Sex Differences in Salt Appetite: Perspectives from Animal Models and Human Studies
Abstract
:1. Introduction
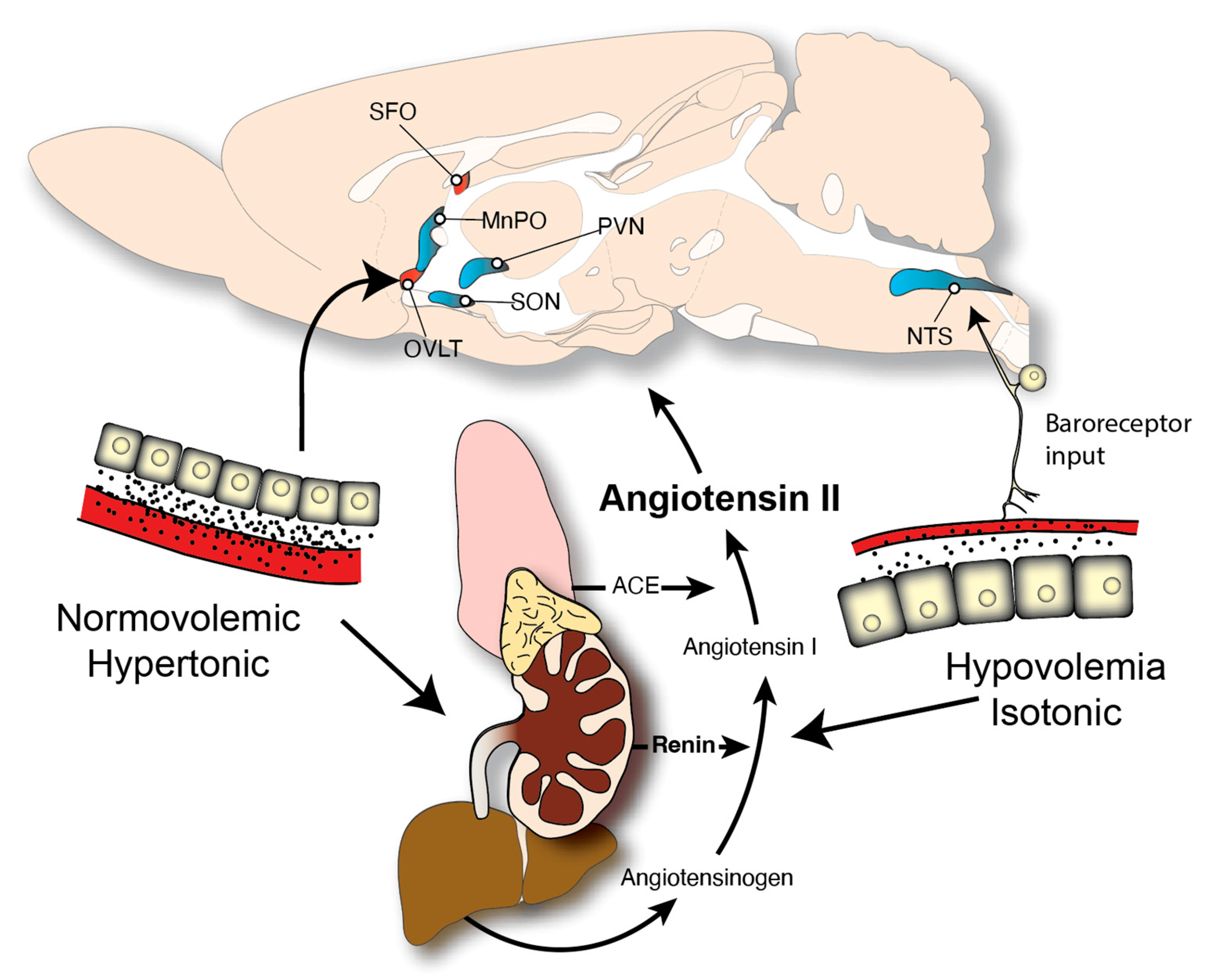
2. Neuroendocrine Controls of Fluid Balance

3. Mechanisms Underlying Sex Differences
4. Sex Differences in Unstimulated/Need-Free Sodium Intake
5. Sex Differences in Stimulated Sodium Intake
6. Changes in Sodium Intake during Pregnancy
7. Neural Controls of Sodium Intake: Implications for Sex Differences
8. Human Salt Intake
9. Sex Differences Related to Reproduction in Humans
10. Sex Differences in Dietary Sodium Intake
11. Sex Differences in Salting of Foods
12. Sex Differences in Salt Intake in the Elderly
13. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denton, D.A. Hunger for Salt: An Anthropological Physiological and Medical Analysis; Springer London Limited: London, UK, 1982. [Google Scholar]
- Schulkin, J. Sodium Hunger: The Search for a Salty Taste; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Wilkins, L.; Richter, C.P. A great craving for salt by a child with cortico adrenal insufficiency. JAMA 1940, 114, 866. [Google Scholar] [CrossRef]
- McCance, R.A. Experimental sodium chloride deficiency in man. Proc. R. Soc. Lond. B Biol. Sci. 1936, 119, 245–268. [Google Scholar] [CrossRef]
- Fitzsimons, J.T. The Physiology of Thirst and Sodium Appetite; Cambridge University Press: Cambridge, UK, 1979. [Google Scholar]
- Henkin, R.I.; Gill, J.R.; Bartter, F.C. Studies on Taste Thresholds in Normal Man and in Patients with Adrenal Cortical Insufficiency: The Role of Adrenal Cortical Steroids and of Serum Sodium Concentration. J. Clin. Investig. 1963, 42, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Blair-West, J.R.; Carey, K.D.; Denton, D.A.; Weisinger, R.S.; Shade, R.E. Evidence that brain angiotensin II is involved in both thirst and sodium appetite in baboons. Am. J. Physiol. 1998, 275, R1639–R1646. [Google Scholar] [CrossRef]
- Belovsky, G.E.; Jordan, P.A. Sodium dynamics and adaptations of a moose population. J. Mammal. 1981, 62, 613–621. [Google Scholar] [CrossRef]
- Aldrich, E. Notes on the salt-feeding habits of the Red Crossbill. Condor 1939, 41, 172–173. [Google Scholar]
- Goodall, J. The Chimpanzees of Gombe: Patterns of Behavior; Harvard University Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Schaller, G.E. The Mountain Gorilla: Ecology and Behavior; University of Chicago Press: Chicago, IL, USA, 1963. [Google Scholar]
- Wolf, G. Innate mechanisms for regulation of sodium intake. Olfaction Taste 1969, 3, 548–553. [Google Scholar]
- Richter, C.P. Salt appetite of mammals: Its dependence on instinct and metabolism. In L’instinct Dans le Comportement des Animaux et de L’homme; Masson et Cie: Paris, France, 1956; p. 577. [Google Scholar]
- Nachman, M. Taste preferences for sodium salts by adrenalectomized rats. J. Comp. Physiol. Psychol. 1962, 55, 1124–1129. [Google Scholar] [CrossRef]
- Leshem, M.; Epstein, A.N. Ontogeny of renin-induced salt appetite in the rat pup. Dev. Psychobiol. 1989, 22, 437–445. [Google Scholar] [CrossRef]
- Berridge, K.C.; Flynn, F.W.; Schulkin, J.; Grill, H.J. Sodium depletion enhances salt palatability in rats. Behav. Neurosci. 1984, 98, 652–660. [Google Scholar] [CrossRef]
- Krieckhaus, E.E.; Wolf, G. Acquisition of sodium by rats: Interaction of innate mechanisms and latent learning. J. Comp. Physiol. Psychol. 1968, 65, 197–201. [Google Scholar] [CrossRef]
- Berridge, K.C.; Schulkin, J. Palatability shift of a salt-associated incentive during sodium depletion. Q. J. Exp. Psychol. B 1989, 41, 121–138. [Google Scholar]
- Dethier, V.G. The taste of salt. Am. Sci. 1977, 65, 744–751. [Google Scholar]
- Geran, L.C.; Spector, A.C. Sodium taste detectability in rats is independent of anion size: The psychophysical characteristics of the transcellular sodium taste transduction pathway. Behav. Neurosci. 2000, 114, 1229–1238. [Google Scholar] [CrossRef]
