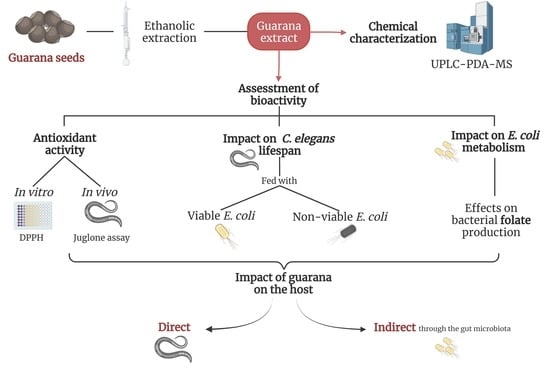
Alterations in Bacterial Metabolism Contribute to the Lifespan Extension Exerted by Guarana in Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. UPLC-PDA-MS Analyses
2.3. Strains and Culture Conditions
2.4. Antioxidant Activity In Vitro: DPPH Method
2.5. Stress-Resistance Assays
2.6. Measurement of Catalase (CAT) and Superoxide Dismutase (SOD) Activity
2.7. Quantification of Total Glutathione in C. elegans
2.8. Lifespan Analysis
2.9. Impact of Guarana on E. coli Viability
2.10. E. coli Folate Extraction
2.11. Folate Liquid Chromatography -Tandem Mass Spectrometry (LC-MS/MS)Analysis
2.12. Statistical Analysis
3. Results
3.1. Chemical Characterization of the Guarana Extract
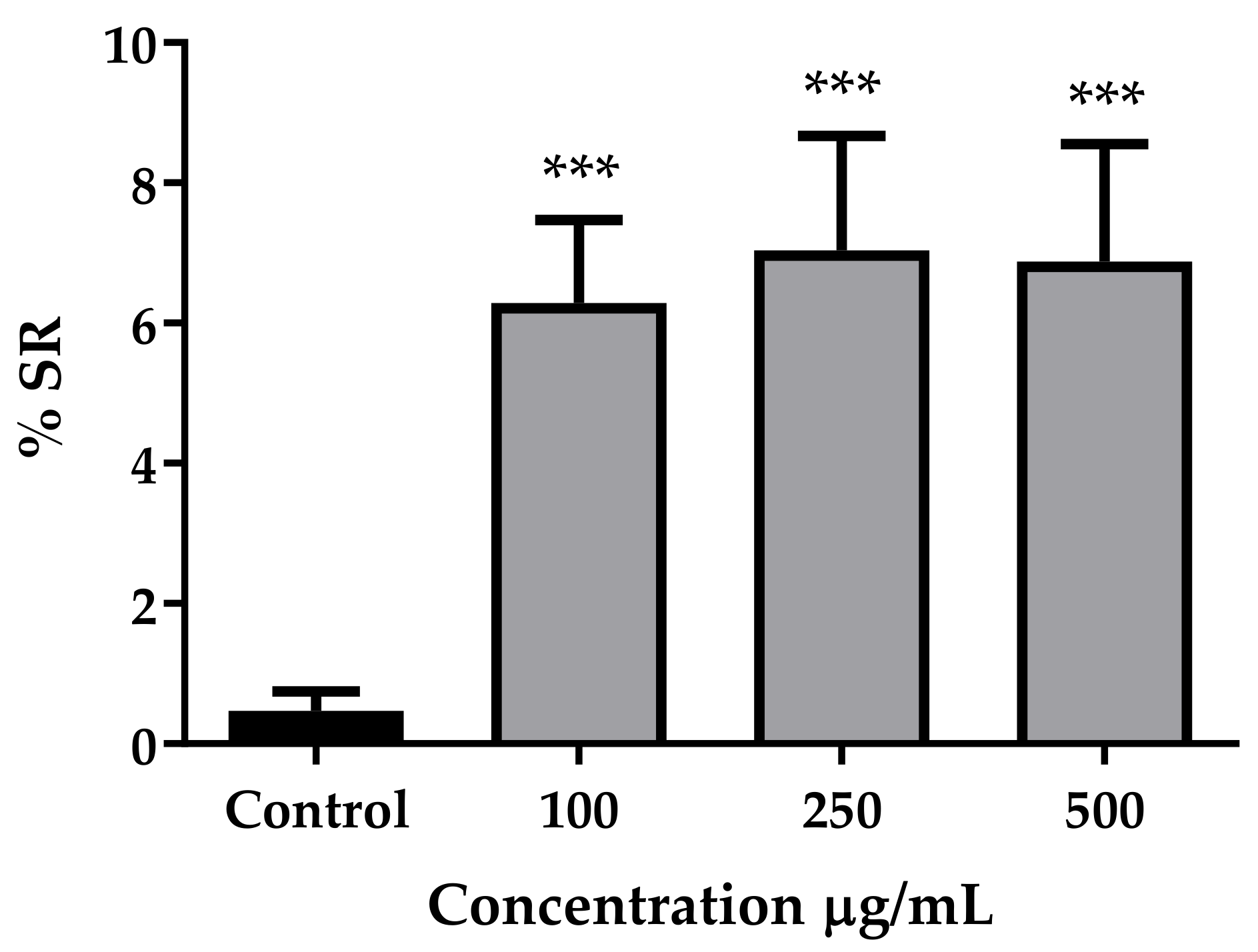
3.2. Antioxidant Activity In Vitro and In Vivo
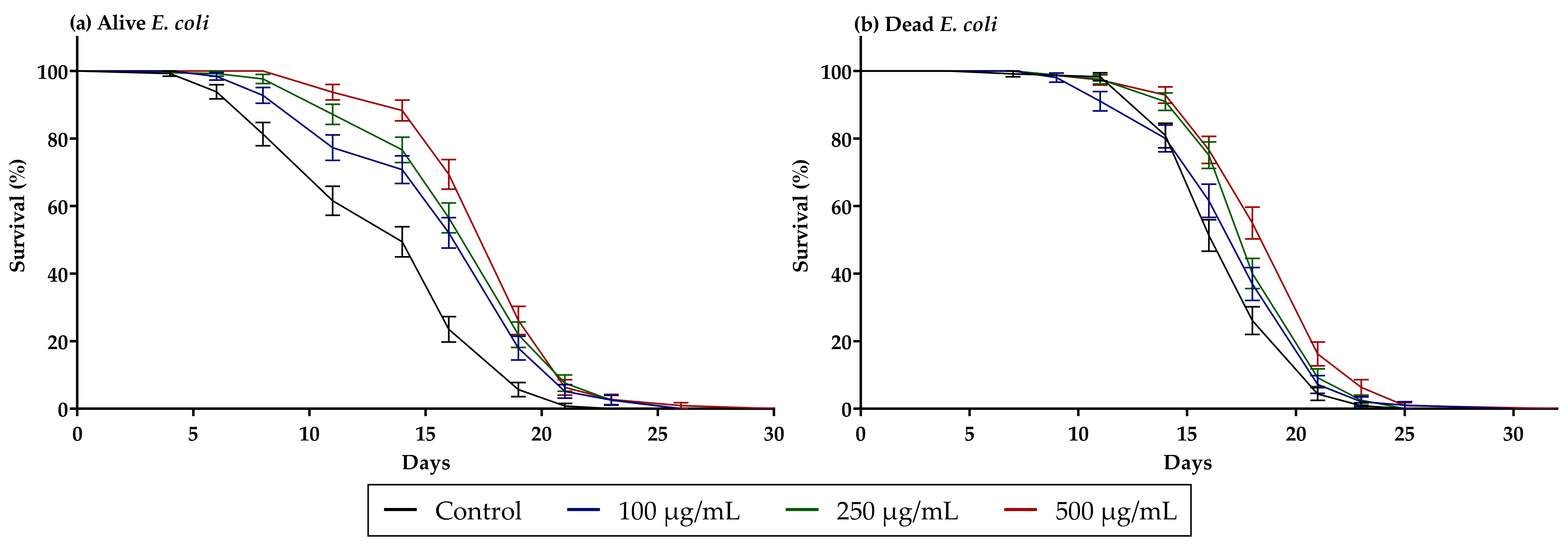
3.3. Guarana Extract Increases C. elegans Lifespan
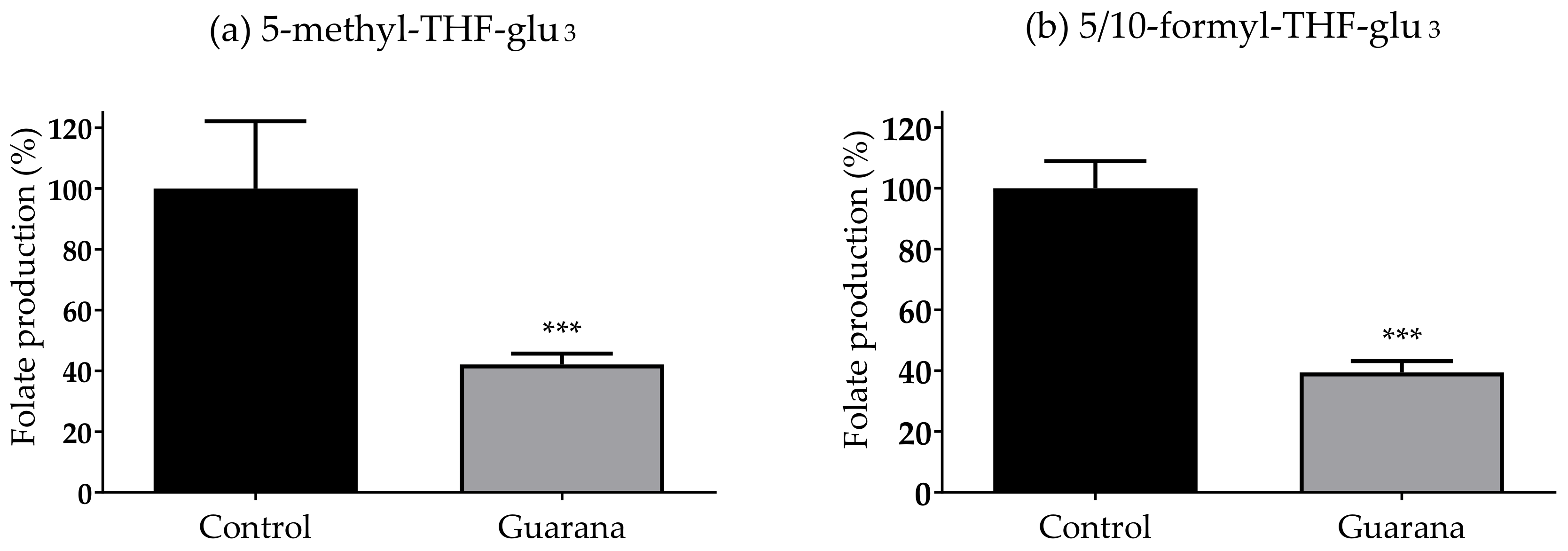
3.4. Guarana Extract Reduces the Folate Production of E. coli
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, N.; Atroch, A.L. Guaraná’s Journey from Regional Tonic to Aphrodisiac and Global Energy Drink. Evid.-Based Complement. Altern. Med. 2010, 7, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, M.; Kim, H.A.; Oketch-Rabah, H.; Marles, R.J.; Roe, A.L.; Calderón, A.I. Safety of Guarana Seed as a Dietary Ingredient: A Review. J. Agric. Food Chem. 2019, 67, 11281–11287. [Google Scholar] [CrossRef]
- Kola, P.; Metowogo, K.; Manjula, S.N.; Katawa, G.; Elkhenany, H.; Mruthunjaya, K.M.; Eklu-Gadegbeku, K.; Aklikokou, K.A. Ethnopharmacological evaluation of antioxidant, anti-angiogenic, and anti-inflammatory activity of some traditional medicinal plants used for treatment of cancer in Togo/Africa. J. Ethnopharmacol. 2022, 283, 114673. [Google Scholar] [CrossRef] [PubMed]
