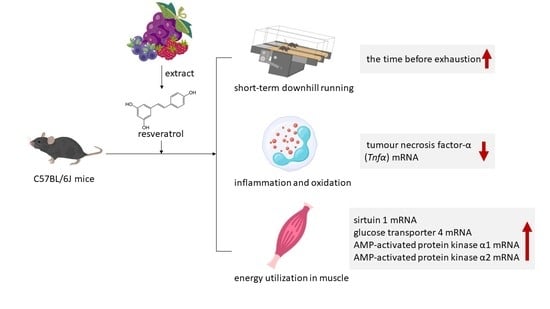
Effects of Resveratrol on Muscle Inflammation, Energy Utilisation, and Exercise Performance in an Eccentric Contraction Exercise Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Experiment Design
2.4. ILT, Exhaustion Test, and Downhill Running
2.5. Sample Collection
2.6. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.7. Next Generation Sequencing Analysis
2.8. Statistical Analysis
3. Results
3.1. Average Body Weight, Food Intake, and Tissue Relative Weight
3.2. Exercise Performance in Exhaustion Test
3.3. Lactate Dehydrogenase (LDH) and Creatine Kinase (CK)
3.4. Gene Expression of Inflammatory Factors in Muscles
3.4.1. Gastrocnemius Muscle
3.4.2. Tibialis Anterior Muscle
3.4.3. Soleus Muscle
3.5. Gene Expression of Energy Metabolism and Antioxidant Factors in Muscles
3.5.1. Gastrocnemius Muscle
3.5.2. Tibialis Anterior Muscle
3.5.3. Soleus Muscle
3.6. MA Plot, Volcano Plot, and Bar Charts for EX vs. EX + RES150 Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mujika, I.; Rønnestad, B.R.; Martin, D.T. Effects of Increased Muscle Strength and Muscle Mass on Endurance-Cycling Performance. Int. J. Sports Physiol. Perform. 2016, 11, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Stricker, P.R.; Faigenbaum, A.D.; McCambridge, T.M.; LaBella, C.R.; Brooks, M.A.; Canty, G.; Diamond, A.B.; Hennrikus, W.; Logan, K.; Moffatt, K.; et al. Resistance Training for Children and Adolescents. Pediatrics 2020, 145, e20201011. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Clevel. Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. Ser. A 2017, 72, 1595–1606. [Google Scholar] [CrossRef]
- Morgan, D.L.; Allen, D.G. Early events in stretch-induced muscle damage. J. Appl. Physiol. 1999, 87, 2007–2015. [Google Scholar] [CrossRef]
- Warren, G.L.; Ingalls, C.P.; Lowe, D.A.; Armstrong, R.B. Excitation-Contraction Uncoupling: Major Role in Contraction-Induced Muscle Injury. Exerc. Sport Sci. Rev. 2001, 29, 82–87. [Google Scholar] [CrossRef]
- Thomson, D. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Liu, C.-C.; Tsao, J.-P.; Hsu, C.-L.; Cheng, I.-S. Effects of Oral Resveratrol Supplementation on Glycogen Replenishment and Mitochondria Biogenesis in Exercised Human Skeletal Muscle. Nutrients 2020, 12, 3721. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.H.; Reid, M.B.; Allen, D.G.; Westerblad, H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J. Physiol. 1998, 509, 565–575. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M. Studies of Mitochondrial and Nonmitochondrial Sources Implicate Nicotinamide Adenine Dinucleotide Phosphate Oxidase(s) in the Increased Skeletal Muscle Superoxide Generation That Occurs During Contractile Activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef]
- Cheng, A.J.; Bruton, J.D.; Lanner, J.T.; Westerblad, H. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J. Physiol. 2015, 593, 457–472. [Google Scholar] [CrossRef]
- Tanskanen, M.; Atalay, M.; Uusitalo, A. Altered oxidative stress in overtrained athletes. J. Sports Sci. 2010, 28, 309–317. [Google Scholar] [CrossRef]
- Yamada, T.; Abe, M.; Lee, J.; Tatebayashi, D.; Himori, K.; Kanzaki, K.; Wada, M.; Bruton, J.D.; Westerblad, H.; Lanner, J.T. Muscle dysfunction associated with adjuvant-induced arthritis is prevented by antioxidant treatment. Skelet. Muscle 2015, 5, 20. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Williams, L.D.; Burdock, G.A.; Edwards, J.A.; Beck, M.; Bausch, J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 2009, 47, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Wahl, D.; Diéguez, C.; Bernier, M.; De Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites—Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.B.; Rolim, N.P.L.; Bartholomeu, J.B.; Gobatto, C.; Kokubun, E.; Brum, P.C. Maximal Lactate Steady State in Running Mice: Effect of Exercise Training. Clin. Exp. Pharmacol. Physiol. 