- Norgren, R.; Leonard, C.M. Taste pathways in rat brainstem. Science 1971, 173, 1136–1139. [Google Scholar] [CrossRef]
- Roper, S.D. The taste of table salt. Pflug. Arch. 2015, 467, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Lossow, K.; Hermans-Borgmeyer, I.; Meyerhof, W.; Behrens, M. Segregating expression of ENaC subunits in taste cells. Chem. Senses 2020, 45, 235–248. [Google Scholar] [CrossRef]
- Rolls, E.T. Taste and smell processing in the brain. Handb. Clin. Neurol. 2019, 164, 97–118. [Google Scholar]
- Oka, Y.; Ye, M.; Zuker, C.S. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature 2015, 520, 349–352. [Google Scholar] [CrossRef]
- Krieckhaus, E.E. “Innate recognition” aids rats in sodium regulation. J. Comp. Physiol. Psychol. 1970, 73, 117–122. [Google Scholar] [CrossRef]
- Fitzsimons, J.T. Angiotensin, thirst, and sodium appetite. Physiol. Rev. 1998, 78, 583–686. [Google Scholar] [CrossRef]
- Epstein, A.N. Epilogue: Retrospect and prognosis. In The Neuropsychology of Thirst: New Findings and Advances in Concepts; Epstein, A.N., Kissileff, H.R., Stellar, E., Eds.; V.H. Winston & Sons: Washington, DC, USA, 1973; pp. 315–332. [Google Scholar]
- Fitzsimons, J.T. Some historical perspectives in the physiology of thirst. In The Neuropsychology of Thirst: New Findings and Advances in Concepts; Epstein, A.N., Kissileff, H.R., Stellar, E., Eds.; V.H. Winston & Sons: Washington, DC, USA, 1973; pp. 3–33. [Google Scholar]
- Daniels, D. Neuropeptides and Regulation of Water intake. In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Fluharty, S.J.; Epstein, A.N. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: II. Synergistic interaction with systemic mineralocorticoids. Behav. Neurosci. 1983, 97, 746–758. [Google Scholar] [CrossRef]
- Sakai, R.R.; Nicolaidis, S.; Epstein, A.N. Salt appetite is suppressed by interference with angiotensin II and aldosterone. Am. J. Physiol. 1986, 251, R762–R768. [Google Scholar] [CrossRef]
- Wolf, G. Effect of deoxycorticosterone on sodium appetite of intact and adrenalectomized rats. Am. J. Physiol. 1965, 208, 1281–1285. [Google Scholar] [CrossRef]
- Fregly, M.J.; Rowland, N.E. Role of renin-angiotensin-aldosterone system in NaCl appetite of rats. Am. J. Physiol. 1985, 248, R1–R11. [Google Scholar] [CrossRef]
- Schulkin, J. Mineralocorticoids, dietary conditions, and sodium appetite. Behav. Biol. 1978, 23, 197–205. [Google Scholar] [CrossRef]
- Geerling, J.C.; Loewy, A.D. Aldosterone-sensitive NTS neurons are inhibited by saline ingestion during chronic mineralocorticoid treatment. Brain. Res. 2006, 1115, 54–64. [Google Scholar] [CrossRef]
- Morris, M.J.; Na, E.S.; Johnson, A.K. Mineralocorticoid receptor antagonism prevents hedonic deficits induced by a chronic sodium appetite. Behav. Neurosci. 2010, 124, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Na, E.S.; Morris, M.J.; Johnson, A.K. Opioid mechanisms that mediate the palatability of and appetite for salt in sodium replete and deficient states. Physiol. Behav. 2012, 106, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Bare, J.K. The specific hunger for sodium chloride in normal and adrenalectomized white rats. J. Comp. Physiol. Psychol. 1949, 42, 242–253. [Google Scholar] [CrossRef]
- Pfaffmann, C.; Bare, J.K. Gustatory nerve discharges in normal and adrenalectomized rats. J. Comp. Physiol. Psychol. 1950, 43, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.J. Changes in gustatory nerve discharges with sodium deficiency: A single unit analysis. Brain. Res. 1977, 121, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Franze, G.M.; Gasparini, S.; de Luca, L.A., Jr.; de Paula, P.M.; Colombari, D.S.; Colombari, E.; Andrade, C.A.; Menani, J.V. Lateral parabrachial nucleus and opioid mechanisms of the central nucleus of the amygdala in the control of sodium intake. Behav. Brain. Res. 2017, 316, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.C. The history of the “Davis Rig”. Appetite 2001, 36, 93–98. [Google Scholar] [CrossRef] [PubMed]
