HM-Chromanone Ameliorates Hyperglycemia and Dyslipidemia in Type 2 Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
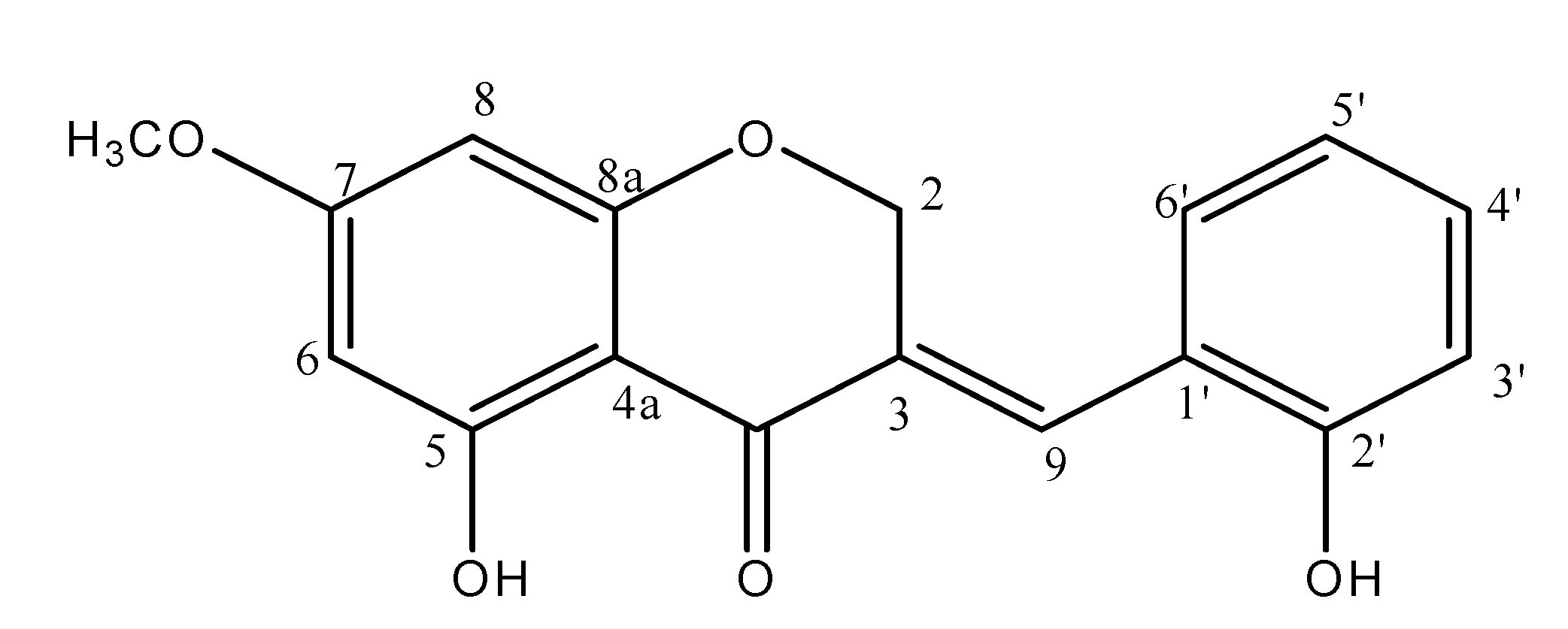
2.1. Preparation of Materials
2.2. Animals
2.3. Blood Glucose and Glycosylated Hemoglobin Levels
2.4. Plasma Insulin Level
2.5. Homeostatic Index of Insulin Resistance
2.6. Serum Lipid Levels
2.7. Plasma Membrane Fraction of Skeletal Muscle
2.8. Western Blot Analysis
2.9. Statistical Analyses
3. Results
3.1. Body Weight, Food Intake, and Water Intake
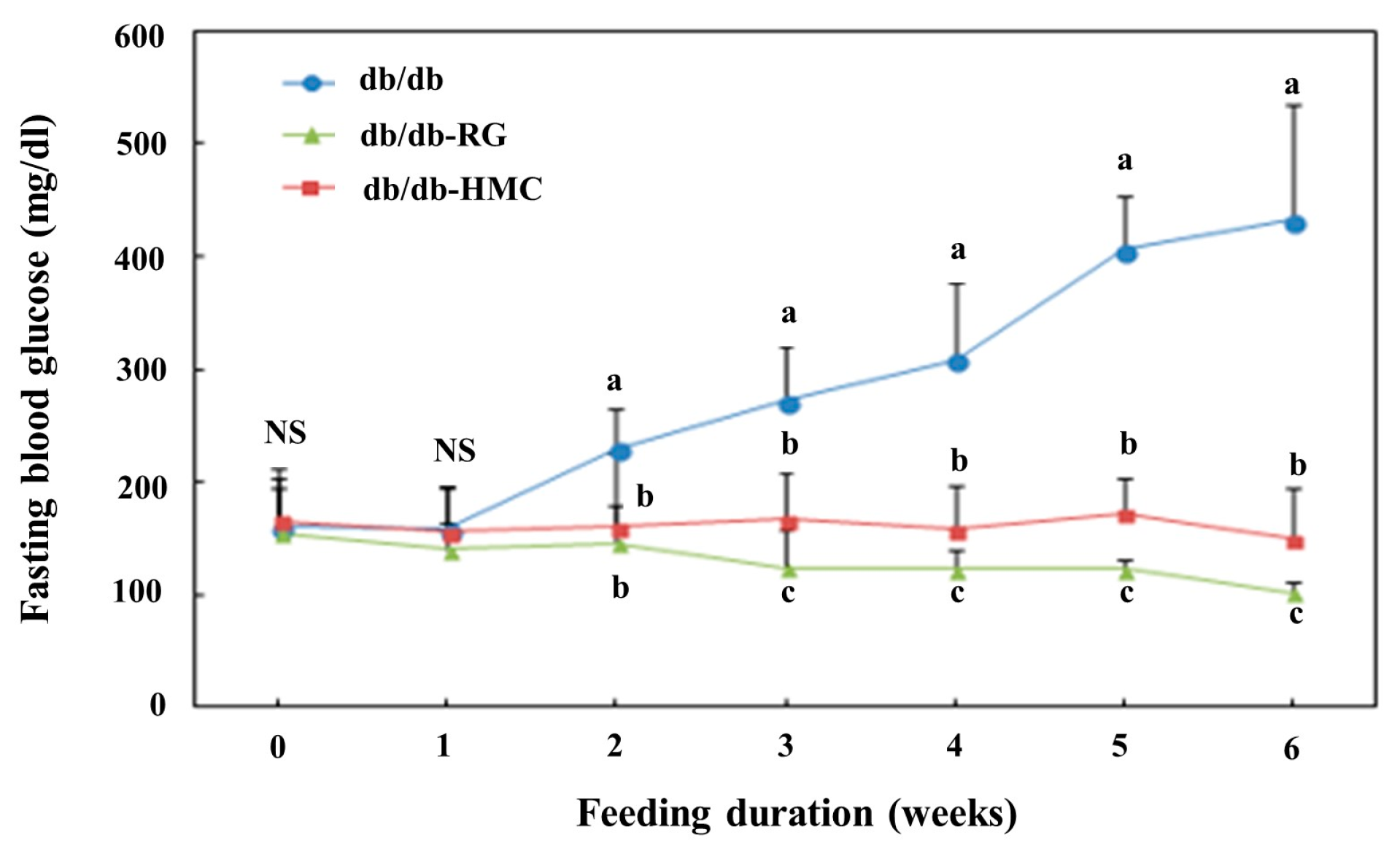
3.2. Blood Glucose and HbA1c Values
3.3. Plasma Insulin Levels and HOMA-IR
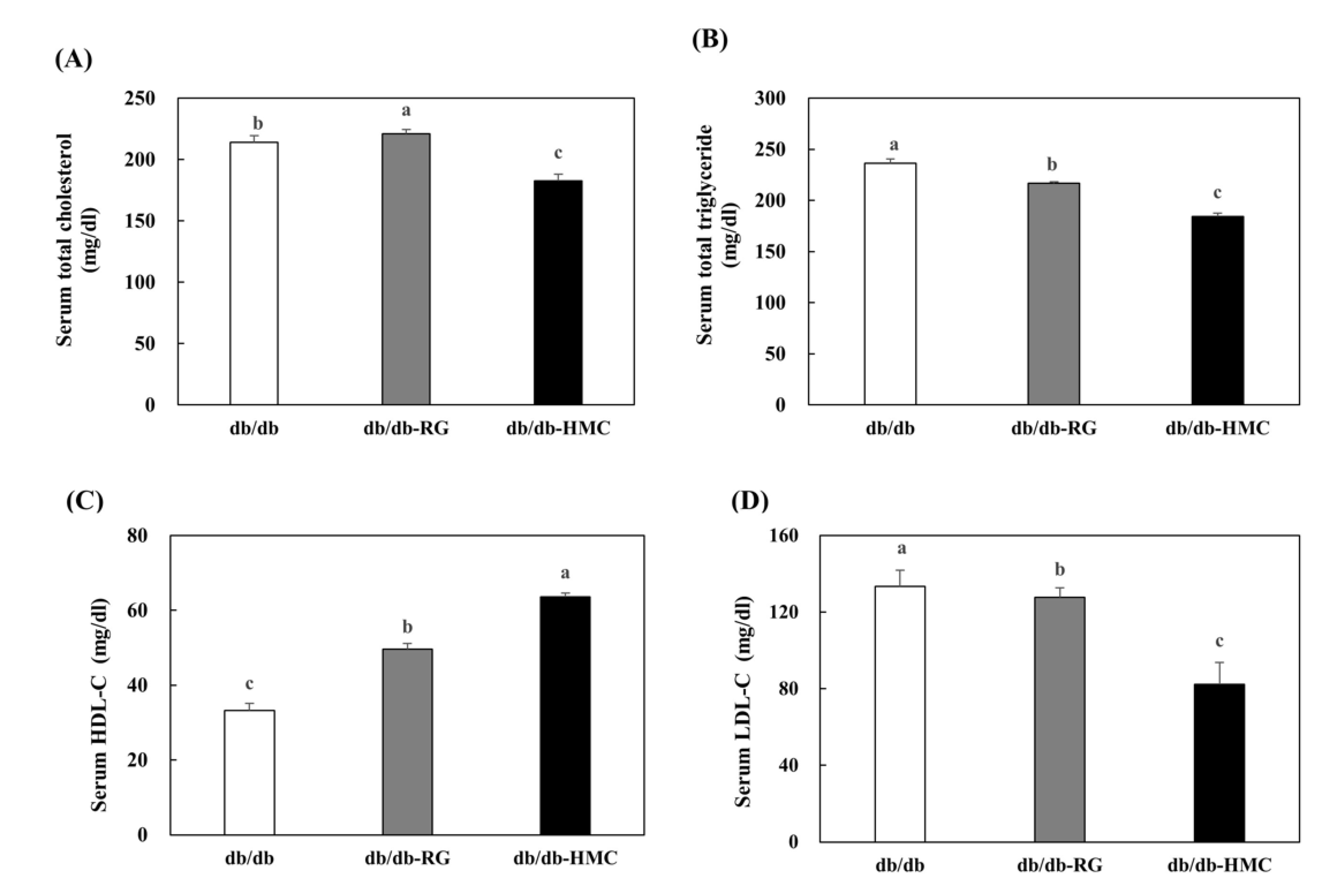
3.4. Serum Lipid Profiles
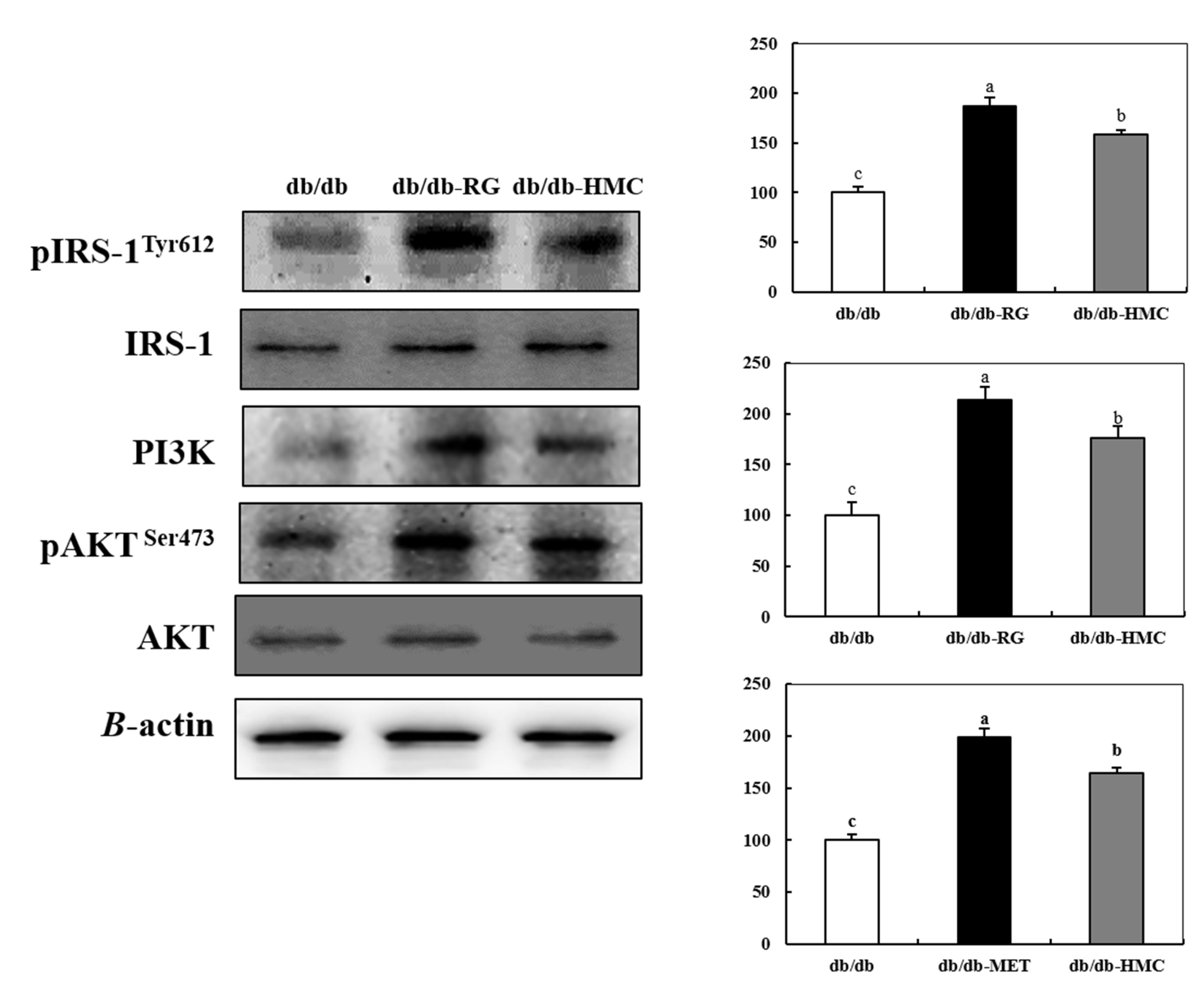
3.5. Expression of Insulin Signaling Pathway
3.6. Expression of AMPK
3.7. Expression of PM-GLUT4
3.8. Expression of AMPK, PPARα, SREBP-1c, and FAS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cheng, D. Prevalence, predisposition and prevention of type II diabetes. Nutr. Metab. 2005, 2, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2005, 36, S67–S74. [Google Scholar]
- Murea, M.; Ma, L.; Freedman, B.I. Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev. Diabet. Stud. RDS 2012, 9, 6–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, S.; Saberi, M.; Olefsky, J.M. Insulin sensitivity: Modulation by nutrients and inflammation. J. Clin. Invest. 2008, 118, 2992–3002. [Google Scholar] [CrossRef] [Green Version]
- Saltiel. A.R. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 2001, 104, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle—A focus on the molecular mechanisms of insulin resistance. Mol. Cell Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef]