- Ruchel, J.B.; Bernardes, V.M.; Braun, J.B.S.; Manzoni, A.G.; Passos, D.F.; Castilhos, L.G.; Abdalla, F.H.; de Oliveira, J.S.; de Andrade, C.M.; Casali, E.A.; et al. Lipotoxicity-associated inflammation is prevented by guarana (Paullinia cupana) in a model of hyperlipidemia. Drug Chem. Toxicol. 2021, 44, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Veloso, C.F.; Machado, A.K.; Cadoná, F.C.; Azzolin, V.F.; Cruz, I.B.M.; Silveira, A.F. Neuroprotective Effects of Guarana (Paullinia cupana Mart.) against Vincristine in Vitro Exposure. J. Prev. Alzheimers Dis. 2018, 5, 65–70. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, D.P.; Pereira, P.; Fontes, A.J.C.; Marques, K.D.S.; de Moraes, É.B.; Guerra, R.N.M.; Garcia, J.B.S. The use of guarana (Paullinia cupana) as a dietary supplement for fatigue in cancer patients: A systematic review with a meta-analysis. Support. Care Cancer 2021, 29, 7171–7182. [Google Scholar] [CrossRef]
- Ruchel, J.B.; Rezer, J.F.; Thorstenberg, M.L.; Dos Santos, C.B.; Cabral, F.L.; Lopes, S.T.; da Silva, C.B.; Machado, A.K.; da Cruz, I.B.; Schetinger, M.R.; et al. Hypercholesterolemia and Ecto-enzymes of Purinergic System: Effects of Paullinia cupana. Phytother. Res. 2016, 30, 49–57. [Google Scholar] [CrossRef]
- Torres, E.; Pinaffi-Langley, A.; Figueira, M.S.; Cordeiro, K.S.; Negrão, L.D.; Soares, M.J.; da Silva, C.P.; Alfino, M.C.Z.; Sampaio, G.R.; de Camargo, A.C. Effects of the consumption of guarana on human health: A narrative review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 272–295. [Google Scholar] [CrossRef]
- Krewer Cda, C.; Ribeiro, E.E.; Ribeiro, E.A.; Moresco, R.N.; da Rocha, M.I.; Montagner, G.F.; Machado, M.M.; Viegas, K.; Brito, E.; da Cruz, I.B. Habitual intake of guaraná and metabolic morbidities: An epidemiological study of an elderly Amazonian population. Phytother. Res. 2011, 25, 1367–1374. [Google Scholar] [CrossRef]
- Yilmaz, L.S.; Walhout, A.J. Worms, bacteria, and micronutrients: An elegant model of our diet. Trends Genet. 2014, 30, 496–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, C.; Weinkove, D. The Gut Microbiota and Ageing. Sub-Cell. Biochem. 2018, 90, 351–371. [Google Scholar] [CrossRef]