- St. John, S.J. The Perceptual Characteristics of Sodium Chloride to Sodium-Depleted Rats. Chem. Senses 2017, 42, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Berridge, K.C. Measuring hedonic impact in animals and infants: Microstructure of affective taste reactivity patterns. Neurosci. Biobehav. Rev. 2000, 24, 173–198. [Google Scholar] [CrossRef]
- Daniels, D. Thirst and Water Balance; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Eckel, L.A.; Houpt, T.A.; Geary, N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol. Behav. 2000, 70, 397–405. [Google Scholar] [CrossRef]
- Wang, G.H.; Richter, C.P.; Guttmacher, A.F. Activity s.studies on male castrated rats with ovarian transplants, and correlation of the activity with the histology of the grafts. Am. J. Physiol. 1925, 73, 581–599. [Google Scholar] [CrossRef] [Green Version]
- Gentry, R.T.; Wade, G.N. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. J. Comp. Physiol. Psychol. 1976, 90, 747–754. [Google Scholar] [CrossRef]
- Sisk, C.L.; Zehr, J.L. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005, 26, 163–174. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.; Niesink, R.J.; van Ree, J.M. The neurobiology of social play behavior in rats. Neurosci. Biobehav. Rev. 1997, 21, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, C.H.; Goy, R.W.; Gerall, A.A.; Young, W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959, 65, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P. Four Core Genotypes and XY* mouse models: Update on impact on SABV research. Neurosci. Biobehav. Rev. 2020, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krecek, J. Sex differences in salt taste: The effect of testosterone. Physiol. Behav. 1973, 10, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Krecek, J.; Novakova, V.; Stibral, K. Sex differences in the taste preference for a salt solution in the rat. Physiol. Behav. 1972, 8, 183–188. [Google Scholar] [CrossRef]
- Sakai, R.R.; Frankmann, S.P.; Fine, W.B.; Epstein, A.N. Prior episodes of sodium depletion increase the need-free sodium intake of the rat. Behav. Neurosci. 1989, 103, 186–192. [Google Scholar] [CrossRef]
- Chow, S.Y.; Sakai, R.R.; Witcher, J.A.; Adler, N.T.; Epstein, A.N. Sex and sodium intake in the rat. Behav. Neurosci. 1992, 106, 172–180. [Google Scholar] [CrossRef]
- Flynn, F.W.; Schulkin, J.; Havens, M. Sex differences in salt preference and taste reactivity in rats. Brain. Res. Bull. 1993, 32, 91–95. [Google Scholar] [CrossRef]
- Curtis, K.S.; Contreras, R.J. Sex differences in electrophysiological and behavioral responses to NaCl taste. Behav. Neurosci. 2006, 120, 917–924. [Google Scholar] [CrossRef] [Green Version]
- Curtis, K.S.; Davis, L.M.; Johnson, A.L.; Therrien, K.L.; Contreras, R.J. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol. Behav. 2004, 80, 657–664. [Google Scholar] [CrossRef]
- Krecek, J. The pineal gland and the development of salt intake patterns in male rats. Dev. Psychobiol. 1976, 9, 181–188. [Google Scholar] [CrossRef]
- Santollo, J.; Edwards, A.A. How predictive is body weight on fluid intake in rats? It depends on sex. Physiol. Behav. 2021, 229, 113262. [Google Scholar] [CrossRef]
- Santollo, J.; Torregrossa, A.M.; Daniels, D. Sex differences in the drinking response to angiotensin II (AngII): Effect of body weight. Horm. Behav. 2017, 93, 128–136. [Google Scholar] [CrossRef]