2007, 34, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; da Rocha, A.L.; Cabrera, E.M.; Marafon, B.B.; Kohama, E.B.; Rovina, R.L.; Simabuco, F.M.; Junior, C.R.B.; de Moura, L.P.; Pauli, J.R.; et al. Role of interleukin-6 in inhibiting hepatic autophagy markers in exercised mice. Cytokine 2020, 130, 155085. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Machado, C.D.S.M.; De Marchi, T.; Casalechi, H.L.; Bjordal, J.M.; Carvalho, P.D.T.C.D.; Leal-Junior, E.C.P. Infrared Low-Level Laser Therapy (Photobiomodulation Therapy) before Intense Progressive Running Test of High-Level Soccer Players: Effects on Functional, Muscle Damage, Inflammatory, and Oxidative Stress Markers—A Randomized Controlled Trial. Oxidative Med. Cell. Longev. 2019, 2019, 6239058. [Google Scholar] [CrossRef]
- Tsao, J.-P.; Liu, C.-C.; Wang, H.-F.; Bernard, J.R.; Huang, C.-C.; Cheng, I.-S. Oral Resveratrol supplementation attenuates exercise-induced Interleukin-6 but not Oxidative Stress after a high intensity cycling challenge in adults. Int. J. Med. Sci. 2021, 18, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Helge, J.W.; Stallknecht, B.; Pedersen, B.K.; Galbo, H.; Kiens, B.; Richter, E.A. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J. Physiol. 2003, 546, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Marko, D.M.; Foran, G.; Vlavcheski, F.; Baron, D.C.; Hayward, G.C.; Baranowski, B.J.; Necakov, A.; Tsiani, E.; MacPherson, R.E.K. Interleukin-6 Treatment Results in GLUT4 Translocation and AMPK Phosphorylation in Neuronal SH-SY5Y Cells. Cells 2020, 9, 1114. [Google Scholar] [CrossRef]
- Ikeda, S.-I.; Tamura, Y.; Kakehi, S.; Sanada, H.; Kawamori, R.; Watada, H. Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 2016, 473, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Lundby, C.; Cotter, J.D.; Burke, L.M. Maximizing Cellular Adaptation to Endurance Exercise in Skeletal Muscle. Cell Metab. 2018, 27, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Chen, H.; Luo, Y.; He, J.; Zheng, P.; Yu, J.; Yu, B. Resveratrol regulates muscle fiber type conversion via miR-22-3p and AMPK/SIRT1/PGC-1α pathway. J. Nutr. Biochem. 2020, 77, 108297. [Google Scholar] [CrossRef] [PubMed]
- Ruple, B.A.; Godwin, J.S.; Mesquita, P.H.C.; Osburn, S.C.; Sexton, C.L.; Smith, M.A.; Ogletree, J.C.; Goodlett, M.D.; Edison, J.L.; Ferrando, A.A.; et al. Myofibril and Mitochondrial Area Changes in Type I and II Fibers Following 10 Weeks of Resistance Training in Previously Untrained Men. Front. Physiol. 2021, 12, 728683. [Google Scholar] [CrossRef]
- Tarini, V.A.F.; Carnevali, L.C.; Arida, R.M.; Cunha, C.A.; Alves, E.S.; Seeleander, M.C.L.; Schmidt, B.; Faloppa, F. Effect of exhaustive ultra-endurance exercise in muscular glycogen and both Alpha1 and Alpha2 Ampk protein expression in trained rats. J. Physiol. Biochem. 2013, 69, 429–440. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Hartogh, D.J.D.; Giacca, A.; Tsiani, E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef]
- Stuart, C.A.; Howell, M.E.A.; Baker, J.D.; Dykes, R.J.; Duffourc, M.M.; Ramsey, M.W.; Stone, M.H. Cycle Training Increased GLUT4 and Activation of Mammalian Target of Rapamycin in Fast Twitch Muscle Fibers. Med. Sci. Sports Exerc. 2010, 42, 96–106. [Google Scholar] [CrossRef]
- Pereira, B.C.; da Rocha, A.L.; Pinto, A.P.; Pauli, J.R.; Moura, L.P.; Mekary, R.A.; de Freitas, E.C.; da Silva, A.S.R. Excessive training impairs the insulin signal transduction in mice skeletal muscles. J. Endocrinol. 2016, 230, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Predominant expression of Sir2α, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004, 556, 281–286. [Google Scholar] [CrossRef]
- Waldman, M.; Cohen, K.; Yadin, D.; Nudelman, V.; Gorfil, D.; Laniado-Schwartzman, M.; Kornwoski, R.; Aravot, D.; Abraham, N.G.; Arad, M.; et al. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1α’. Cardiovasc. Diabetol. 2018, 17, 111. [Google Scholar] [CrossRef]
