2008, 60, 184–191. [Google Scholar] [CrossRef]
- Desarnaud, F.; Jakovcevski, M.; Morellini, F.; Schachner, M. Stress downregulates hippocampal expression of the adhesion molecules NCAM and CHL1 in mice by mechanisms independent of DNA methylation of their promoters. Cell Adhes. Migr. 2008, 2, 38–44. [Google Scholar] [CrossRef]
- Valente, P.; Castroflorio, E.; Rossi, P.; Fadda, M.; Sterlini, B.; Cervigni, R.I.; Prestigio, C.; Giovedì, S.; Onofri, F.; Mura, E.; et al. PRRT2 Is a Key Component of the Ca(2+)-Dependent Neurotransmitter Release Machinery. Cell Rep. 2016, 15, 117–131. [Google Scholar] [CrossRef]
- Block, R.I.; Farnham, S.; Braverman, K.; Hinrichs, J.V.; Ghoneim, M.M. Norms for free associations and five types of constrained associations. Psychol. Rep. 1989, 64, 1065–1066. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, F.; Gao, J.; Guan, X.; Liu, B.; Wang, X.; Qin, Z.; Tang, K.; Liu, S. Proteomics-based screening of the target proteins associated with antidepressant-like effect and mechanism of Saikosaponin A. J. Cell. Mol. Med. 2020, 24, 174–188. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 3, 22–37. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Tornese, P.; Sala, N.; Bonini, D.; Bonifacino, T.; La Via, L.; Milanese, M.; Treccani, G.; Seguini, M.; Ieraci, A.; Mingardi, J.; et al. Chronic mild stress induces anhedonic behavior and changes in glutamate release, BDNF trafficking and dendrite morphology only in stress vulnerable rats. The rapid restorative action of ketamine. Neurobiol. Stress 2019, 10, 100160. [Google Scholar] [CrossRef]
- Crema, L.M.; Vendite, D.; Horn, A.P.; Diehl, L.A.; Aguiar, A.P.; Nunes, E.; Vinade, L.; Fontella, F.U.; Salbego, C.; Dalmaz, C. Effects of chronic restraint stress and estradiol replacement on glutamate release and uptake in the spinal cord from ovariectomized female rats. Neurochem. Res. 2009, 34, 499–507. [Google Scholar] [CrossRef]