- Bergman, R.N.; Ader, M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol. Metab. 2000, 11, 351–356. [Google Scholar] [CrossRef]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139. [Google Scholar] [CrossRef] [Green Version]
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 2007, 87, 507–520. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care 2003, 26, 583–586. [Google Scholar]
- Javad, M.A. Curcumin increases insulin sensitivity in C2C12 muscle cells via AKT and AMPK signaling pathways. Cogent. Food Agric. 2019, 5, 1. [Google Scholar]
- Ben Djoudi Ouadda, A.; Levy, E.; Ziv, E. Increased hepatic lipogenesis in insulin resistance and Type 2 diabetes is associated with AMPK signalling pathway up-regulation in Psammomys obesus. Biosci. Rep. 2009, 29, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foretz, M.; Pacot, C.; Dugail, I. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol. Cell Biol. 1999, 19, 3760–3768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linden, A.G.; Li, S.; Choi, H.Y. Interplay between ChREBP and SREBP-1c coordinates postprandial glycolysis and lipogenesis in livers of mice. J. Lipid Res. 2018, 59, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, P.K.; Doble, M. A target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr. Diabetes Rev. 2008, 4, 291–308. [Google Scholar] [CrossRef]
- Xu, X.; Liang, T.; Lin, X.; Wen, Q.; Liang, X.; Li, W.; Qin, F.; Zheng, N.; Ming, J.; Huang, R. Effect of the total extract of averrhoacarambola (oxalidaceae) root on the expression levels of tlr4 and nf-kappab in streptozotocin-induced diabetic mice. Cell Physiol. Biochem. 2015, 36, 2307–2316. [Google Scholar] [CrossRef] [Green Version]
- Hsu, F.L.; Huang, C.F.; Chen, Y.W.; Yen, Y.P.; Wu, C.T.; Uang, B.J.; Yang, R.S.; Liu, S.H. Antidiabetic effects of pterosin a, a small-molecular-weight natural product, on diabetic mouse models. Diabetes 2013, 62, 628–638. [Google Scholar] [CrossRef] [Green Version]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [Green Version]