- Camilo, E.; Zimmerman, J.; Mason, J.B.; Golner, B.; Russell, R.; Selhub, J.; Rosenberg, I.H. Folate synthesized by bacteria in the human upper small intestine is assimilated by the host. Gastroenterology 1996, 110, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Dukowicz, A.C.; Lacy, B.E.; Levine, G.M. Small intestinal bacterial overgrowth: A comprehensive review. Gastroenterol. Hepatol. 2007, 3, 112–122. [Google Scholar]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Maynard, C.; Weinkove, D. Bacteria increase host micronutrient availability: Mechanisms revealed by studies in C. elegans. Genes Nutr. 2020, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Papaevgeniou, N.; Chondrogianni, N. Anti-aging and Anti-aggregation Properties of Polyphenolic Compounds in C. elegans. Curr. Pharm. Des. 2018, 24, 2107–2120. [Google Scholar] [CrossRef]
- Arantes, L.P.; Machado, M.L.; Zamberlan, D.C.; da Silveira, T.L.; da Silva, T.C.; da Cruz, I.B.M.; Ribeiro, E.E.; Aschner, M.; Soares, F.A.A. Mechanisms involved in anti-aging effects of guarana (Paullinia cupana) in Caenorhabditis elegans. Braz. J. Med. Biol. Res. 2018, 51, e7552. [Google Scholar] [CrossRef]
- Boasquívis, P.F.; Silva, G.M.M.; Paiva, F.A.; Cavalcanti, R.M.; Nunez, C.V.; de Paula Oliveira, R. Guarana (Paullinia cupana) Extract Protects Caenorhabditis elegans Models for Alzheimer Disease and Huntington Disease through Activation of Antioxidant and Protein Degradation Pathways. Oxid. Med. Cell Longev. 2018, 2018, 9241308. [Google Scholar] [CrossRef] [Green Version]
- Zamberlan, D.C.; Arantes, L.P.; Machado, M.L.; da Silveira, T.L.; da Silva, A.F.; da Cruz, I.B.M.; Figueiredo, C.P.; Soares, F.A.A. Guarana (Paullinia cupana Mart.) protects against amyloid-β toxicity in Caenorhabditis elegans through heat shock protein response activation. Nutr. Neurosci. 2020, 23, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Martinez-Martinez, D.; Cabreiro, F. C. elegans: A biosensor for host-microbe interactions. Lab Anim. 2021, 50, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Flavell, S.W. Host-microbe interactions and the behavior of Caenorhabditis elegans. J. Neurogenet. 2020, 34, 500–509. [Google Scholar] [CrossRef]
