The Effectiveness of a Combined Healthy Eating, Physical Activity, and Sleep Hygiene Lifestyle Intervention on Health and Fitness of Overweight Airline Pilots: A Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
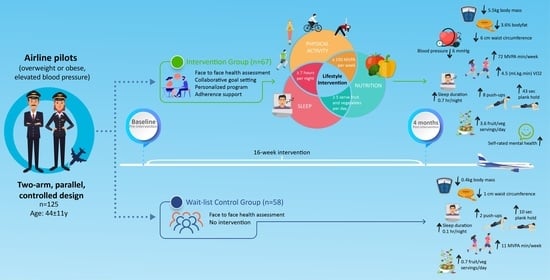
2.1. Design
2.2. Participants
2.3. Intervention
2.4. Outcome Measures
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
3.2. Intervention Adherence
3.3. Body Mass, Skinfolds, Waist Girth, Bodyfat Percentage, Blood Pressure and Pulse
3.4. VO2max, Pushups and Plank Hold
3.5. Health Behaviors and Self-Rated Health
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- 1.
- Emphasize whole foods
- 2.
- Reduce sugar where possible
- 3.
- Eat a rainbow of foods
- 4.
- Reduce white
- 5.
- Eat lean protein with each meal
- 6.
- Caution with your portions
- 7.
- Eat slowly and mindfully
- 8.
- Think about your drinks
- 9.
- Choose good fats
- 10.
- Setup your healthy environment
Appendix B
| Sleep Hygiene Strategies for Enhancing Sleep | YES Achieving | NOT Achieving |
|---|---|---|
| 1. Sleep at least 7 h | ||
| 2. Sleep routine or depower hour | ||
| 3. Regular sleep and wake time | ||
| 4. Dim lights near bedtime and turn off electronics >30 min before bed | ||
| 5. Avoid sleep disruptors 4–6 h before bed e.g., caffeine, large meals, alcohol | ||
| 6. Have a dark, cool, quiet sleep environment | ||
| 7. Exercise every day, not too close to bedtime | ||
| 8. Use the bedroom only for sleeping and intimacy | ||
| 9. Do a brain dump on paper before bed | ||
| 10. Early morning light exposure |
References
- Wilson, D.; Driller, M.; Johnston, B.; Gill, N. The Prevalence of Cardiometabolic Health Risk Factors among Airline Pilots: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 4848. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Driller, M.; Winwood, P.; Johnston, B.; Gill, N. The Effects of a Brief Lifestyle Intervention on the Health of Overweight Airline Pilots during COVID-19: A 12-Month Follow-Up Study. Nutrients 2021, 13, 4288. [Google Scholar] [CrossRef] [PubMed]
- Van Drongelen, A.; Boot, C.R.; Hlobil, H.; Twisk, J.W.; Smid, T.; van der Beek, A.J. Evaluation of an mHealth intervention aiming to improve health-related behavior and sleep and reduce fatigue among airline pilots. Scand. J. Work. Environ. Health 2014, 40, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, J.J.; Barrientos-Gutiérrez, T.; Corvalan, C.; Hyder, A.A.; Lazo-Porras, M.; Oni, T.; Wells, J.C.K. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat. Med. 2019, 25, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.; Bell, R. Social determinants and non-communicable diseases: Time for integrated action. BMJ 2019, 364, l251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasserre, A.M.; Strippoli, M.P.; Glaus, J.; Gholam-Rezaee, M.; Vandeleur, C.L.; Castelao, E.; Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Preisig, M. Prospective associations of depression subtypes with cardio-metabolic risk factors in the general population. Mol. Psychiatry 2017, 22, 1026–1034. [Google Scholar] [CrossRef] [Green Version]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab.—Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.; Driller, M.; Johnston, B.; Gill, N. The prevalence and distribution of health risk factors in airline pilots: A cross-sectional comparison with the general population. Aust. N. Z. J. Public Health 2022. [Google Scholar] [CrossRef]