- Do, G.M.; Jung, U.J.; Park, H.J.; Kwon, E.Y.; Jeon, S.M.; McGregor, R.A.; Choi, M.S. Resveratrol ameliorates diabetes-related metabolic changes via activation of amp-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 2012, 56, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Park, J.Y.; Seo, Y.; Han, J.S. A new chromanone isolated from Portulaca oleracea L. increases glucose uptake by stimulating GLUT4 translocation to the plasma membrane in 3T3-L1 adipocytes. Int. J. Biol. Macromol. 2019, 123, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D.; Zhu, J.S.; Zhu, J.H.; Weldon, H.; Maianu, L.; Garvey, W.T. Glucosamine induces insulin resistance in vivo by affecting GLUT4 translocation in skeletal muscle: Implications for glucose toxicity. J. Clin. Invest. 1995, 96, 2792–2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klip, A.; Ramlal, T.; Young, D.A.; Holloszy, J.O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987, 224, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Chen, X.; Sun, Y.; Shi, J.; Xu, X.; Shi, Y.C. Hypoglycemic effects of pyrodextrins with different molecular weights and digestibilities in mice with diet-induced obesity. J. Agric. Food Chem. 2018, 66, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Akindele, A.J.; Otuguor, E.; Singh, D.; Ota, D.; Benebo, A.S. Hypoglycemic, antilipidemic and antioxidant effects of valproic acid in alloxan-induced diabetic rats. Eur. J. Pharmacol. 2015, 762, 174–183. [Google Scholar] [CrossRef]
- Lee, S.M.; Bustamante, S.; Flores, C.; Bezerra, J.; Goda, T.; Koldovský, O. Chronic effects of an alpha-glucosidase inhibitor (Bay o 1248) on intestinal disaccharidase activity in normal and diabetic mice. J. Pharmacol. Exp. Ther. 1987, 240, 132–137. [Google Scholar]