- Santollo, J.; Myers, K.E.; Rainer, I.L.; Edwards, A.A. Gonadal hormones in female rats protect against dehydration-induced memory impairments in the novel object recognition paradigm. Horm. Behav. 2019, 114, 104547. [Google Scholar] [CrossRef]
- Romeo, R.D. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front. Neuroendocrinol. 2010, 31, 232–240. [Google Scholar] [CrossRef]
- Antunes Rodrigues, J.; Covian, M.R. Hypothalamic Control of Sodium Chloride and Water Intake. Acta. Physiol. Lat. Am. 1963, 13, 94–100. [Google Scholar]
- Kensicki, E.; Dunphy, G.; Ely, D. Estradiol increases salt intake in female normotensive and hypertensive rats. J. Appl. Physiol. 2002, 93, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Fregly, M.J. Effect of an oral contraceptive on NaCl appetite and preference threshold in rats. Pharmacol. Biochem. Behav. 1973, 1, 61–65. [Google Scholar] [CrossRef]
- Santollo, J.; Edwards, A.A.; Howell, J.A.; Myers, K.E. Bidirectional effects of estradiol on the control of water intake in female rats. Horm. Behav. 2021, 133, 104996. [Google Scholar] [CrossRef]
- Yu, K.; He, Y.; Hyseni, I.; Pei, Z.; Yang, Y.; Xu, P.; Cai, X.; Liu, H.; Qu, N.; Liu, H.; et al. 17beta-estradiol promotes acute refeeding in hungry mice via membrane-initiated ERalpha signaling. Mol. Metab. 2020, 42, 101053. [Google Scholar] [CrossRef]
- Wolf, G. Refined salt appetite methodology for rats demonstrated by assessing sex differences. J. Comp. Physiol. Psychol. 1982, 96, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Begg, D.P.; Sinclair, A.J.; Weisinger, R.S. Reductions in water and sodium intake by aged male and female rats. Nutr. Res. 2012, 32, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Omouessi, S.T.; Chapleur, M.; Leshem, M.; Thornton, S.N. Gender and obesity influence sodium intake and fluid regulation in Zucker rats following repeated sodium depletions. Physiol. Behav. 2006, 89, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Leshem, M.; Kavushansky, A.; Devys, J.M.; Thornton, S. Enhancement revisited: The effects of multiple depletions on sodium intake in rats vary with strain, substrain, and gender. Physiol. Behav. 2004, 82, 571–580. [Google Scholar] [CrossRef]
- Stricker, E.M.; Thiels, E.; Verbalis, J.G. Sodium appetite in rats after prolonged dietary sodium deprivation: A sexually dimorphic phenomenon. Am. J. Physiol. 1991, 260, R1082–R1088. [Google Scholar] [CrossRef]
- Scheidler, M.G.; Verbalis, J.G.; Stricker, E.M. Inhibitory effects of estrogen on stimulated salt appetite in rats. Behav. Neurosci. 1994, 108, 141–150. [Google Scholar] [CrossRef]
- Dadam, F.M.; Caeiro, X.E.; Cisternas, C.D.; Macchione, A.F.; Cambiasso, M.J.; Vivas, L. Effect of sex chromosome complement on sodium appetite and Fos-immunoreactivity induced by sodium depletion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R175–R184. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Kuhn, C.; Oka, Y.; Yarmolinsky, D.A.; Hummler, E.; Ryba, N.J.P.; Zuker, C.Z. The cells and peripheral representations of sodium taste in mice. Nature 2010, 464, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Findlay, A.L.; Fitzsimons, J.T.; Kucharczyk, J. Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. J. Endocrinol. 1979, 82, 215–225. [Google Scholar] [CrossRef]
- Jonklaas, J.; Buggy, J. Angiotensin-estrogen interaction in female brain reduces drinking and pressor responses. Am. J. Physiol. 1984, 247, R167–R172. [Google Scholar] [CrossRef]
- Santollo, J.; Daniels, D. Activation of G protein-coupled estrogen receptor 1 (GPER-1) decreases fluid intake in female rats. Horm. Behav. 2015, 73, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santollo, J.; Marshall, A.; Curtis, K.S.; Speth, R.C.; Clark, S.D.; Daniels, D. Divergent effects of ERalpha and ERbeta on fluid intake by female rats are not dependent on concomitant changes in AT1R expression or body weight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R14–R23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santollo, J.; Collett, J.A.; Edwards, A.A. The anti-dipsogenic and anti-natriorexigenic effects of estradiol, but not the anti-pressor effect, are lost in aged female rats. Physiol. Rep. 2021, 9, e14948. [Google Scholar] [CrossRef] [PubMed]
