RNA-Seq Analysis of Protection against Chronic Alcohol Liver Injury by Rosa roxburghii Fruit Juice (Cili) in Mice
Abstract
:1. Introduction
2. Materials and Methods
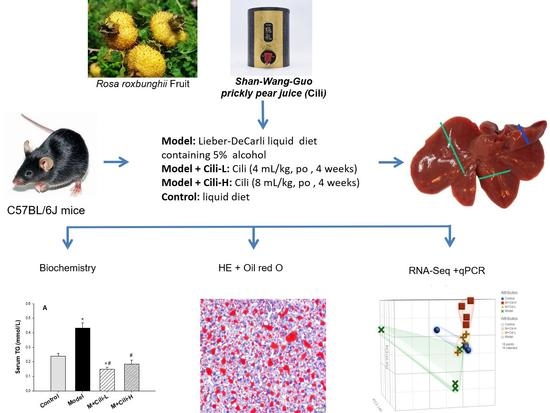
2.1. Cili, Liquid Diets and Chemicals
2.2. Experimental Animals
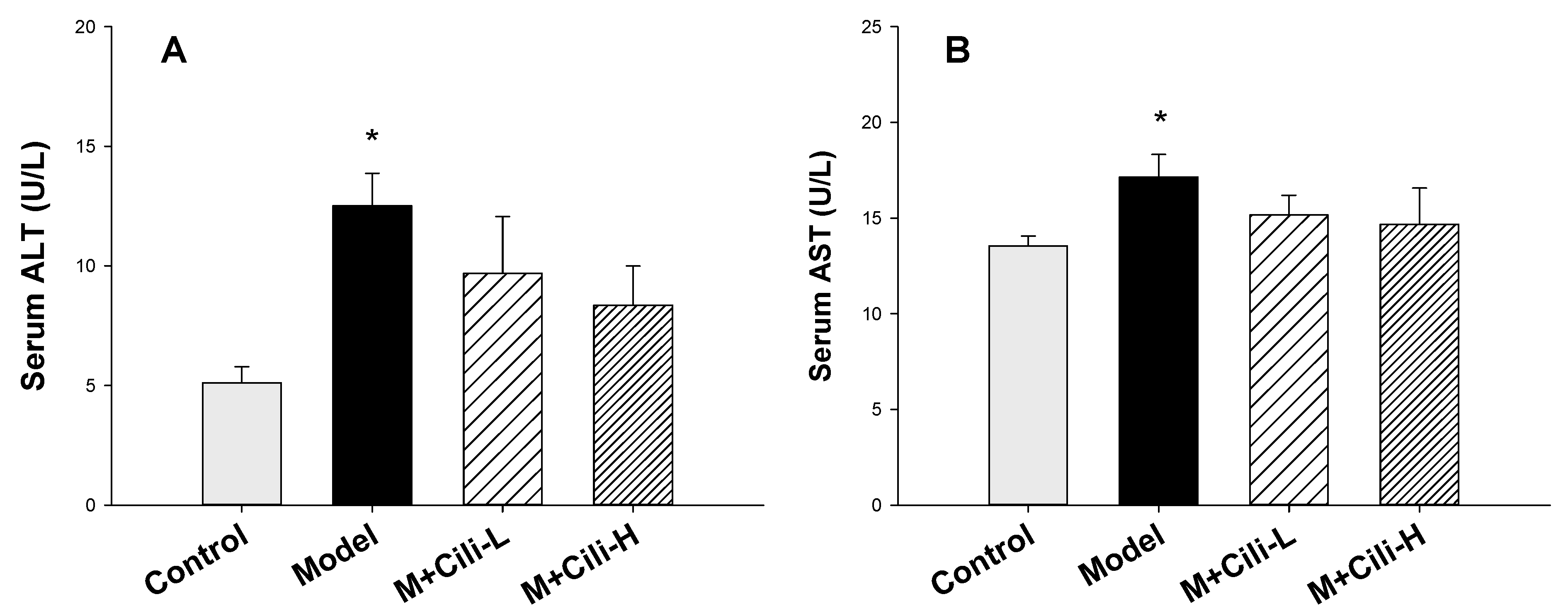
2.3. Serum Enzyme Activities
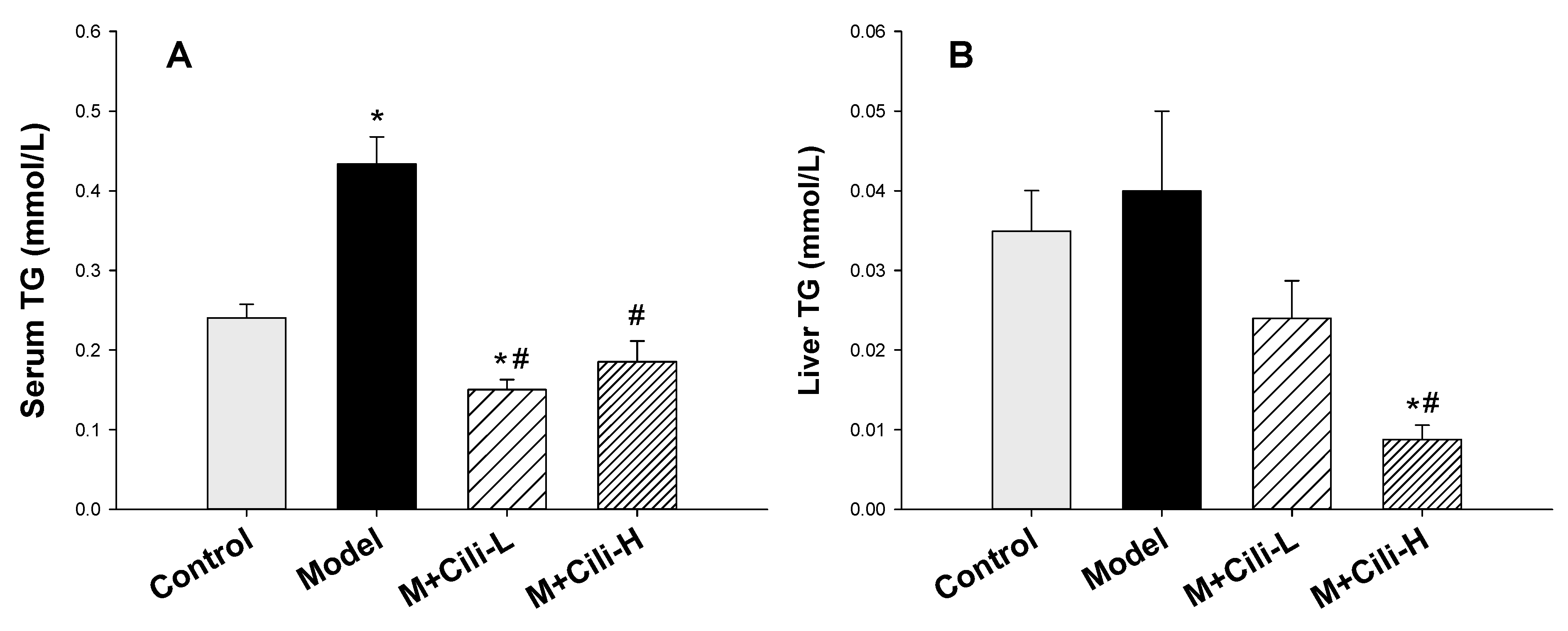
2.4. Triglyceride Determination
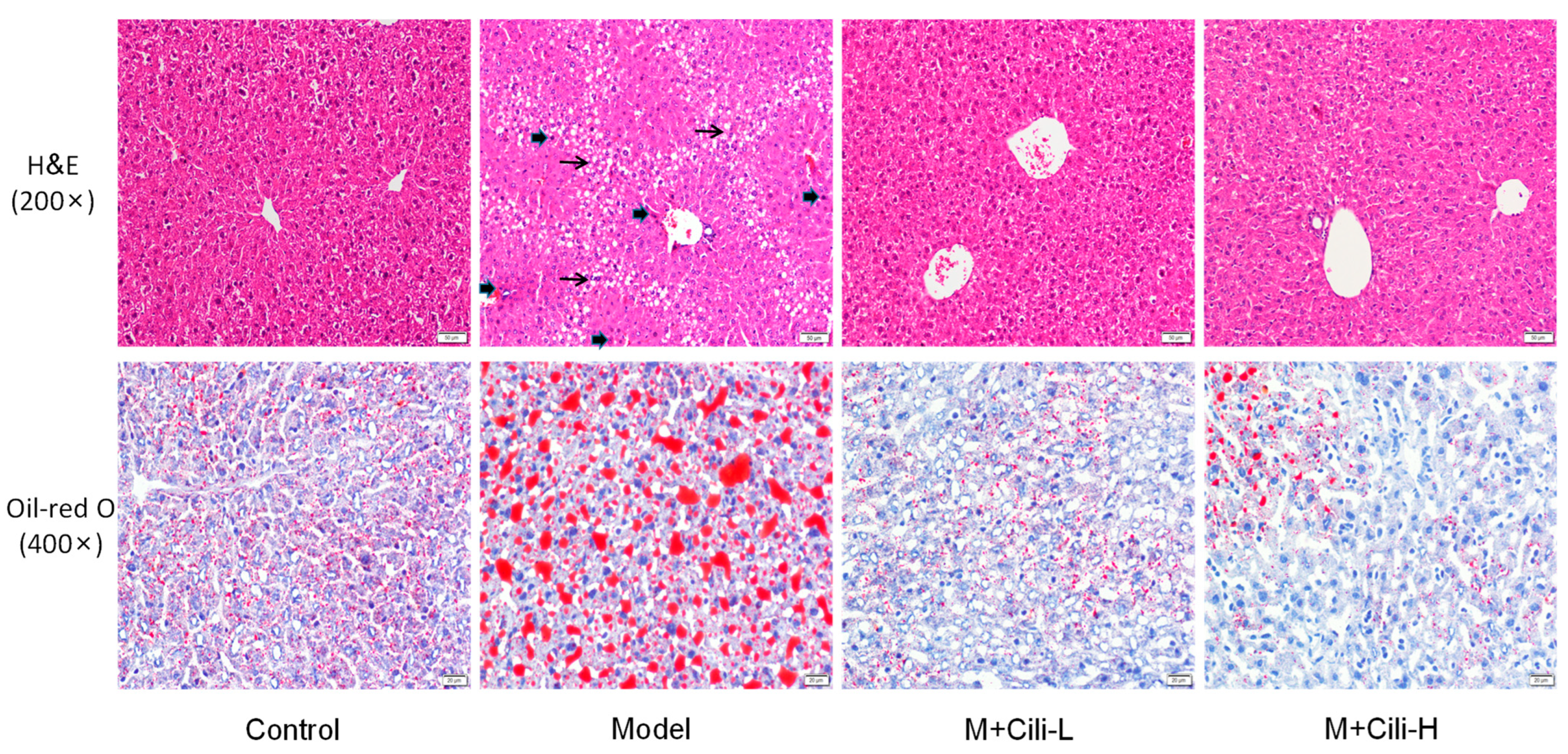
2.5. Haematoxylin and Eosin Staining and Oil-Red O Staining
2.6. RNA Extraction and Easy RNA-Seq
2.7. Principal Component Analysis
2.8. Heatmap Visualization of Differentially Expressed Genes
2.9. Real-Time qPCR
2.10. Statistics
3. Results
3.1. Cili Protected against Alcohol-Induced Elevation of Triglyceride
3.2. Cili Improved Alcohol-Induced Pathology and Lipid Accumulation
3.3. Cili Reversed Alcohol-Induced Aberrant Gene Expression
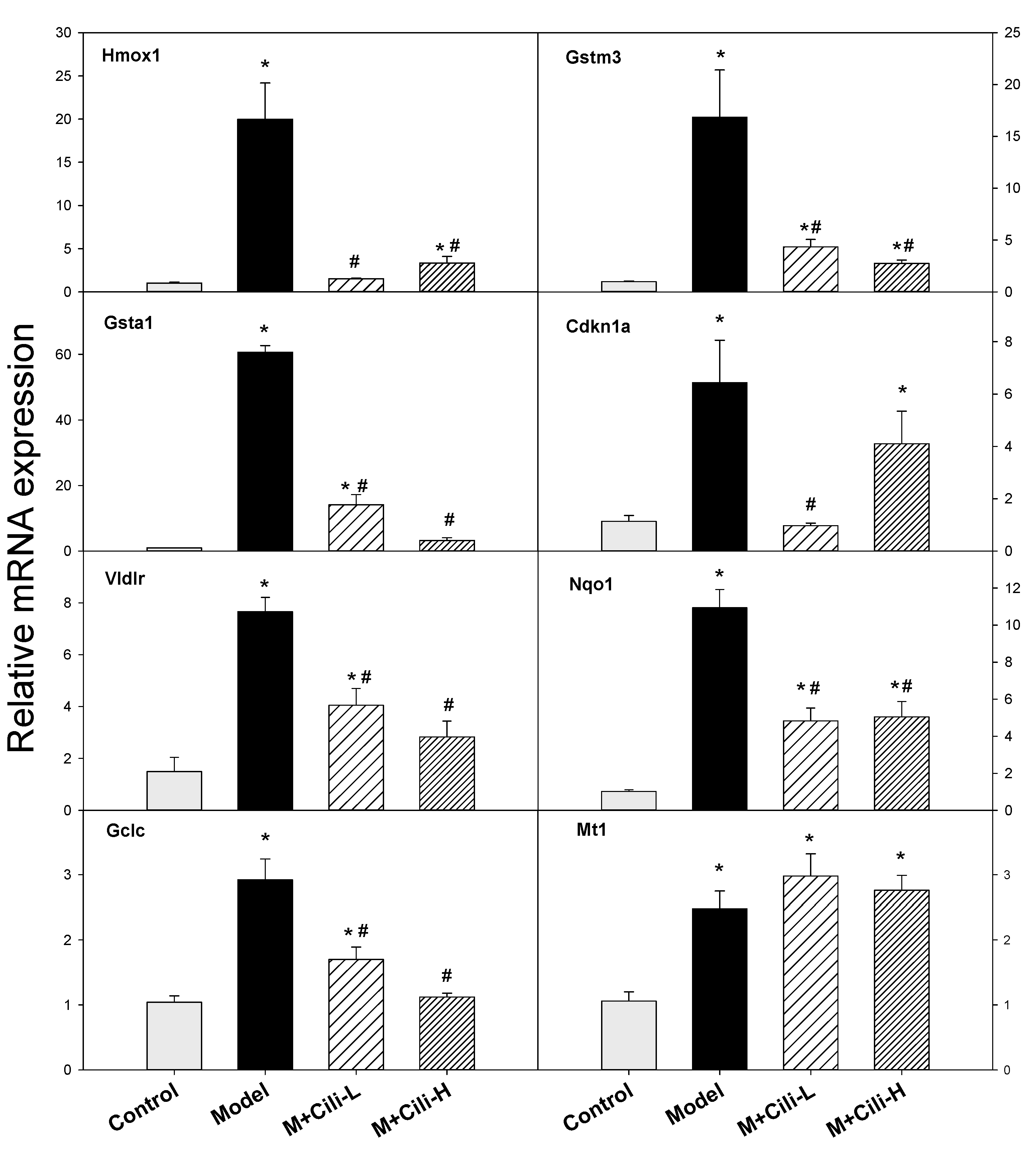
3.4. The qPCR Analysis of Selected Genes
4. Discussion
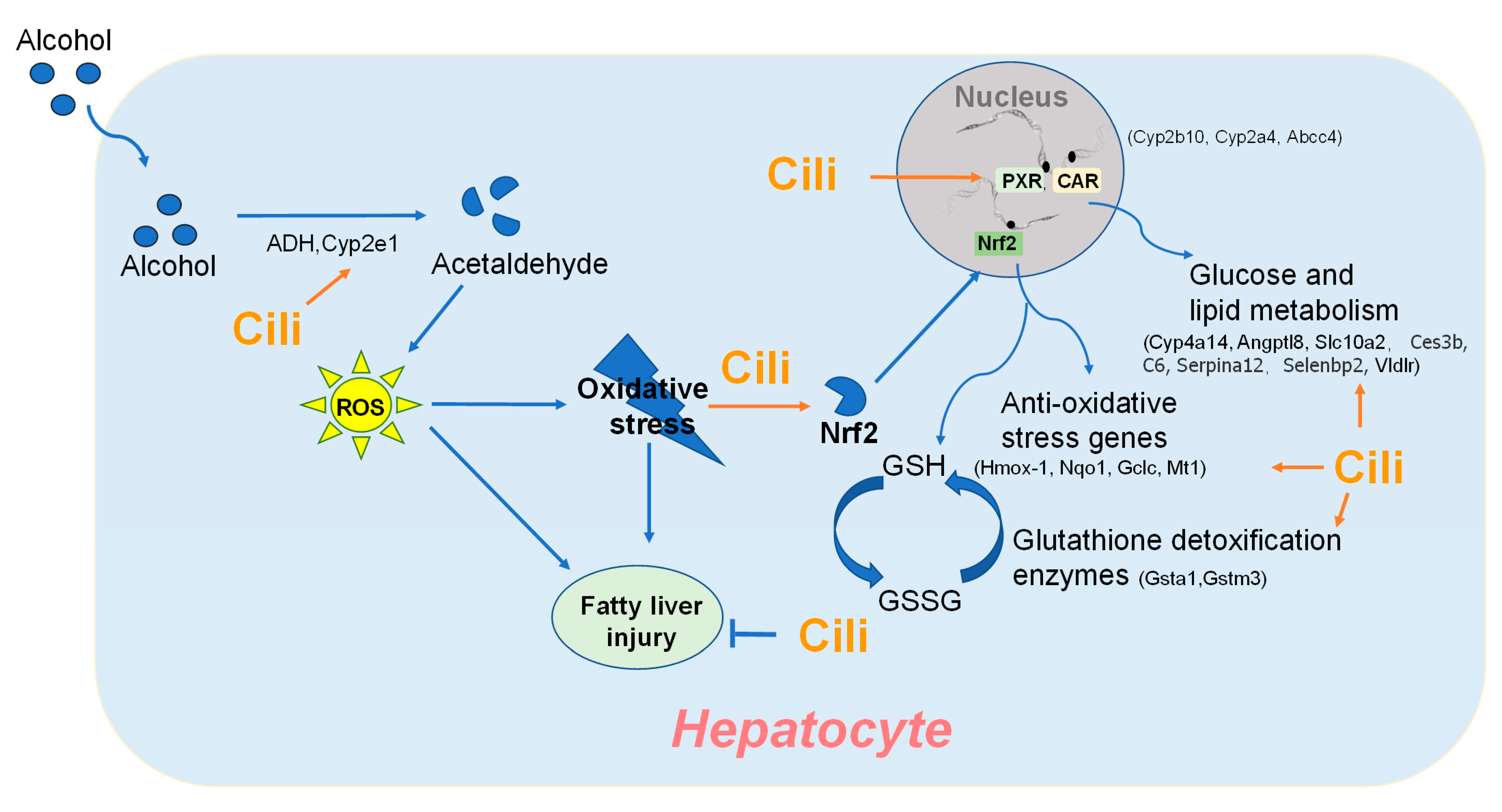
4.1. Cili Protected against Chronic Alcohol Liver Injury
4.2. Cili Alleviated Chronic Alcohol-Induced Aberrant Gene Expression
4.3. Multiple Components in Cili Could Function in an Intergraded Manner to Achieve Beneficial Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Committee, Z.B.E. Zhonghua Bencao; Guizhou Science and Technology Press: Guiyang, China, 2005; pp. 334–335. [Google Scholar]
- Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; Chinese Medical Press: Beijing, China, 2020; Volume 1. [Google Scholar]