- Cai, D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol. Metab. 2013, 24, 40–47. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Mattson, M.P. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011, 10, 187–198. [Google Scholar] [CrossRef]
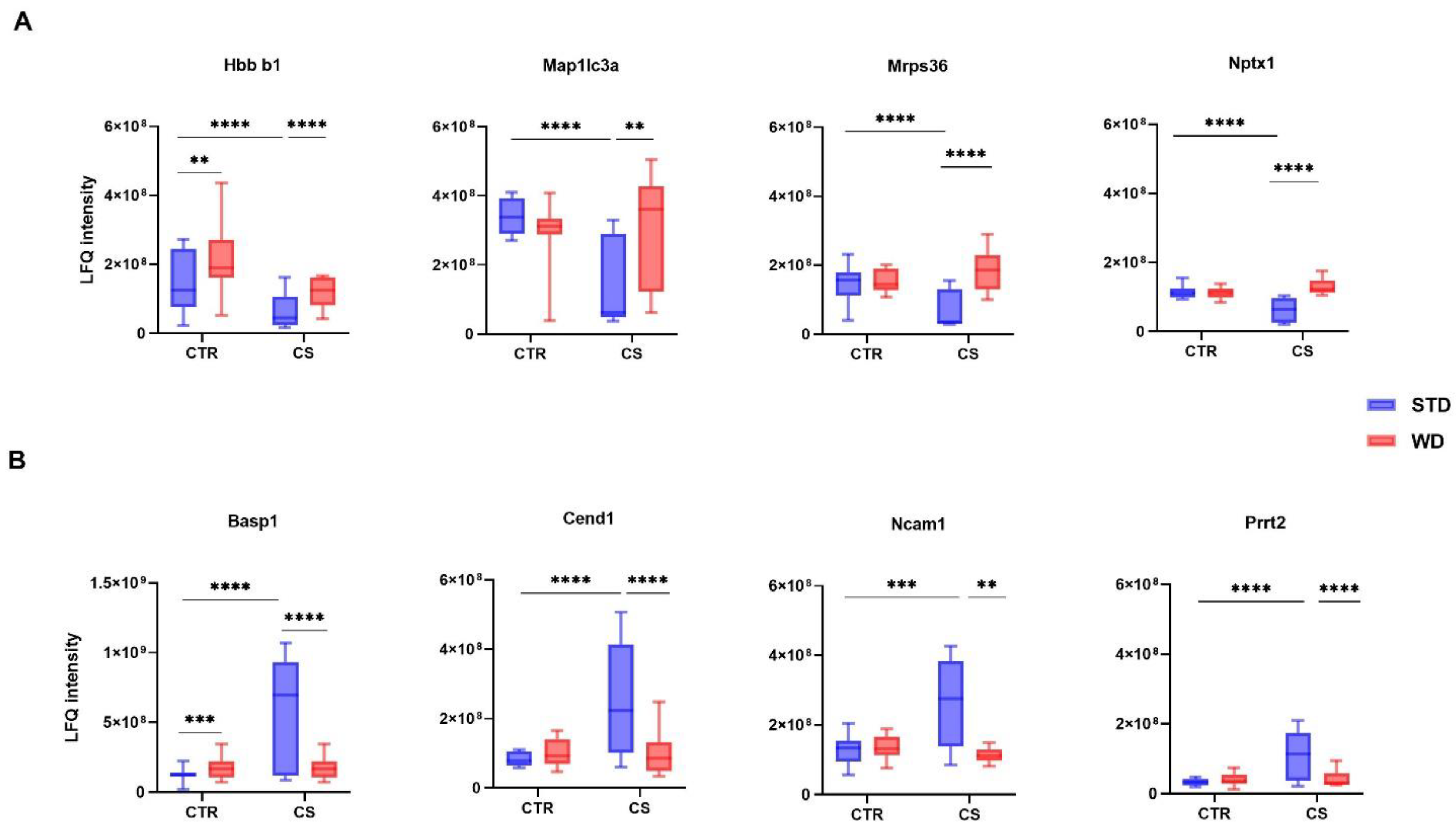



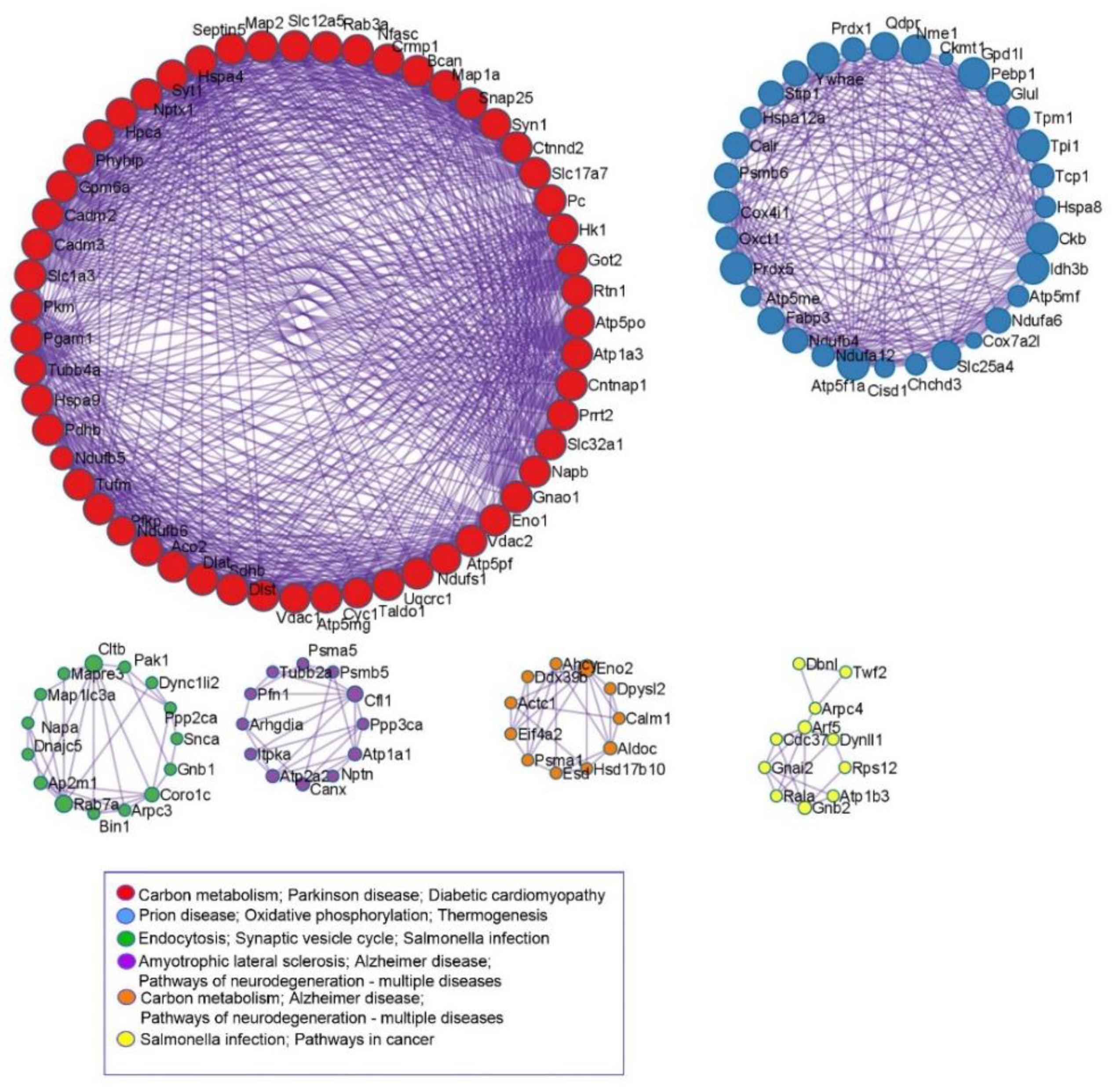
| Groups | CTR | WD | CS | WD/CS | Significance | ||
|---|---|---|---|---|---|---|---|
| Interaction | Diet | Stress | |||||
| Energy intake (kcal/day) | 65.95 ± 9.23 | 103.18 ± 16.64 **** | 60.78 ± 21.14 | 96.04 ± 43.7 ****,#### | ns | p < 0.0001 | ns |
| Final body weight (g) | 273.99 ± 23.69 | 359.90 ± 29.89 **** | 278.11 ± 10.08 | 325.75 ± 33.80 **,# | p = 0.0482 | p < 0.0001 | ns |
| Adipose (g) | 10.14 ± 1.80 | 41.11 ± 12.56 **** | 7.03 ± 1.94 | 21.92 ± 6.98 *,#,&&& | p = 0.0075 | p < 0.0001 | p = 0.0004 |
| Trunk (g) | 132.12 ± 10.19 | 151.86 ± 17.02 ** | 134.62 ± 5.10 | 150.66 ± 16.26 * | ns | p = 0.0007 | ns |
| Heart (g) | 0.93 ± 0.11 | 1.09 ± 0.16 | 0.91 ± 0.04 | 1.11 ± 0.15 | ns | ns | ns |
| Liver (g) | 10.71 ± 1.20 | 11.41 ± 1.86 | 11.5 ± 2.34 | 13.13 ± 3.99 | ns | ns | ns |
| Groups | CTR | WD | CS | WD/CS | Significance | ||
|---|---|---|---|---|---|---|---|
| Serum | Interaction | Diet | Stress | ||||
| Glucose (mg/dl) | 296.8 ± 33.36 | 317.3 ± 34.97 | 335.8 ± 46.54 | 512.98 ± 137.36 ***,##,&& | p = 0.020 | p = 0.004 | p = 0.001 |
| Insulin (ulU/mL) | 22.57 ± 2.92 | 23.13 ± 0.38 | 24.54 ± 5.09 | 21.68 ± 3.36 | ns | ns | ns |
| QUICKY index | 0.262 ± 0.002 | 0.258 ± 0.003 | 0.256 ± 0.01 | 0.248 ± 0.008 * | ns | p = 0.049 | p = 0.015 |
| Leptin (pg/mL) | 1742.5 ± 679.3 | 12897 ± 3107.6 **** | 4222.3 ± 2489.1 | 1412.6 ± 80.2 &&&& | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Cholesterol (mg/dl) | 79.28 ± 10.89 | 77.01 ± 10.06 | 75.03 ± 7.24 | 75.73 ± 10.38 | ns | ns | ns |