- Virk, B.; Jia, J.; Maynard, C.A.; Raimundo, A.; Lefebvre, J.; Richards, S.A.; Chetina, N.; Liang, Y.; Helliwell, N.; Cipinska, M.; et al. Folate Acts in E. coli to Accelerate C. elegans Aging Independently of Bacterial Biosynthesis. Cell Rep. 2016, 14, 1611–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabreiro, F.; Au, C.; Leung, K.Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, L.; Yanez, K.; Vivanco, M.; Melo, R.; Cotoras, M. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Ind. Crops Prod. 2013, 43, 360–364. [Google Scholar] [CrossRef]
- Virk, B.; Correia, G.; Dixon, D.P.; Feyst, I.; Jia, J.; Oberleitner, N.; Briggs, Z.; Hodge, E.; Edwards, R.; Ward, J.; et al. Excessive folate synthesis limits lifespan in the C. elegans: E. coli aging model. BMC Biol. 2012, 10, 67. [Google Scholar] [CrossRef] [Green Version]
- Lopez, V.; Akerreta, S.; Casanova, E.; Garcia-Mina, J.M.; Cavero, R.Y.; Calvo, M.I. In vitro antioxidant and anti-rhizopus activities of lamiaceae herbal extracts. Plant Food Hum. Nutr. 2007, 62, 151–155. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Reigada, I.; Moliner, C.; Valero, M.S.; Weinkove, D.; Langa, E.; Gomez Rincon, C. Antioxidant and Antiaging Effects of Licorice on the Caenorhabditis elegans Model. J. Med. Food 2019, 23, 1. [Google Scholar] [CrossRef]
- Jenkins, N.L.; James, S.A.; Salim, A.; Sumardy, F.; Speed, T.P.; Conrad, M.; Richardson, D.R.; Bush, A.I.; McColl, G. Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans. eLife 2020, 9, e56580. [Google Scholar] [CrossRef] [PubMed]
- Kortesoja, M.; Karhu, E.; Olafsdottir, E.S.; Freysdottir, J.; Hanski, L. Impact of dibenzocyclooctadiene lignans from Schisandra chinensis on the redox status and activation of human innate immune system cells. Free Radic. Biol. Med. 2019, 131, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Reigada, I.; Perez-Tanoira, R.; Patel, J.Z.; Savijoki, K.; Yli-Kauhaluoma, J.; Kinnari, T.J.; Fallarero, A. Strategies to Prevent Biofilm Infections on Biomaterials: Effect of Novel Naturally-Derived Biofilm Inhibitors on a Competitive Colonization Model of Titanium by Staphylococcus aureus and SaOS-2 Cells. Microorganisms 2020, 8, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, C.; Cummins, I.; Green, J.; Weinkove, D. A bacterial route for folic acid supplementation. BMC Biol. 2018, 16, 67. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Kwon, Y.K.; Rabinowitz, J.D. Isotope ratio-based profiling of microbial folates. J. Am. Soc. Mass Spectrom. 2007, 18, 898–909. [Google Scholar] [CrossRef] [Green Version]
