Long-Term High-Fat Diet Consumption Induces Cognitive Decline Accompanied by Tau Hyper-Phosphorylation and Microglial Activation in Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
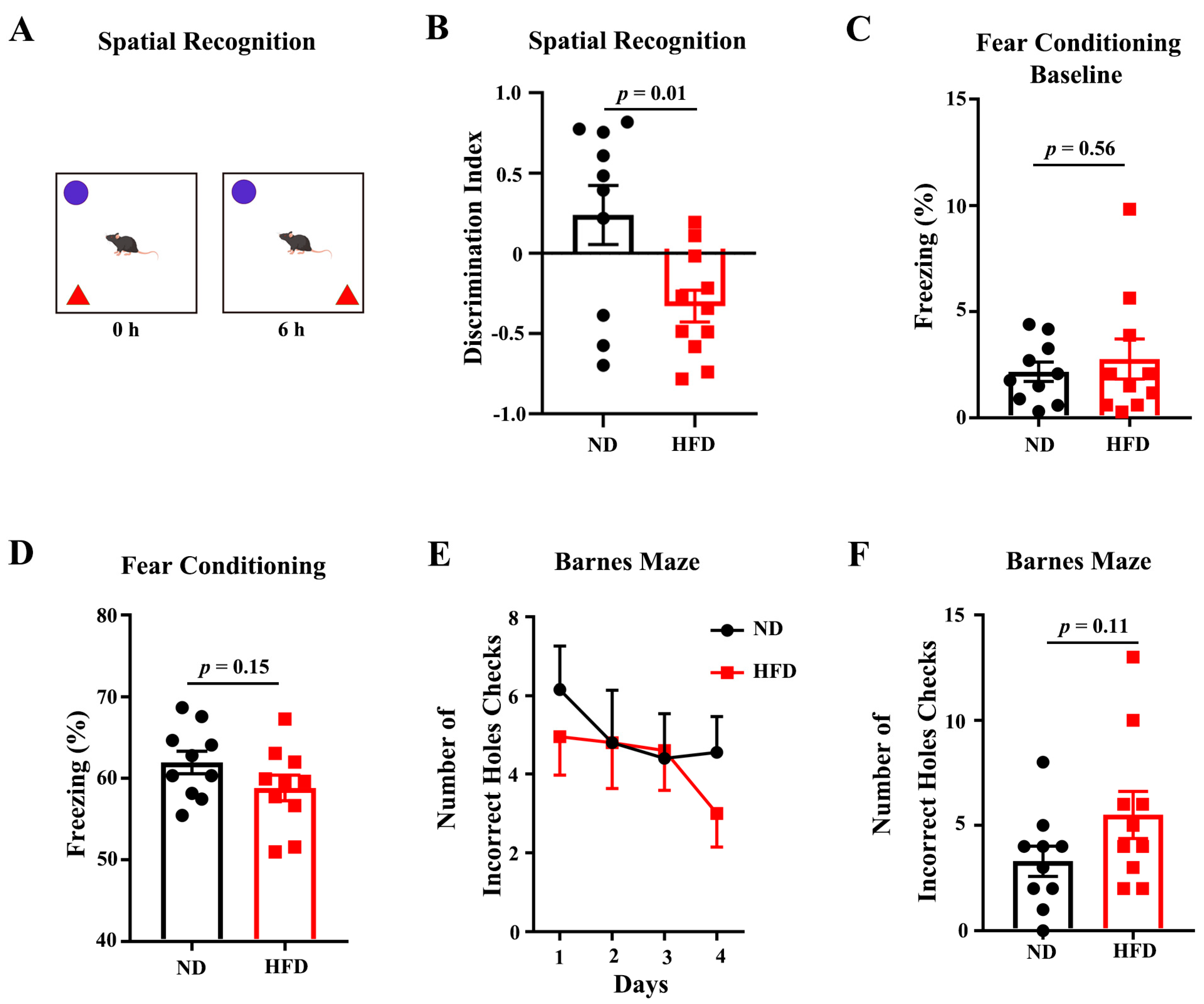
2.2. Object-Place Recognition