- Li, H.; Fang, W.; Wang, Z.; Chen, Y. Physicochemical, biological properties, and flavour profile of Rosa roxburghii Tratt, Pyracantha fortuneana, and Rosa laevigata Michx fruits: A comprehensive review. Food Chem. 2022, 366, 130509. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Lv, M.J.; An, J.Y.; Fan, X.H.; Dong, M.Z.; Zhang, S.D.; Wang, J.D.; Wang, Y.Q.; Cai, Z.H.; Fu, Y.J. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhang, Q.; Zhang, Y.H.; Lu, X.Y.; Fu, W.M.; He, J.Y. Chemical Analysis of Dietary Constituents in Rosa roxburghii and Rosa sterilis Fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef]
- Song, P.; Shen, X. Juice from Fructus Rosae Roxburghii normalizes blood lipids in mice with diet-induced hyperlipidemia. Food Sci. Nutr. 2020, 8, 6069–6082. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Shen, X. Proteomic analysis of liver in diet-induced Hyperlipidemic mice under Fructus Rosa roxburghii action. J. Proteom. 2021, 230, 103982. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Han, S.C.; Wu, M.H. Beneficial Effects of Hydroalcoholic Extract from Rosa Roxburghii Tratt Fruit on Hyperlipidemia in High-Fat-Fed Rats. Acta Cardiol. Sin. 2020, 36, 148–159. [Google Scholar] [CrossRef]
- Wang, S.; Xia, J.; De Paepe, K.; Zhang, B.; Fu, X.; Huang, Q.; Van de Wiele, T. Ultra-high Pressure Treatment Controls In Vitro Fecal Fermentation Rate of Insoluble Dietary Fiber from Rosa Roxburghii Tratt Pomace and Induces Butyrogenic Shifts in Microbiota Composition. J. Agric. Food Chem. 2021, 69, 10638–10647. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic db/db Mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, C.; Zeng, Q.; Yao, M.; Chen, X.; Zhang, A. Assessing the potential value of Rosa Roxburghii Tratt in arsenic-induced liver damage based on elemental imbalance and oxidative damage. Environ. Geochem. Health 2021, 43, 1165–1175. [Google Scholar] [CrossRef]