- Park, K.S.; Lee, D.E.; Sung, J.H.; Chung, S.H. Comparisons of antidiabetic effect of Panax ginseng on MLD STZ-induced diabetic rats in terms of time of administration. J. Ginseng Res. 2002, 26, 191–195. [Google Scholar]
- Lebovitz, H.E.; Dole, J.F.; Patwardhan, R.; Rappaport, E.B.; Freed, M.I. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. Rosiglitazone Clinical Trials Study Group. J. Clin. Endocrinol. Metab. 2001, 86, 280–288. [Google Scholar] [CrossRef]
- Goldstein, B.J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 3–10. [Google Scholar] [CrossRef]
- Jiang, G.Q.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, E.J.; Khil, L.Y.; Kwak, W.J.; Won, H.S.; Chae, S.H.; Lee, B.H.; Moon, C.K. Effects of brazilin on the production of fructose-2, 6-bisphosphate in rat hepatocytes. J. Ethnopharmacol. 2005, 102, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fowler, M.I.; Messenger, D.J.; Terry, L.A.; Gu, X.; Zhou, L.; Liu, R.; Su, J.; Shi, S.; Ordaz-Ortiz, J.J.; et al. Homoisoflavonoids Are Potent Glucose Transporter 2 (GLUT2) Inhibitors: A Potential Mechanism for the Glucose-Lowering Properties of Polygonatum odoratum. J. Agric. Food Chem. 2018, 28, 3137–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahbar, S. An abnormal hemoglobin in red cells of diabetics. Clin. Chim. Acta 1968, 22, 296–298. [Google Scholar] [CrossRef]
- Khalafallah, A.; Phuah, E.; Al-Barazan, A.M.; Nikakis, I.; Radford, A.; Clarkson, W.; Trevett, C.; Brain, T.; Gebski, V.; Corbould, A. Glycosylated haemoglobin for screening and diagnosis of gestational diabetes mellitus. BMJ Open 2016, 6, e011059. [Google Scholar] [CrossRef]