- Klatt, M.D.; Sieck, C.; Gascon, G.; Malarkey, W.; Huerta, T. A healthcare utilization cost comparison between employees receiving a worksite mindfulness or a diet/exercise lifestyle intervention to matched controls 5 years post intervention. Complementary Ther. Med. 2016, 27, 139–144. [Google Scholar] [CrossRef]
- International Civil Aviation Authority. Manual of Civil Aviation Medicine, 3rd ed.; International Civil Aviation Authority: Montreal, QC, Canada, 2012; p. 580. [Google Scholar]
- Keyes, C.L.M.; Grzywacz, J.G. Health as a Complete State: The Added Value in Work Performance and Healthcare Costs. J. Occup. Environ. Med. 2005, 47, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.; Driller, M.; Johnston, B.; Gill, N. The effectiveness of a 17-week lifestyle intervention on health behaviors among airline pilots during COVID-19. J. Sport Health Sci. 2021, 10, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Jamnik, V.; Bredin, S.S.; Shephard, R.J.; Gledhill, N. The 2020 Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+): 2020 PAR-Q+. Health Fit. J. Can. 2019, 12, 58–61. [Google Scholar]
- Beeken, R.J.; Croker, H.; Morris, S.; Leurent, B.; Omar, R.; Nazareth, I.; Wardle, J. Study protocol for the 10 Top Tips (10TT) Trial: Randomised controlled trial of habit-based advice for weight control in general practice. BMC Public Health 2012, 12, 667. [Google Scholar] [CrossRef] [Green Version]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barisic, A.; Leatherdale, S.T.; Kreiger, N. Importance of Frequency, Intensity, Time and Type (FITT) in physical activity assessment for epidemiological research. Can. J. Public Health 2011, 102, 174–175. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Esparza-Ros, F.; Vaquero-Cristobal, R.; Marfell-Jones, M. International Standards for Anthropometric Assessment (2019); The International Society for the Advancement of Kinanthropometry: Murcia, Spain, 2019. [Google Scholar]
- Davidson, L.E.; Wang, J.; Thornton, J.C.; Kaleem, Z.; Silva-Palacios, F.; Pierson, R.N.; Heymsfield, S.B.; Gallagher, D. Predicting Fat Percent by Skinfolds in Racial Groups: Durnin and Womersley Revisited. Med. Sci. Sports Exerc. 2011, 43, 542. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Clemons, J. Construct Validity of Two Different Methods of Scoring and Performing Push-ups. J. Strength Cond. Res. 2019, 33, 2971–2980. [Google Scholar] [CrossRef]
- Tong, T.K.; Wu, S.; Nie, J. Sport-specific endurance plank test for evaluation of global core muscle function. Phys. Ther. Sport 2014, 15, 58–63. [Google Scholar] [CrossRef]
- Hanson, N.J.; Scheadler, C.M.; Katsavelis, D.; Miller, M.G. Validity of the Wattbike 3-Minute Aerobic Test: Measurement and Estimation of VO2max. J. Strength Cond. Res. 2022, 36, 400–404. [Google Scholar] [CrossRef]
- Storer, T.W.; Davis, J.A.; Caiozzo, V.J. Accurate prediction of VO2max in cycle ergometry. Med. Sci. Sports Exerc. 1990, 22, 704–712. [Google Scholar] [CrossRef]
- Atkinson, G.; Nevill, A.M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, J.E.; Keller, S.D.; Kosinski, M. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales; Health Institute, New England Medical Center: Boston, MA, USA, 1995. [Google Scholar]
- Ministry of Health. Methodology Report 2017/18: New Zealand Health Survey. 2019. Available online: https://www.health.govt.nz/publication/methodology-report-2017-18-new-zealand-health-survey (accessed on 1 January 2020).