- Axley, P.D.; Richardson, C.T.; Singal, A.K. Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease. Clin. Liver Dis. 2019, 23, 39–50. [Google Scholar] [CrossRef]
- Fuster, D.; Samet, J.H. Alcohol Use in Patients with Chronic Liver Disease. N. Engl. J. Med. 2018, 379, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.M.; Lu, Y. Alcoholic Liver Disease: From CYP2E1 to CYP2A5. Curr. Mol. Pharmacol. 2017, 10, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zheng, K.; Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. The Lieber-DeCarli Diet-A Flagship Model for Experimental Alcoholic Liver Disease. Alcohol Clin. Exp. Res. 2018, 42, 1828–1840. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.R.; Hsu, Y.W.; Pan, T.M.; Lee, C.L. Monascin and Ankaflavin of Monascus purpureus Prevent Alcoholic Liver Disease through Regulating AMPK-Mediated Lipid Metabolism and Enhancing Both Anti-Inflammatory and Anti-Oxidative Systems. Molecules 2021, 26, 6301. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Lin, H.H.; Hsu, C.C.; Chen, B.C.; Chen, J.H. Aqueous Extract of Pepino (Solanum muriactum Ait) Leaves Ameliorate Lipid Accumulation and Oxidative Stress in Alcoholic Fatty Liver Disease. Nutrients 2018, 10, 931. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lei, S.S.; Su, J.; Cai, X.M.; Xu, H.; He, X.; Chen, Y.H.; Lu, H.X.; Li, H.; Qian, L.Q.; et al. Alcohol Induces More Severe Fatty Liver Disease by Influencing Cholesterol Metabolism. Evid. Based Complementary Altern. Med. 2019, 2019, 7095684. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Yao, R.; Yasuoka, A.; Kamei, A.; Kitagawa, Y.; Tateishi, N.; Tsuruoka, N.; Kiso, Y.; Sueyoshi, T.; Negishi, M.; Misaka, T.; et al. Dietary flavonoids activate the constitutive androstane receptor (CAR). J. Agric. Food Chem. 2010, 58, 2168–2173. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Meng, Z.; Wang, X.; Zeng, S.; Huang, W. The nuclear receptor CAR modulates alcohol-induced liver injury. Lab. Investig. 2011, 91, 1136–1145. [Google Scholar] [CrossRef]
- Choi, S.; Neequaye, P.; French, S.W.; Gonzalez, F.J.; Gyamfi, M.A. Pregnane X receptor promotes ethanol-induced hepatosteatosis in mice. J. Biol. Chem. 2018, 293, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wei, P.; Zhang, J.; Dowhan, D.H.; Han, Y.; Moore, D.D. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharm. J. 2002, 2, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, G.; Nie, Y.; Han, S.; Li, J.; Zhong, X.B.; Zhang, L. Epigenetic Memory Is Involved in the Persistent Alterations of Drug-Processing Genes in Adult Mice due to PCN-Activated PXR during Early Life. Toxicol. Sci. 2019, 172, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Yasuoka, A.; Kamei, A.; Ushiama, S.; Kitagawa, Y.; Rogi, T.; Shibata, H.; Abe, K.; Misaka, T. Nuclear receptor-mediated alleviation of alcoholic fatty liver by polyphenols contained in alcoholic beverages. PLoS ONE 2014, 9, e87142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Wang, M.; Chen, W.S.; Su, H.; Wang, Y.T.; Wan, J.B. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef]