- Park, H.R.; Cho, J.S. Effects of a natural medicinal multi-plant extract on blood glucose, insulin levels, and serum malondialdehyde concentrations in streptozotocin-induced diabetic rats. J. East Asian Soc. Diet Life 2007, 17, 205–212. [Google Scholar]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Treebak, J.T.; Glund, S.; Deshmukh, A.; Klein, D.K.; Long, Y.C.; Jensen, T.E.; Jørgensen, S.B.; Viollet, B.; Andersson, L.; Neumann, D.; et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 2006, 55, 2051–2058. [Google Scholar] [CrossRef] [Green Version]
- Baus, D.; Heermeier, K.; De, H.M.; Metz-Weidmann, C.; Gassenhuber, J.; Dittrich, W.; Welte, S.; Tennagels, N. Identification of a novel AS160 splice variant that regulates GLUT4 translocation and glucose-uptake in rat muscle cells. Cell Signal. 2008, 20, 2237–2246. [Google Scholar] [CrossRef]
- Hu, X.; Wang, S.; Xu, J.; Wang, D.B.; Chen, Y.; Yang, G.Z. Triterpenoid saponins from Stauntonia chinensis ameliorate insulin resistance via the AMP-activated protein kinase and IR/IRS-1/PI3K/AKT pathways in insulin-resistant HepG2 cells. Int. J. Mol. Sci. 2014, 15, 10446–10458. [Google Scholar] [CrossRef]
- Khil, L.Y.; Han, S.S.; Kim, S.G.; Chang, T.S.; Jeon, S.D.; So, D.S.; Moon, C.K. Effects of Brazilin on GLUT4 recruitment in isolated rat epididymal adipocytes. Biochem. Pharmacol. 1999, 58, 1705–1712. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, F.; Qi, J.; Song, X.C.; Hu, Z.F.; Zhu, D.N.; Yu, B.Y. Homoisoflavonoids from the fibrous roots of Polygonatum odoratum with glucose uptake-stimulatory activity in 3T3-L1 adipocytes. J. Nat. Prod. 2010, 73, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Lee-Young, R.S.; Bonner, J.S.; Mayes, W.H.; Iwueke, I.; Barrick, B.A.; Hasenour, C.M.; Kang, L.; Wasserman, D.H. AMP-activated protein kinase (AMPK)alpha2 plays a role in determining the cellular fate of glucose in insulin-resistant mouse skeletal