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise Capacity and Mortality among Men Referred for Exercise Testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Egbewale, B.E.; Lewis, M.; Sim, J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: A simulation study. BMC Med. Res. Methodol. 2014, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Taylor & Francis Group: Florence, Italy, 1988. [Google Scholar]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. Appropriate Physical Activity Intervention Strategies for Weight Loss and Prevention of Weight Regain for Adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Nooyens, A.C.J.; Koppes, L.L.; Visscher, T.L.; Twisk, J.W.; Kemper, H.C.; Schuit, A.J.; van Mechelen, W.; Seidell, J.C. Adolescent skinfold thickness is a better predictor of high body fatness in adults than is body mass index: The Amsterdam Growth and Health Longitudinal Study. Am. J. Clin. Nutr. 2007, 85, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Lemes, Í.R.; Turi-Lynch, B.C.; Cavero-Redondo, I.; Linares, S.N.; Monteiro, H.L. Aerobic training reduces blood pressure and waist circumference and increases HDL-c in metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. J. Am. Soc. Hypertens. 2018, 12, 580–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.; Gibson, C.A.; Tran, Z.V.; Osness, W.H. Controlled Endurance Exercise Training and VO2max Changes in Older Adults: A Meta-Analysis. Prev. Cardiol. 2005, 8, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Duffecy, J.; Reutrakul, S.; Levenson, J.C.; McFarland, M.M.; Lee, S.; Qeadan, F. Behavioral interventions to extend sleep duration: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 60, 101532. [Google Scholar] [CrossRef] [PubMed]
- Kettle, V.E.; Madigan, C.D.; Coombe, A.; Graham, H.; Thomas, J.J.C.; Chalkley, A.E.; Daley, A.J. Effectiveness of physical activity interventions delivered or prompted by health professionals in primary care settings: Systematic review and meta-analysis of randomised controlled trials. BMJ 2022, 376, e068465. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Ravia, J. A Systematic Review of Behavioral Interventions to Promote Intake of Fruit and Vegetables. J. Am. Diet. Assoc. 2011, 111, 1523–1535. [Google Scholar] [CrossRef]
- Samdal, G.B.; Eide, G.E.; Barth, T.; Williams, G.; Meland, E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 42. [Google Scholar] [CrossRef] [Green Version]


| Parameters | All subjects (n = 125) | Intervention (n = 67) | Control (n = 58) |
|---|---|---|---|
| Sex (female/male) | 12/113 | 6/61 | 6/52 |
| Age (years) | 44.5 ± 10.7 | 43.7 ± 10.0 | 45.6 ± 11.4 |
| Short haul (n) | 60 | 34 | 26 |
| Long haul (n) | 65 | 33 | 32 |
| Height (cm) | 178.4 ± 7.4 | 179.2 ± 6.9 | 177.4 ± 7.8 |
| Systolic BP (mmHg) | 132.3 ± 5.6 | 133.3 ± 6.0 | 131.1 ± 4.9 * |
| Diastolic BP (mmHg) | 85.6 ± 3.8 | 86.0 ± 3.9 | 85.0 ± 3.6 |
| MAP (mmHg) | 101.1 ± 3.8 | 101.8 ± 3.8 | 100.4 ± 3.6 * |
| Pulse (bpm) | 66.9 ± 6.6 | 67.4 ± 6.1 | 66.4 ± 7.2 |