- Ushirobira, T.M.A.; Yamaguti, E.; Uemura, L.M.; Nakamura, C.V.; Dias, B.P.; de Mello, J.C.P. Chemical and Microbiological Study of Extract from Seeds of Guaraná (Paullinia cupana var. sorbilis). Lat. Am. J. Pharm. 2007, 26, 5–9. [Google Scholar]
- Da Silva, G.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Brito, E.S.; Nascimento, M.M.; Zocolo, G.J.; Coutinho, J.P.; de Jesus, R.M. Chemical profiling of guarana seeds (Paullinia cupana) from different geographical origins using UPLC-QTOF-MS combined with chemometrics. Food Res. Int. 2017, 102, 700–709. [Google Scholar] [CrossRef]
- Silva, M.P.; Thomazini, M.; Holkem, A.T.; Pinho, L.S.; Genovese, M.I.; Fávaro-Trindade, C.S. Production and characterization of solid lipid microparticles loaded with guaraná (Paullinia cupana) seed extract. Food Res. Int. 2019, 123, 144–152. [Google Scholar] [CrossRef]
- Schimpl, F.C.; Kiyota, E.; Mazzafera, P. Production of Recombinant Caffeine Synthase from Guarana (Paullinia cupana var. sorbilis) in Escherichia coli. Methods Mol. Biol. 2016, 1405, 49–57. [Google Scholar] [CrossRef]
- Yamaguti-Sasaki, E.; Ito, L.A.; Canteli, V.C.; Ushirobira, T.M.; Ueda-Nakamura, T.; Dias Filho, B.P.; Nakamura, C.V.; de Mello, J.C. Antioxidant capacity and in vitro prevention of dental plaque formation by extracts and condensed tannins of Paullinia cupana. Molecules 2007, 12, 1950–1963. [Google Scholar] [CrossRef] [Green Version]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Belló, C.; Prestes, A.P.; Schemberger, J.A.; Hacke, A.C.M.; Pereira, R.P.; Manente, F.A.; Carlos, I.Z.; de Andrade, C.R.; Fernandes, D.; da Cruz, I.B.M.; et al. Aqueous extract of Paullinia cupana attenuates renal and hematological effects associated with ketoprofen. J. Food Biochem. 2021, 45, e13560. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.N.; Paula Barbosa, A.; de Freitas, A.A.; Alvarenga, L.F.; Pádua, R.M.; Gomes Faraco, A.A.; Braga, F.C.; Vianna-Soares, C.D.; Castilho, R.O. TNF-α inhibition, antioxidant effects and chemical analysis of extracts and fraction from Brazilian guaraná seed powder. Food Chem. 2021, 355, 129563. [Google Scholar] [CrossRef] [PubMed]