- Kisley, L.R.; Sakai, R.R.; Ma, L.Y.; Fluharty, S.J. Ovarian steroid regulation of angiotensin II-induced water intake in the rat. Am. J. Physiol. 1999, 276, R90–R96. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, C.; Amigone, J.L.; Vivas, L. Serotonergic system involvement in the inhibitory action of estrogen on induced sodium appetite in female rats. Physiol. Behav. 2011, 104, 398–407. [Google Scholar] [CrossRef]
- Pereira, E.D., Jr.; Dantas, R.M.; Andrade-Franze, G.M.F.; de Luca, L.A., Jr.; Menani, J.V.; Andrade, C.A.F. Estradiol modulates the palatability of 0.3 M NaCl in female rats during sodium appetite. Appetite 2019, 133, 252–261. [Google Scholar] [CrossRef]
- Bursey, R.G.; Watson, M.L. The effect of sodium restriction during gestation of offspring brain development in rats. Am. J. Clin. Nutr. 1983, 37, 43–51. [Google Scholar] [CrossRef]
- Koleganova, N.; Piecha, G.; Ritz, E.; Becker, L.E.; Muller, A.; Weckbach, M.; Nyengaard, J.R.; Schirmacher, P.; Gross-Weissmann, M.L. Both high and low maternal salt intake in pregnancy alter kidney development in the offspring. Am. J. Physiol. Renal. Physiol. 2011, 301, F344–F354. [Google Scholar] [CrossRef] [Green Version]
- Denton, D.A.; Nelson, J.F. The effects of pregnancy and lactation on the mineral appetites of wild rabbits (Oryctolagus cuniculus (L.)). Endocrinology 1971, 88, 31–40. [Google Scholar] [CrossRef]
- Churchill, S.; Bengele, H.H.; Melby, J.C.; Alexander, E.A. Role of aldosterone in sodium retention of pregnancy in the rat. Am. J. Physiol. 1981, 240, R175–R181. [Google Scholar] [CrossRef]
- Churchi-l, S.E.; Bengele, H.H.; Alexander, E.A. Sodium balance during pregnancy in the rat. Am. J. Physiol. 1980, 239, R143–R148. [Google Scholar] [CrossRef] [PubMed]
- McBurnie, M.; Denton, D.; Tarjan, E. Influence of pregnancy and lactation on Na appetite of BALB/c mice. Am. J. Physiol. 1988, 255, R1020–R1024. [Google Scholar] [CrossRef] [PubMed]
- Leshem, M.; Levin, T.; Schulkin, J. Intake and hedonics of calcium and sodium during pregnancy and lactation in the rat. Physiol. Behav. 2002, 75, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Thiels, E.; Verbalis, J.G.; Stricker, E.M. Sodium appetite in lactating rats. Behav. Neurosci. 1990, 104, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Covelli, M.D.; Denton, D.A.; Nelson, J.F.; Shulkes, A.A. Hormonal factors influencing salt appetite in pregnancy. Endocrinology 1973, 93, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.N.; Bernstein, I.L. NaCl preference increases during pregnancy and lactation: Assessment using brief access tests. Pharmacol. Biochem. Behav. 2001, 68, 555–563. [Google Scholar] [CrossRef]
- Shulkes, A.A.; Covelli, M.D.; Denton, D.A.; Nelson, J.F. Hormonal factors influencing salt appetite in lactation. Aust. J. Exp. Biol. Med. Sci. 1972, 50, 819–826. [Google Scholar] [CrossRef]
- Frankmann, S.P.; Ulrich, P.; Epstein, A.N. Transient and lasting effects of reproductive episodes on NaCl intake of the female rat. Appetite 1991, 16, 193–204. [Google Scholar] [CrossRef]
- Nation, H.L.; Nicoleau, M.; Kinsman, B.J.; Browning, K.N.; Stocker, S.D. DREADD-induced activation of subfornical organ neurons stimulates thirst and salt appetite. J. Neurophysiol. 2016, 115, 3123–3129. [Google Scholar] [CrossRef] [Green Version]
- McKinley, M.J.; Albiston, A.L.; Allen, A.M.; Mathai, M.L.; May, C.N.; McAllen, R.M.; Oldfield, B.J.; Mendelsohn, F.A.; Chai, S.Y. The brain renin-angiotensin system: Location and physiological roles. Int. J. Biochem. Cell Biol. 2003, 35, 901–918. [Google Scholar] [CrossRef]