- Xiong, Z.E.; Dong, W.G.; Wang, B.Y.; Tong, Q.Y.; Li, Z.Y. Curcumin attenuates chronic ethanol-induced liver injury by inhibition of oxidative stress via mitogen-activated protein kinase/nuclear factor E2-related factor 2 pathway in mice. Pharmacogn. Mag. 2015, 11, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Zhang, J.; Yao, J.; Zhang, B.; Duan, W.; Zhao, C.; Du, P.; Song, J.; Zheng, Y.; Wang, M. Shanxi Aged Vinegar Protects against Alcohol-Induced Liver Injury via Activating Nrf2-Mediated Antioxidant and Inhibiting TLR4-Induced Inflammatory Response. Nutrients 2018, 10, 805. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dou, X.; Li, S.; Zhang, X.; Sun, X.; Zhou, Z.; Song, Z. Nuclear factor (erythroid-derived 2)-like 2 activation-induced hepatic very-low-density lipoprotein receptor overexpression in response to oxidative stress contributes to alcoholic liver disease in mice. Hepatology 2014, 59, 1381–1392. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Liu, X.; Wang, Y.; Ren, X.; Ma, J.; Song, R.; Wang, X.; Dong, Y.; Fan, Q.; Wei, J.; et al. Multi-omics integration reveals the hepatoprotective mechanisms of ursolic acid intake against chronic alcohol consumption. Eur. J. Nutr. 2021, 61, 115–126. [Google Scholar] [CrossRef]
- Brind, A.M.; Hurlstone, A.; Edrisinghe, D.; Gilmore, I.; Fisher, N.; Pirmohamed, M.; Fryer, A.A. The role of polymorphisms of glutathione S-transferases GSTM1, M3, P1, T1 and A1 in susceptibility to alcoholic liver disease. Alcohol Alcohol. 2004, 39, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Dong, H.; Thompson, D.C.; Shertzer, H.G.; Nebert, D.W.; Vasiliou, V. Glutathione defense mechanism in liver injury: Insights from animal models. Food Chem. Toxicol. 2013, 60, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Lei, P.; Zhao, W.; Pang, B.; Yang, X.; Li, B.L.; Ren, M.; Shan, Y.J. Broccoli Sprout Extract Alleviates Alcohol-Induced Oxidative Stress and Endoplasmic Reticulum Stress in C57BL/6 Mice. J. Agric. Food Chem. 2018, 66, 5574–5580. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, K.; Cederbaum, A.I.; Lu, Y. Suppressed hepatocyte proliferation via a ROS-HNE-P21 pathway is associated with nicotine- and cotinine-enhanced alcoholic fatty liver in mice. Biochem. Biophys. Res. Commun. 2019, 512, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, X.; James Kang, Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp. Biol. Med. 2002, 227, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Smalling, R.V.; Huang, Y.; Jiang, Y.; Kusumanchi, P.; Bogaert, W.; Wang, L.; Delker, D.A.; Skill, N.J.; Han, S.; et al. The role of SHP/REV-ERBα/CYP4A axis in the pathogenesis of alcohol-associated liver disease. JCI Insight 2021, 6, e140687. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Weymouth, N.; Shi, Z. Smooth muscle α actin (Acta2) and myofibroblast function during hepatic wound healing. PLoS ONE 2013, 8, e77166. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Peng, D. ANGPTL8: An Important Regulator in Metabolic Disorders. Front. Endocrinol. 2018, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- van de Peppel, I.P.; Rao, A.; Dommerholt, M.B.; Bongiovanni, L.; Thomas, R.; de Bruin, A.; Karpen, S.J.; Dawson, P.A.; Verkade, H.J.; Jonker, J.W. The Beneficial Effects of Apical Sodium-Dependent Bile Acid Transporter Inactivation Depend on Dietary Fat Composition. Mol. Nutr. Food Res. 2020, 64, e2000750. [Google Scholar] [CrossRef]
- Ham, J.R.; Choi, R.Y.; Lee, Y.; Lee, M.K. Effects of Edible Insect Tenebrio molitor Larva Fermentation Extract as a Substitute Protein on Hepatosteatogenesis and Proteomic Changes in Obese Mice Induced by High-Fat Diet. Int. J. Mol. Sci. 2021, 22, 3615. [Google Scholar] [CrossRef]