- Nishigaki, A.; Kido, T.; Kida, N.; Kakita-Kobayashi, M.; Tsubokura, H.; Hisamatsu, Y.; Okada, H. Resveratrol protects mitochondrial quantity by activating SIRT1/PGC-1α expression during ovarian hypoxia. Reprod. Med. Biol. 2020, 19, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Soung, Y.H.; Lee, J.W.; Kim, S.Y.; Nam, S.W.; Park, W.S.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. Mutational analysis of the kinase domain of MYLK2 gene in common human cancers. Pathol. Res. Pract. 2006, 202, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cao, J.; Zhang, J.; Ge, L.; Zhang, H.; Liu, X. Skeletal Muscle Transcriptome Analysis of Hanzhong Ma Duck at Different Growth Stages Using RNA-Seq. Biomolecules 2021, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Bourges, I.; Ramus, C.; de Camaret, B.M.; Beugnot, R.; Remacle, C.; Cardol, P.; Hofhaus, G.; Issartel, J.-P. Structural organization of mitochondrial human complex I: Role of the ND4 and ND5 mitochondria-encoded subunits and interaction with prohibitin. Biochem. J. 2004, 383, 491–499. [Google Scholar] [CrossRef] [PubMed]









| Genes | Direction | Primer Sequence (5′to 3′) |
|---|---|---|
| Gapdh | Forward | TGGTGAAGGTCGGTGTGAAC |
| Reverse | AATGAAGGGGTCGTTGATGG | |
| Tnfα | Forward | CCACCACGCTCTTCTGTCTAC |
| Reverse | AGGGTCTGGGCCATAGAACT | |
| Il6 | Forward | GCTTAATTACACATGTTCTCTGGGAAA |
| Reverse | CAAGTGCATCATCGTTGTTCATAC | |
| Il1β | Forward | TGGACCTTCCAGGATGAGGACA |
| Reverse | GTTCATCTCGGAGCCTGTAGTG | |
| Sirt1 | Forward | CAGACCCTCAAGCCATGTTT |
| Reverse | ACACAGAGACGGCTGGAACT | |
| Glut4 | Forward | GTAACTTCATTGTCGGCATGG |
| Reverse | AGCTGAGATCTGGTCAAACG | |
| Ampkα1 | Forward | CTCAGTTCCTGGAGAAAGATGG |
| Reverse | CTGCCGGTTGAGTATCTTCAC | |
| Ampkα2 | Forward | CAGGCCATAAAGTGGCAGTTA |
| Reverse | AAAAGTCTGTCGGAGTGCTGA | |
| Pgc1α | Forward | TGATGTGAATGACTTGGATACAGACA |
| Reverse | GCTCATTGTTGTACTGGTTGGATATG |
| Parameters | NC | EX | EX + RES25 | EX + RES150 |
|---|---|---|---|---|
| Body weight (g) | 23.2 ± 0.5 | 23.5 ± 0.5 | 23.3 ± 0.4 | 23.3 ± 0.5 |
| Food intake (g/mice/day) | 6.10 ± 0.71 | 6.60 ± 0.59 | 6.60 ± 0.61 | 6.50 ± 0.81 |
| Water intake (mL/mice/day) | 3.80 ± 0.06 | 4.00 ± 0.03 | 4.00 ± 0.03 | 4.00 ± 0.09 |
| Liver (% of BW) | 3.80 ± 0.05 | 3.80 ± 0.22 | 3.50 ± 0.07 | 3.50 ± 0.08 |
| Gastrocnemius muscle (% of BW) | 1.10 ± 0.03 | 1.10 ± 0.03 | 1.10 ± 0.02 | 1.10 ± 0.02 |
| Tibialis anterior muscle (% of BW) | 0.36 ± 0.03 | 0.36 ± 0.03 | 0.34 ± 0.02 | 0.37 ± 0.03 |
| eWAT (% of BW) | 1.00 ± 0.05 a | 0.61 ± 0.05 b | 0.60 ± 0.03 b | 0.68 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L.-Y.; Huang, W.-C.; Kan, N.-W.; Tung, T.-H.; Huynh, L.B.P.; Huang, S.-Y. Effects of Resveratrol on Muscle Inflammation, Energy Utilisation, and Exercise Performance in an Eccentric Contraction Exercise Mouse Model. Nutrients 2023, 15, 249. https://doi.org/10.3390/nu15010249
Su L-Y, Huang W-C, Kan N-W, Tung T-H, Huynh LBP, Huang S-Y. Effects of Resveratrol on Muscle Inflammation, Energy Utilisation, and Exercise Performance in an Eccentric Contraction Exercise Mouse Model. Nutrients. 2023; 15(1):249. https://doi.org/10.3390/nu15010249
Chicago/Turabian StyleSu, Liang-Yu, Wen-Ching Huang, Nai-Wen Kan, Te-Hsuan Tung, Linh Ba Phuong Huynh, and Shih-Yi Huang. 2023. "Effects of Resveratrol on Muscle Inflammation, Energy Utilisation, and Exercise Performance in an Eccentric Contraction Exercise Mouse Model" Nutrients 15, no. 1: 249. https://doi.org/10.3390/nu15010249
APA StyleSu, L.-Y., Huang, W.-C., Kan, N.-W., Tung, T.-H., Huynh, L. B. P., & Huang, S.-Y. (2023). Effects of Resveratrol on Muscle Inflammation, Energy Utilisation, and Exercise Performance in an Eccentric Contraction Exercise Mouse Model. Nutrients, 15(1), 249. https://doi.org/10.3390/nu15010249