| Body mass (kg) | 90.5 ± 9.2 | 91.1 ± 8.0 | 89.8 ± 10.5 |
| BMI (kg/m2) | 28.4 ± 2.0 | 28.3 ± 1.7 | 28.5 ± 3.4 |
| Skinfold sum × 8 sites (mm) | 136.5 ± 24.1 | 138.3 ± 17.7 | 134.4 ± 29.9 |
| Bodyfat (%) | 24.3 ± 3.6 | 24.7 ± 3.2 | 23.9 ± 4.0 |
| Waist girth (cm) | 96.6 ± 7.6 | 97.8 ± 8.1 | 95.2 ± 6.8 |
| Waist to hip ratio | 0.93 ± 0.07 | 0.94 ± 0.07 | 0.93 ± 0.08 |
| VO2max (mL/kg/min) | 36.3 ± 5.4 | 35.6 ± 5.8 | 37.0 ± 4.8 |
| Push-ups (repetitions) | 17.2 ± 7.3 | 16.4 ± 6.8 | 18.1 ± 7.7 |
| Plank hold (s) | 79.7 ± 24.7 | 77.2 ± 25.5 | 82.5 ± 23.7 |
| Walking per week (min) | 73.8 ± 42.5 | 70.5 ± 32.2 | 77.7 ± 52.0 |
| MVPA per week (min) | 141.8 ± 41.1 | 138.0 ± 41.6 | 146.2 ± 40.3 |
| Fruit intake (serve/day) | 1.3 ± 0.7 | 1.5 ± 0.8 | 1.0 ± 0.6 |
| Vegetable intake (serve/day) | 2.0 ± 0.7 | 1.8 ± 0.7 | 2.4 ± 0.5 |
| F&V intake (serve/day) | 3.3 ± 0.7 | 3.3 ± 0.7 | 3.4 ± 0.7 |
| Sleep per day (h) | 7.0 ± 0.5 | 7.0 ± 0.4 | 7.0 ± 0.6 |
| Global PSQI (score) | 6.3 ± 2.1 | 6.4 ± 2.2 | 6.1 ± 1.9 |
| MCS-12 (score) | 48.9 ± 4.6 | 48.6 ± 5.8 | 49.3 ± 2.8 |
| PCS-12 (score) | 46.7 ± 3.4 | 46.3 ± 3.8 | 47.2 ± 2.8 |
| Intervention | Control | ANCOVA (Group Main Effects) | Between Group ES | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 67) | (n = 58) | ||||||||
| Time (Months) | M | SD | Follow Up Change (95% CI) | M | SD | Follow Up Change (95% CI) | p | d | |
| Body mass (kg) | 0 | 91.1 | 8.0 | 89.8 | 10.5 | 0.14, Trivial | |||
| 4 | 85.6 | 7.7 | 5.5 (4.8–6.1) | 89.4 | 85.6 | 0.4 (0.1–0.7) | <0.001 | −0.41, Small | |
| BMI (kg/m2) | 0 | 28.3 | 1.7 | 28.5 | 3.4 | 0.08, Trivial | |||
| 4 | 26.7 | 1.6 | 1.7 (1.5–1.9) | 28.4 | 2.4 | 0.1 (0.0–0.2) | <0.001 | −0.86, Moderate | |
| Systolic BP (mmHg) | 0 | 133.3 | 6.0 | 131.1 | 4.9 | 0.39, Small | |||
| 4 | 125.2 | 5.8 | 8.1 (7.3–8.9) | 132.5 | 5.9 | 1.3 (0.1–2.8) | <0.001 | −1.25, Large | |
| Diastolic BP (mmHg) | 0 | 86.0 | 3.9 | 85.0 | 3.6 | 0.27, Small | |||
| 4 | 80.8 | 5.4 | 5.2 (4.2–6.2) | 84.8 | 4.7 | 0.2 (0.9–1.4) | <0.001 | −0.77, Moderate | |
| MAP (mmHg) | 0 | 101.8 | 3.8 | 100.4 | 3.6 | 0.38, Small | |||
| 4 | 95.6 | 5.0 | 6.2 (5.4–6.9) | 100.7 | 4.7 | 0.3 (0.8–1.4) | <0.001 | −1.04, Moderate | |
| Pulse (bpm) | 0 | 67.4 | 6.1 | 66.4 | 7.2 | 0.15, Trivial | |||
| 4 | 61.0 | 6.5 | 6.3 (4.8–7.8) | 67.0 | 8.8 | 0.6 (1.0–2.2) | <0.001 | −0.78, Moderate | |
| Skinfold sum (mm) | 0 | 138.3 | 17.7 | 134.4 | 29.9 | 0.16, Trivial | |||
| 4 | 110.1 | 14.5 | 28.2 (26–30.5) | 133.0 | 29.8 | 1.5 (0.5–3.4) | <0.001 | −1.00, Moderate | |
| Bodyfat (%) | 0 | 24.7 | 3.2 | 23.9 | 4.0 | 0.21, Small | |||
| 4 | 21.0 | 2.8 | 3.6 (3.3–4.0) | 23.7 | 4.1 | 0.2 (0.1–0.4) | <0.001 | −0.79, Moderate | |
| Waist (cm) | 0 | 97.8 | 8.1 | 95.2 | 6.8 | 0.35, Small | |||
| 4 | 91.8 | 7.9 | 6.0 (5.3–6.8) | 94.3 | 6.9 | 1.0 (0.1–1.8) | <0.001 | −0.34, Small | |
| Waist to hip ratio | 0 | 0.94 | 0.07 | 0.93 | 0.08 | 0.09, Trivial | |||
| 4 | 0.90 | 0.07 | 0.03 (0.02–0.04) | 0.92 | 0.07 | 0.1 (0.0–0.2) | <0.001 | −0.22, Small | |