- Matsuda, T.; Hiyama, T.Y.; Niimura, F.; Matsusaka, T.; Fukamizu, A.; Kobayashi, K.; Noda, M. Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nat. Neurosci. 2017, 20, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Jarvie, B.C.; Palmiter, R.D. HSD2 neurons in the hindbrain drive sodium appetite. Nat. Neurosci. 2017, 20, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Geerling, J.C.; Loewy, A.D. Aldosterone-sensitive neurons in the nucleus of the solitary: Efferent projections. J. Comp. Neurol. 2006, 498, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Herbert, H.; Moga, M.M.; Saper, C.B. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol. 1990, 293, 540–580. [Google Scholar] [CrossRef] [PubMed]
- Tokita, K.; Inoue, T.; Boughter, J.D., Jr. Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience 2009, 161, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Nitabach, M.N.; Schulkin, J.; Epstein, A.N. The medial amygdala is part of a mineralocorticoid-sensitive circuit controlling NaCl intake in the rat. Behav. Brain. Res. 1989, 35, 127–134. [Google Scholar] [CrossRef]
- Sakai, R.R.; McEwen, B.S.; Fluharty, S.J.; Ma, L.Y. The amygdala: Site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney Int. 2000, 57, 1337–1345. [Google Scholar] [CrossRef] [Green Version]
- Reilly, J.J.; Maki, R.; Nardozzi, J.; Schulkin, J. The effects of lesions of the bed nucleus of the stria terminalis on sodium appetite. Acta Neurobiol. Exp. 1994, 54, 253–257. [Google Scholar]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997, 388, 507–525. [Google Scholar] [CrossRef]
- Leshem, M. Biobehavior of the human love of salt. Neurosci. Biobehav. Rev. 2009, 33, 1–17. [Google Scholar] [CrossRef]
- Morimoto, S.; Cassell, M.D.; Sigmund, C.D. Neuron-specific expression of human angiotensinogen in brain causes increased salt appetite. Physiol. Genom. 2002, 9, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, J.L. Serial sodium depletion and NaCl solution intake. Physiol. Behav. 1966, 1, 75–77. [Google Scholar] [CrossRef] [Green Version]
- Metheny, N.A.; Krieger, M.M. Salt toxicity: A systematic review and case reports. J. Emerg. Nurs. 2020, 46, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Bertino, M. Rats (Rattus norvegicus) do not prefer salted solid food. J. Comp. Psychol. 1985, 99, 240–247. [Google Scholar] [CrossRef]
- Bertino, M.; Tordoff, M.G. Sodium depletion increases rats’ preferences for salted food. Behav. Neurosci. 1988, 102, 565–573. [Google Scholar] [CrossRef]
- Leshem, M.; Neufeld, M.; del Canho, S. Ontogeny of the ionic specificity of sodium appetite in the rat pup. Dev. Psychobiol. 1994, 27, 381–394. [Google Scholar] [CrossRef]
- Redmond, I. Underground Elephants. Anim. Kingd. 1984, 87, 30–37. [Google Scholar]
- Kratz, A.; Siegel, A.J.; Verbalis, J.G.; Adner, M.M.; Shirey, T.; Lee-Lewandrowski, E.; Lewandrowski, K.B. Sodium status of collapsed marathon runners. Arch. Pathol. Lab. Med. 2005, 129, 227–230. [Google Scholar] [CrossRef]
- Almond, C.S.; Shin, A.Y.; Fortescue, E.B.; Mannix, R.C.; Wypij, D.; Binstadt, B.A.; Duncan, C.N.; Olson, D.P.; Salerno, A.E.; Newburger, J.W.; et al. Hyponatremia among runners in the Boston Marathon. N. Engl. J. Med. 2005, 352, 1550–1556. [Google Scholar] [CrossRef] [Green Version]
- Al-Dahhan, J.; Jannoun, L.; Haycock, G.B. Effect of salt supplementation of newborn premature infants on neurodevelopmental outcome at 10-13 years of age. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 86, F120–F123. [Google Scholar] [CrossRef] [Green Version]