- Mattei, R.; Dias, R.F.; Espínola, E.B.; Carlini, E.A.; Barros, S.B. Guarana (Paullinia cupana): Toxic behavioral effects in laboratory animals and antioxidants activity in vitro. J. Ethnopharmacol. 1998, 60, 111–116. [Google Scholar] [CrossRef]
- Sereia, A.L.; de Oliveira, M.T.; Baranoski, A.; Marques, L.L.M.; Ribeiro, F.M.; Isolani, R.G.; de Medeiros, D.C.; Chierrito, D.; Lazarin-Bidóia, D.; Zielinski, A.A.F.; et al. In vitro evaluation of the protective effects of plant extracts against amyloid-beta peptide-induced toxicity in human neuroblastoma SH-SY5Y cells. PLoS ONE 2019, 14, e0212089. [Google Scholar] [CrossRef]
- Hamerski, L.; Somner, G.V.; Tamaio, N.J. Paullinia cupana Kunth (Sapindaceae): A review of its ethnopharmacology, phytochemistry and pharmacology. J. Med. Plants Res. 2013, 7, 2221–2229. [Google Scholar]
- Lee, C.Y.; Nanah, C.N.; Held, R.A.; Clark, A.R.; Huynh, U.G.T.; Maraskine, M.C.; Uzarski, R.L.; McCracken, J.; Sharma, A. Effect of electron donating groups on polyphenol-based antioxidant dendrimers. Biochimie 2015, 111, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, H.; Roxo, M.; Röhrig, T.; Richling, E.; Wang, X.; Wink, M. Anti-Aging and Antioxidant Potential of Paullinia cupana var. sorbilis: Findings in Caenorhabditis elegans Indicate a New Utilization for Roasted Seeds of Guarana. Medicines 2017, 4, 61. [Google Scholar] [CrossRef]
- Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Caernohabditis elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals. Molecules 2020, 25, 3194. [Google Scholar] [CrossRef]
- Zeidán-Chuliá, F.; Gelain, D.P.; Kolling, E.A.; Rybarczyk-Filho, J.L.; Ambrosi, P.; Terra, S.R.; Pires, A.S.; da Rocha, J.B.; Behr, G.A.; Moreira, J.C. Major components of energy drinks (caffeine, taurine, and guarana) exert cytotoxic effects on human neuronal SH-SY5Y cells by decreasing reactive oxygen species production. Oxid. Med. Cell Longev. 