| VO2max (mL/kg/min) | 0 | 35.6 | 5.8 | 37.0 | 4.8 | −0.26, Small | |||
| 4 | 40.2 | 5.9 | 4.5 (4.0–5.0) | 37.3 | 5.1 | 0.2 (0.1–0.6) | <0.001 | 0.52, Small | |
| Push-ups (repetitions) | 0 | 16.4 | 6.8 | 18.1 | 7.7 | −0.22, Small | |||
| 4 | 24.3 | 7.1 | 7.8 (6.5–9.1) | 19.9 | 8.1 | 1.9 (1.2–2.6) | <0.001 | 0.57, Small | |
| Plank hold (s) | 0 | 77.2 | 25.5 | 82.5 | 23.7 | −0.21, Small | |||
| 4 | 120.0 | 39.6 | 42.8 (34.4–51.3) | 92.1 | 32.1 | 9.5 (3.8–15.1) | <0.001 | 0.77, Moderate | |
| Hours slept (h/day) | 0 | 7.0 | 0.4 | 7.0 | 0.6 | −0.17, Trivial | |||
| 4 | 7.6 | 0.5 | 0.7 (0.6–0.8) | 7.1 | 0.5 | 0.1 (0.0–0.2) | <0.001 | 1.00, Moderate | |
| PSQI Global (score) | 0 | 6.4 | 2.2 | 6.1 | 1.9 | 0.14, Trivial | |||
| 4 | 4.0 | 1.3 | 2.4 (2.0–2.8) | 5.8 | 1.8 | 0.3 (0.1–0.5) | <0.001 | −1.16, Moderate | |
| IPAQ-walk (min) | 0 | 70.5 | 32.2 | 77.7 | 52.0 | −0.17, Trivial | |||
| 4 | 97.0 | 30.0 | 26.5 (18.1–34.9) | 95.4 | 49.0 | 17.8 (8.0–27.6) | 0.163 | 0.04, Trivial | |
| IPAQ-MVPA (min) | 0 | 138.0 | 41.6 | 146.2 | 40.3 | −0.20, Small | |||
| 4 | 210.3 | 44.3 | 72.4 (60.0–84.8) | 156.9 | 46.4 | 10.8 (5.0–16.5) | <0.001 | 1.18, Moderate | |
| F&V Intake (serve/day) | 0 | 3.3 | 0.7 | 3.4 | 0.7 | −0.17, Trivial | |||
| 4 | 6.9 | 1.3 | 3.6 (3.3–4.0) | 3.8 | 0.9 | 0.4 (0.1–0.7) | <0.001 | 2.69, Large | |
| PCS-12 (score) | 0 | 46.3 | 3.8 | 47.2 | 2.8 | −0.28, Small | |||
| 4 | 51.5 | 3.4 | 5.2 (4.4–5.9) | 47.9 | 2.8 | 0.7 (0.3–1.1) | <0.001 | 1.14, Moderate | |
| MCS-12 (score) | 0 | 48.6 | 5.8 | 49.3 | 2.8 | −0.15, Trivial | |||
| 4 | 53.3 | 3.6 | 4.7 (3.7–5.8) | 49.5 | 2.9 | 0.2 (0.2–0.7) | <0.001 | 1.15, Moderate | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, D.; Driller, M.; Winwood, P.; Clissold, T.; Johnston, B.; Gill, N. The Effectiveness of a Combined Healthy Eating, Physical Activity, and Sleep Hygiene Lifestyle Intervention on Health and Fitness of Overweight Airline Pilots: A Controlled Trial. Nutrients 2022, 14, 1988. https://doi.org/10.3390/nu14091988
Wilson D, Driller M, Winwood P, Clissold T, Johnston B, Gill N. The Effectiveness of a Combined Healthy Eating, Physical Activity, and Sleep Hygiene Lifestyle Intervention on Health and Fitness of Overweight Airline Pilots: A Controlled Trial. Nutrients. 2022; 14(9):1988. https://doi.org/10.3390/nu14091988
Chicago/Turabian StyleWilson, Daniel, Matthew Driller, Paul Winwood, Tracey Clissold, Ben Johnston, and Nicholas Gill. 2022. "The Effectiveness of a Combined Healthy Eating, Physical Activity, and Sleep Hygiene Lifestyle Intervention on Health and Fitness of Overweight Airline Pilots: A Controlled Trial" Nutrients 14, no. 9: 1988. https://doi.org/10.3390/nu14091988
APA StyleWilson, D., Driller, M., Winwood, P., Clissold, T., Johnston, B., & Gill, N. (2022). The Effectiveness of a Combined Healthy Eating, Physical Activity, and Sleep Hygiene Lifestyle Intervention on Health and Fitness of Overweight Airline Pilots: A Controlled Trial. Nutrients, 14(9), 1988. https://doi.org/10.3390/nu14091988