- Shirazki, A.; Weintraub, Z.; Reich, D.; Gershon, E.; Leshem, M. Lowest neonatal serum sodium predicts sodium intake in low birth weight children. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1683–R1689. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, J.; Diaz, J.J.; Malaga, I.; Perillan, C.; Costales, M.; Vijande, M. Sodium taste threshold in children and its relationship to blood pressure. Braz. J. Med. Biol. Res. 2007, 40, 721–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, D.J. Taste and food preference changes across the course of pregnancy. Appetite 1992, 19, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Toma, R.B. Taste changes during pregnancy. Am. J. Clin. Nutr. 1986, 43, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Bartoshuk, L.M.; Striegel-Moore, R.; Rodin, J. Taste changes across pregnancy. Ann. N. Y. Acad. Sci. 1998, 855, 805–809. [Google Scholar] [CrossRef]
- Leshem, M. The excess salt appetite of humans is not due to sodium loss in adulthood. Physiol. Behav. 2009, 98, 331–337. [Google Scholar] [CrossRef]
- Niegowska, J.; Barylko-Pikielna, N. Salt taste perception in women during physiological pregnancy. Ginekol. Pol. 1998, 69, 168–174. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Sodium and Potassium; The National Academies Press: Washington, DC, USA, 2019; p. 594. [Google Scholar] [CrossRef]
- Verd, S.; Nadal-Amat, J.; Gich, I.; Leshem, M. Salt preference of nursing mothers is associated with earlier cessation of exclusive breastfeeding. Appetite 2010, 54, 233–236. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Lucchina, L.A.; Prutkin, J.; Fast, K. PROP (6-n-propylthiouracil) supertasters and the saltiness of NaCl. Ann. N. Y. Acad. Sci. 1998, 855, 793–796. [Google Scholar] [CrossRef]
- Crystal, S.R.; Bowen, D.J.; Bernstein, I.L. Morning sickness and salt intake, food cravings, and food aversions. Physiol. Behav. 1999, 67, 181–187. [Google Scholar] [CrossRef]
- Frye, C.A.; Demolar, G.L. Menstrual cycle and sex differences influence salt preference. Physiol. Behav. 1994, 55, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, R.B.; Ryu, M.; Przypek, J. Preferences for foods with varying levels of salt and fat differ as a function of dietary restraint and exercise but not menstrual cycle. Physiol. Behav. 1995, 57, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. The taste for salt in humans. Am. J. Clin. Nutr. 1997, 65, 692S–697S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochsenbein-Kolble, N.; von Mering, R.; Zimmermann, R.; Hummel, T. Changes in gustatory function during the course of pregnancy and postpartum. BJOG 2005, 112, 1636–1640. [Google Scholar] [CrossRef]
- Verma, P.; Mahajan, K.K.; Mittal, S.; Ghildiyal, A. Salt preference across different phases of menstrual cycle. Indian. J. Physiol. Pharmacol. 2005, 49, 99–102. [Google Scholar]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [Green Version]
- Lozada, M.; Sanchez-Castillo, C.P.; del A. Cabrera, G.; Mata, I.I.; Pichardo-Ontiveros, E.; James, W.P. Salt: Its goodness and perversities. Rev. Investig. Clin. 2007, 59, 382–393. [Google Scholar]
- Wright, J.D.; Wang, C.Y.; Kennedy-Stephenson, J.; Ervin, R.B. Dietary intake of ten key nutrients for public health, United States: 1999–2000. Adv. Data 2003, 324, 1–4. [Google Scholar]
- Control, I.C.O.D. MABAT First Israeli National Health and Nutrition Survey 1999–2001: Part 1—General Findings; Israeli Center of Disease Control: Ramat Gan, Israel, 2003. [Google Scholar]
- Goldstein, P.; Leshem, M. Dietary sodium, added salt, and serum sodium associations with growth and depression in the U.S. general population. Appetite 2014, 79, 83–90. [Google Scholar] [CrossRef]
- Razaz, J.M.; Balam, F.H.; Karimi, T.; Rahmani, J.; Kalantari, N.; Shariatpanahi, S.P.; Bawadi, H.; Bhagavathula, A.S.; Roudsari, A.H. Sex Differences in Healthy Eating: Investigating the Moderating Effect of Self-Efficacy. J. Nutr. Educ. Behav. 2022, 54, 151–158. [Google Scholar] [CrossRef]