2013, 2013, 791795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesp, K.; Smant, G.; Kammenga, J.E. Caenorhabditis elegans DAF-16/FOXO transcription factor and its mammalian homologs associate with age-related disease. Exp. Gerontol. 2015, 72, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.; Suzuki, Y.J. Juglone in Oxidative Stress and Cell Signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, G.D.; Bridge, W.J. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox. Biol. 2019, 24, 101171. [Google Scholar] [CrossRef]
- Li, H.; Roxo, M.; Cheng, X.; Zhang, S.; Cheng, H.; Wink, M. Pro-oxidant and lifespan extension effects of caffeine and related methylxanthines in Caenorhabditis elegans. Food Chem. X 2019, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Jeffery, I.B. Microbiome-health interactions in older people. Cell. Mol. Life Sci. 2018, 75, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.E.; Bäumler, A.J. Dysbiosis in the inflamed intestine: Chance favors the prepared microbe. Gut Microbes 2014, 5, 71–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppercorn, M.A. Advances in drug therapy for inflammatory bowel disease. Ann. Intern. Med. 1990, 112, 50–60. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W., Jr.; García, F.A.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; Landefeld, C.S.; et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 183–189. [Google Scholar] [CrossRef]
- Cole, B.F.; Baron, J.A.; Sandler, R.S.; Haile, R.W.; Ahnen, D.J.; Bresalier, R.S.; McKeown-Eyssen, G.; Summers, R.W.; Rothstein, R.I.; Burke, C.A.; et al. Folic acid for the prevention of colorectal adenomas: A randomized clinical trial. JAMA 2007, 297, 2351–2359. [Google Scholar] [CrossRef]
- Milne, D.B.; Canfield, W.K.; Mahalko, J.R.; Sandstead, H.H. Effect of oral folic acid supplements on zinc, copper, and iron absorption and excretion. Am. J. Clin. Nutr. 1984, 39, 535–539. [Google Scholar] [CrossRef]