| LDL cholesterol (mg/dl) | 21.08 ± 4.35 | 27.71 ± 2.69 | 17.84 ± 3.03 | 26.29 ± 4.19 # | ns | p = 0.0006 | ns |
| Triglicerides (mg/dl) | 138.88 ± 21.09 | 141.88 ± 32.06 | 192.78 ± 64.6 | 202.63 ± 69.56 | ns | ns | p = 0.016 |
| Estradiol (pg/mL) | 22.17 ± 10.62 | 20.06 ± 13.20 | 27.65 ± 15.30 | 34.14 ± 17.76 | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka-Chmielewska, M.; Liśkiewicz, D.; Liśkiewicz, A.; Przybyła, M.; Marczak, Ł.; Wojakowska, A.; Grabowska, K.; Grabowski, M.; Barski, J.J.; Małecki, A. Global Proteome Profiling of the Temporal Cortex of Female Rats Exposed to Chronic Stress and the Western Diet. Nutrients 2022, 14, 1934. https://doi.org/10.3390/nu14091934
Nowacka-Chmielewska M, Liśkiewicz D, Liśkiewicz A, Przybyła M, Marczak Ł, Wojakowska A, Grabowska K, Grabowski M, Barski JJ, Małecki A. Global Proteome Profiling of the Temporal Cortex of Female Rats Exposed to Chronic Stress and the Western Diet. Nutrients. 2022; 14(9):1934. https://doi.org/10.3390/nu14091934
Chicago/Turabian StyleNowacka-Chmielewska, Marta, Daniela Liśkiewicz, Arkadiusz Liśkiewicz, Marta Przybyła, Łukasz Marczak, Anna Wojakowska, Konstancja Grabowska, Mateusz Grabowski, Jarosław Jerzy Barski, and Andrzej Małecki. 2022. "Global Proteome Profiling of the Temporal Cortex of Female Rats Exposed to Chronic Stress and the Western Diet" Nutrients 14, no. 9: 1934. https://doi.org/10.3390/nu14091934
APA StyleNowacka-Chmielewska, M., Liśkiewicz, D., Liśkiewicz, A., Przybyła, M., Marczak, Ł., Wojakowska, A., Grabowska, K., Grabowski, M., Barski, J. J., & Małecki, A. (2022). Global Proteome Profiling of the Temporal Cortex of Female Rats Exposed to Chronic Stress and the Western Diet. Nutrients, 14(9), 1934. https://doi.org/10.3390/nu14091934