- Oh, H.; Kim, J.; Huh, Y.; Kim, S.H.; Jang, S.I. Association of Household Income Level with Vitamin and Mineral Intake. Nutrients 2021, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.; Peters, S.A.E.; Woodward, M. Sex differences in macronutrient intake and adherence to dietary recommendations: Findings from the UK Biobank. BMJ Open 2018, 8, e020017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henney, J.E.; Taylor, C.L.; Boon, C.S. (Eds.) Strategies to Reduce Sodium Intake in the United States; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- Liem, D.G.; Miremadi, F.; Keast, R.S. Reducing sodium in foods: The effect on flavor. Nutrients 2011, 3, 694–711. [Google Scholar] [CrossRef] [Green Version]
- Venezia, A.; Barba, G.; Russo, O.; Capasso, C.; de Luca, V.; Farinaro, E.; Cappuccio, F.P.; Galletti, F.; Rossi, G.; Strazzullo, P. Dietary sodium intake in a sample of adult male population in southern Italy: Results of the Olivetti Heart Study. Eur. J. Clin. Nutr. 2010, 64, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Oliver, G.; Wardle, J.; Gibson, E.L. Stress and food choice: A laboratory study. Psychosom. Med. 2000, 62, 853–865. [Google Scholar] [CrossRef]
- Garriguet, D. Sodium consumption at all ages. Health Rep. 2007, 18, 47–52. [Google Scholar]
- Leshem, M.; Dessie-Navon, H. Acculturation of immigrant diet, basic taste responses and sodium appetite. J. Nutr. Sci. 2018, 7, e21. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Cho, H.J.; Bae, E.; Kim, Y.C.; Kim, S.; Chin, H.J. Not salt taste perception but self-reported salt eating habit predicts actual salt intake. J. Korean Med. Sci. 2014, 29 (Suppl. S2), S91–S96. [Google Scholar] [CrossRef] [Green Version]
- Drewnowski, A.; Henderson, S.A.; Driscoll, A.; Rolls, B.J. Salt taste perceptions and preferences are unrelated to sodium consumption in healthy older adults. J. Am. Diet. Assoc. 1996, 96, 471–474. [Google Scholar] [CrossRef]
- Shepherd, R.; Farleigh, C.A.; Wharf, S.G. Limited compensation by table salt for reduced salt within a meal. Appetite 1989, 13, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hendi, K.; Leshem, M. Salt appetite in the elderly. Br. J. Nutr. 2014, 112, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Stachenfeld, N.S.; DiPietro, L.; Nadel, E.R.; Mack, G.W. Mechanism of attenuated thirst in aging: Role of central volume receptors. Am. J. Physiol. 1997, 272, R148–R157. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Oparil, S. Hypertension in Women: Recent Advances and Lingering Questions. Hypertension 2017, 70, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.C. Linking gustatory neurobiology to behavior in vertebrates. Neurosci. Biobehav. Rev. 2000, 24, 391–416. [Google Scholar] [CrossRef]
- Wald, N.; Leshem, M. Salt conditions a flavor preference or aversion after exercise depending on NaCl dose and sweat loss. Appetite 2003, 40, 277–284. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santollo, J.; Daniels, D.; Leshem, M.; Schulkin, J. Sex Differences in Salt Appetite: Perspectives from Animal Models and Human Studies. Nutrients 2023, 15, 208. https://doi.org/10.3390/nu15010208
Santollo J, Daniels D, Leshem M, Schulkin J. Sex Differences in Salt Appetite: Perspectives from Animal Models and Human Studies. Nutrients. 2023; 15(1):208. https://doi.org/10.3390/nu15010208
Chicago/Turabian StyleSantollo, Jessica, Derek Daniels, Micah Leshem, and Jay Schulkin. 2023. "Sex Differences in Salt Appetite: Perspectives from Animal Models and Human Studies" Nutrients 15, no. 1: 208. https://doi.org/10.3390/nu15010208
APA StyleSantollo, J., Daniels, D., Leshem, M., & Schulkin, J. (2023). Sex Differences in Salt Appetite: Perspectives from Animal Models and Human Studies. Nutrients, 15(1), 208. https://doi.org/10.3390/nu15010208