- Kleber Silveira, A.; Moresco, K.S.; Mautone Gomes, H.; da Silva Morrone, M.; Kich Grun, L.; Pens Gelain, D.; de Mattos Pereira, L.; Giongo, A.; Rodrigues De Oliveira, R.; Fonseca Moreira, J.C. Guarana (Paullinia cupana Mart.) alters gut microbiota and modulates redox status, partially via caffeine in Wistar rats. Phytother. Res. 2018, 32, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
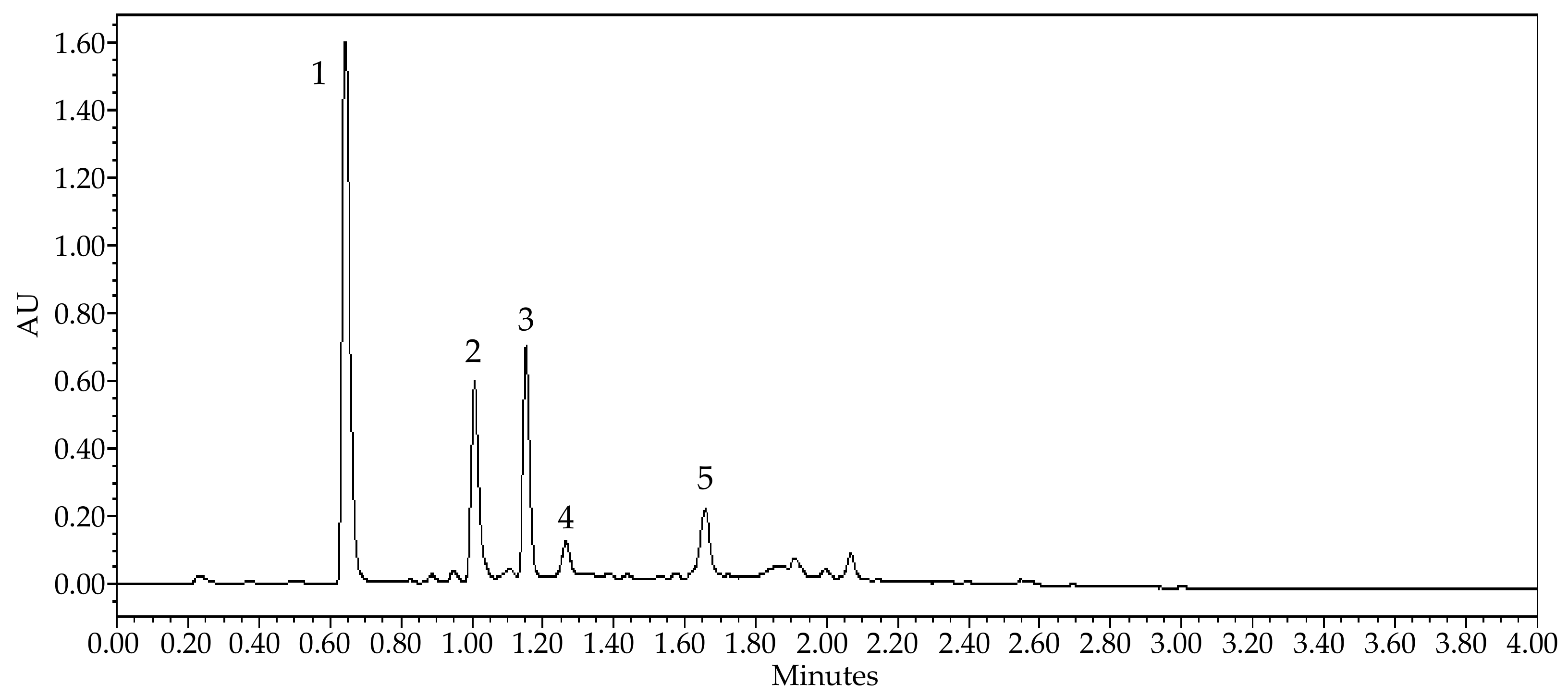
| Peak No. | Retention Time (min) | [M − H]− | [M + H]+ | Product Ions | Identified Compounds | |
|---|---|---|---|---|---|---|
| [M − H]− | [M + H]+ | |||||
| 1 | 0.64 | 195.18 | Caffeine | |||
| 2 | 1.00 | 289.02 | 245.00; 203.07; 587.16; 449.06 | Catechin | ||
| 3 | 1.15 | 289.02 | 203.04; 245.00; 220.95; 587.06; 449.01 | Epicatechin | ||
| 4 | 1.26 | 575.06 | 289.03; 284.89; 449.18; 539.11; 557.03; 575.88 | A-type procyanidin dimer | ||
| 5 | 1.65 | 574.96 | 289.00; 575.68; 285.08; 449.00; 423.06; 407.38 | A-type procyanidin dimer | ||
| Sample | EC50 (µg/mL) |
|---|---|
| Guarana extract | 4.696 to 8.747 |
| Epicatechin | 9.320 to 13.780 |
| Ascorbic acid | 1.719 to 2.021 |
| Average Lifespan (Days) | ||
|---|---|---|
| Sample | Alive E. coli | Dead E. coli |
| Control | 13.90 ± 4.40 | 17.44 ± 2.90 |
| Guarana 100 µg/mL | 16.49 ± 4.04 | 17.84 ± 3.70 |
| Guarana 250 µg/mL | 17.47 ± 3.37 | 18.68 ± 2.86 |
| Guarana 500 µg/mL | 18.42 ± 3.19 | 19.47 ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reigada, I.; Kapp, K.; Maynard, C.; Weinkove, D.; Valero, M.S.; Langa, E.; Hanski, L.; Gómez-Rincón, C. Alterations in Bacterial Metabolism Contribute to the Lifespan Extension Exerted by Guarana in Caenorhabditis elegans. Nutrients 2022, 14, 1986. https://doi.org/10.3390/nu14091986
Reigada I, Kapp K, Maynard C, Weinkove D, Valero MS, Langa E, Hanski L, Gómez-Rincón C. Alterations in Bacterial Metabolism Contribute to the Lifespan Extension Exerted by Guarana in Caenorhabditis elegans. Nutrients. 2022; 14(9):1986. https://doi.org/10.3390/nu14091986
Chicago/Turabian StyleReigada, Inés, Karmen Kapp, Claire Maynard, David Weinkove, Marta Sofía Valero, Elisa Langa, Leena Hanski, and Carlota Gómez-Rincón. 2022. "Alterations in Bacterial Metabolism Contribute to the Lifespan Extension Exerted by Guarana in Caenorhabditis elegans" Nutrients 14, no. 9: 1986. https://doi.org/10.3390/nu14091986