- Arab, J.P.; Cabrera, D.; Sehrawat, T.S.; Jalan-Sakrikar, N.; Verma, V.K.; Simonetto, D.; Cao, S.; Yaqoob, U.; Leon, J.; Freire, M.; et al. Hepatic stellate cell activation promotes alcohol-induced steatohepatitis through Igfbp3 and SerpinA12. J. Hepatol. 2020, 73, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Bykov, I.L.; Väkevä, A.; Järveläinen, H.A.; Meri, S.; Lindros, K.O. Protective function of complement against alcohol-induced rat liver damage. Int. Immunopharmacol. 2004, 4, 1445–1454. [Google Scholar] [CrossRef]
- Song, Y.; Kurose, A.; Li, R.; Takeda, T.; Onomura, Y.; Koga, T.; Mutoh, J.; Ishida, T.; Tanaka, Y.; Ishii, Y. Ablation of Selenbp1 Alters Lipid Metabolism via the Pparα Pathway in Mouse Kidney. Int. J. Mol. Sci. 2021, 22, 5334. [Google Scholar] [CrossRef] [PubMed]
- Dela Peña, I.J.; Yoon, S.Y.; de la Peña, J.B.; Park, S.; Yoon, B.; Kim, H.J.; Paek, S.H.; Seo, Y.K.; Moon, B.S.; Cheong, J.H. The ameliorating effect of Rosa roxburghii against ethanol-induced psychomotor alterations in rats. Am. J. Drug Alcohol Abus. 2014, 40, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Liu, M.; Pan, L.; He, L.; Guo, Y. Oxidative Stress and TGF-β1/Smads Signaling Are Involved in Rosa roxburghii Fruit Extract Alleviating Renal Fibrosis. Evid. Based Complementary Altern. Med. 2019, 2019, 4946580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Kan, J. Ultrasound-assisted extraction, characterization, and antioxidant activity in vitro and in vivo of polysaccharides from Chestnut rose (Rosa roxburghii tratt) fruit. J. Food Sci. Technol. 