muscle. Diabetologia 2013, 56, 608–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Jiang, Y.; Liu, J.; Jin, M.; Qin, N.; Chen, Y.; Niu, W.; Duan, H. AMPK/AS160 mediates tiliroside derivatives-stimulated GLUT4 translocation in muscle cells. Drug Des. Devel. Ther. 2018, 12, 1581–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Alkhateeb, H.; Bonen, A. A component of medicinal herbs, rescues palmitate-induced insulin resistance in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R804–R812. [Google Scholar] [CrossRef] [Green Version]
- Fuliang, H.U.; Hepburn, H.R.; Xuan, H.; Chen, M.; Daya, S.; Radloff, S.E. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol. Res. 2005, 51, 147–152. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, G.D.; Kim, J.O.; Kim, K.S.; Lee, W.Y.; Hong, J.H. Effect of Bulnesia sarmienti water extracts on lipid metabolism in neonatally streptozotocin-induced diabetic rats. J. Life Sci. 2008, 8, 999–1004. [Google Scholar] [CrossRef]
- Durbin, R.J. Thiazolidinedione therapy in the prevention/delay of type 2 diabetes in patients with impaired glucose tolerance and insulin resistance. Diabetes Obes. Metab. 2004, 6, 280–285. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, K.H.; Han, J.S.; Lee, D.H.; Park, D.B.; Jung, W.K.; Park, P.J.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.R.; Kim, Y.; Choi, E.J.; Jung, M.A.; Bae, D.; Jo, A.; Kim, S. Antiobesity effects of unripe Rubus coreanus Miquel and its constituents: An in vitro and in vivo characterization of the underlying mechanism. Evid. Based Complement. Alternat. Med. 2016, 2016, 4357656. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Nakamura, M.T.; Cho, H.P.; Clarke, S.D. Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J. Biol. Chem. 1999, 274, 23577–23583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhalidy, H.; Moore, W.; Zhang, Y.; McMillan, R.; Wang, A.; Ali, M.; Suh, K.S.; Zhen, W.; Cheng, Z.; Jia, Z.; et al. Small molecule kaempferol promotes insulin sensitivity and preserved pancreatic β-cell mass in middle-aged obese diabetic mice. J. Diab. Res. 2015, 14, 532984. [Google Scholar]
- Okabe, Y.; Shimada, T.; Horikawa, T.; Kinoshita, K.; Koyama, K.; Ichinose, K.; Aburada, M.; Takahashi, K. Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferia parviflora. Phytomedicine 2014, 21, 800–806. [Google Scholar] [CrossRef]



| db/db | db/db-RG | db/db-HMC | |
|---|---|---|---|
| Water consumption (mL/day) | 3.54 ± 0.44 a | 1.99 ± 0.26 c | 3.16 ± 0.48 b |
| Food consumption (g/day) | 2.35 ± 0.28 NS | 2.17 ± 0.29 | 2.30 ± 0.28 |
| Body weight (g) | |||
| Initial | 25.71 ± 1.56 NS | 26.54 ± 1.51 | 26.56 ± 1.28 |
| Final | 42.81 ± 5.08 b | 47.53 ± 2.50 a | 40.43 ± 2.72 c |
| Body weight gain (g/day) | 0.40 ± 0.08 b | 0.49 ± 0.02 a | 0.33 ± 0.03 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.E.; Son, J.; Seo, Y.; Han, J.S. HM-Chromanone Ameliorates Hyperglycemia and Dyslipidemia in Type 2 Diabetic Mice. Nutrients 2022, 14, 1951. https://doi.org/10.3390/nu14091951
Park JE, Son J, Seo Y, Han JS. HM-Chromanone Ameliorates Hyperglycemia and Dyslipidemia in Type 2 Diabetic Mice. Nutrients. 2022; 14(9):1951. https://doi.org/10.3390/nu14091951
Chicago/Turabian StylePark, Jae Eun, Jaemin Son, Youngwan Seo, and Ji Sook Han. 2022. "HM-Chromanone Ameliorates Hyperglycemia and Dyslipidemia in Type 2 Diabetic Mice" Nutrients 14, no. 9: 1951. https://doi.org/10.3390/nu14091951
APA StylePark, J. E., Son, J., Seo, Y., & Han, J. S. (2022). HM-Chromanone Ameliorates Hyperglycemia and Dyslipidemia in Type 2 Diabetic Mice. Nutrients, 14(9), 1951. https://doi.org/10.3390/nu14091951