2018, 55, 1083–1092. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Li, C.; Xu, F.; Chen, J. A polysaccharide from Rosa roxburghii Tratt fruit attenuates high-fat diet-induced intestinal barrier dysfunction and inflammation in mice by modulating the gut microbiota. Food Funct. 2021, 13, 530–547. [Google Scholar] [CrossRef]
- Huang, D.; Li, C.; Chen, Q.; Xie, X.; Fu, X.; Chen, C.; Huang, Q.; Huang, Z.; Dong, H. Identification of polyphenols from Rosa roxburghii Tratt pomace and evaluation of in vitro and in vivo antioxidant activity. Food Chem. 2021, 377, 131922. [Google Scholar] [CrossRef]
- Xu, S.J.; Wang, X.; Wang, T.Y.; Lin, Z.Z.; Hu, Y.J.; Huang, Z.L.; Yang, X.J.; Xu, P. Flavonoids from Rosa roxburghii Tratt prevent reactive oxygen species-mediated DNA damage in thymus cells both combined with and without PARP-1 expression after exposure to radiation in vivo. Aging 2020, 12, 16368–16389. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Huang, X.-Y.; Zhou, N.; Wu, Q.; Liu, J.; Shi, J.-S. RNA-Seq Analysis of Protection against Chronic Alcohol Liver Injury by Rosa roxburghii Fruit Juice (Cili) in Mice. Nutrients 2022, 14, 1974. https://doi.org/10.3390/nu14091974
Yang S, Huang X-Y, Zhou N, Wu Q, Liu J, Shi J-S. RNA-Seq Analysis of Protection against Chronic Alcohol Liver Injury by Rosa roxburghii Fruit Juice (Cili) in Mice. Nutrients. 2022; 14(9):1974. https://doi.org/10.3390/nu14091974
Chicago/Turabian StyleYang, Shan, Xian-Yu Huang, Nian Zhou, Qin Wu, Jie Liu, and Jing-Shan Shi. 2022. "RNA-Seq Analysis of Protection against Chronic Alcohol Liver Injury by Rosa roxburghii Fruit Juice (Cili) in Mice" Nutrients 14, no. 9: 1974. https://doi.org/10.3390/nu14091974