2.3. Fear Conditioning
2.4. Barnes Maze
2.5. Immunoblotting
2.6. Immunofluorescence
2.7. Fluoro-Jade C (FJC) Staining
2.8. Statistics
3. Results
3.1. Long-Term HFD Deteriorates Behavioral Performance of Aged Mice
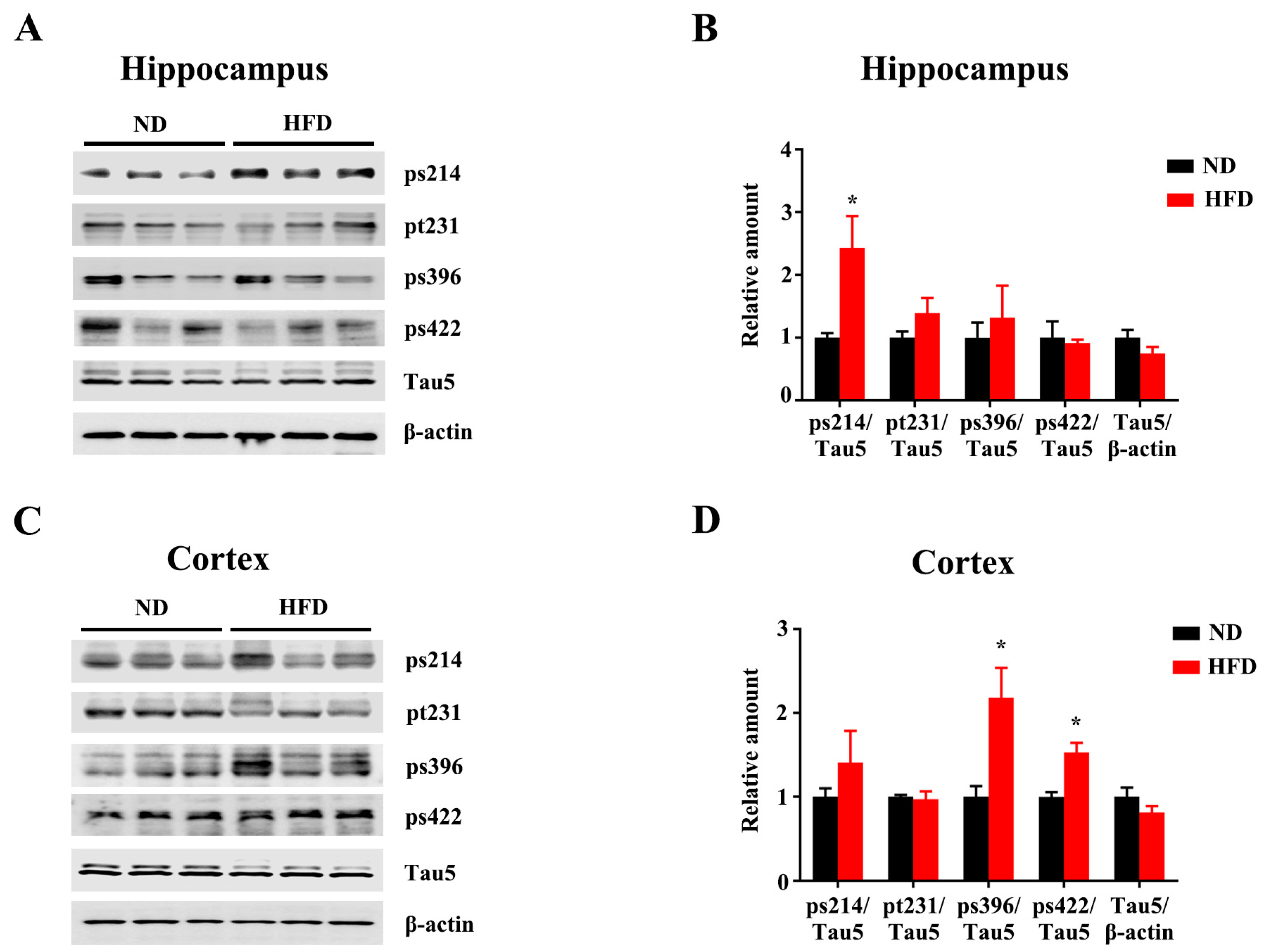
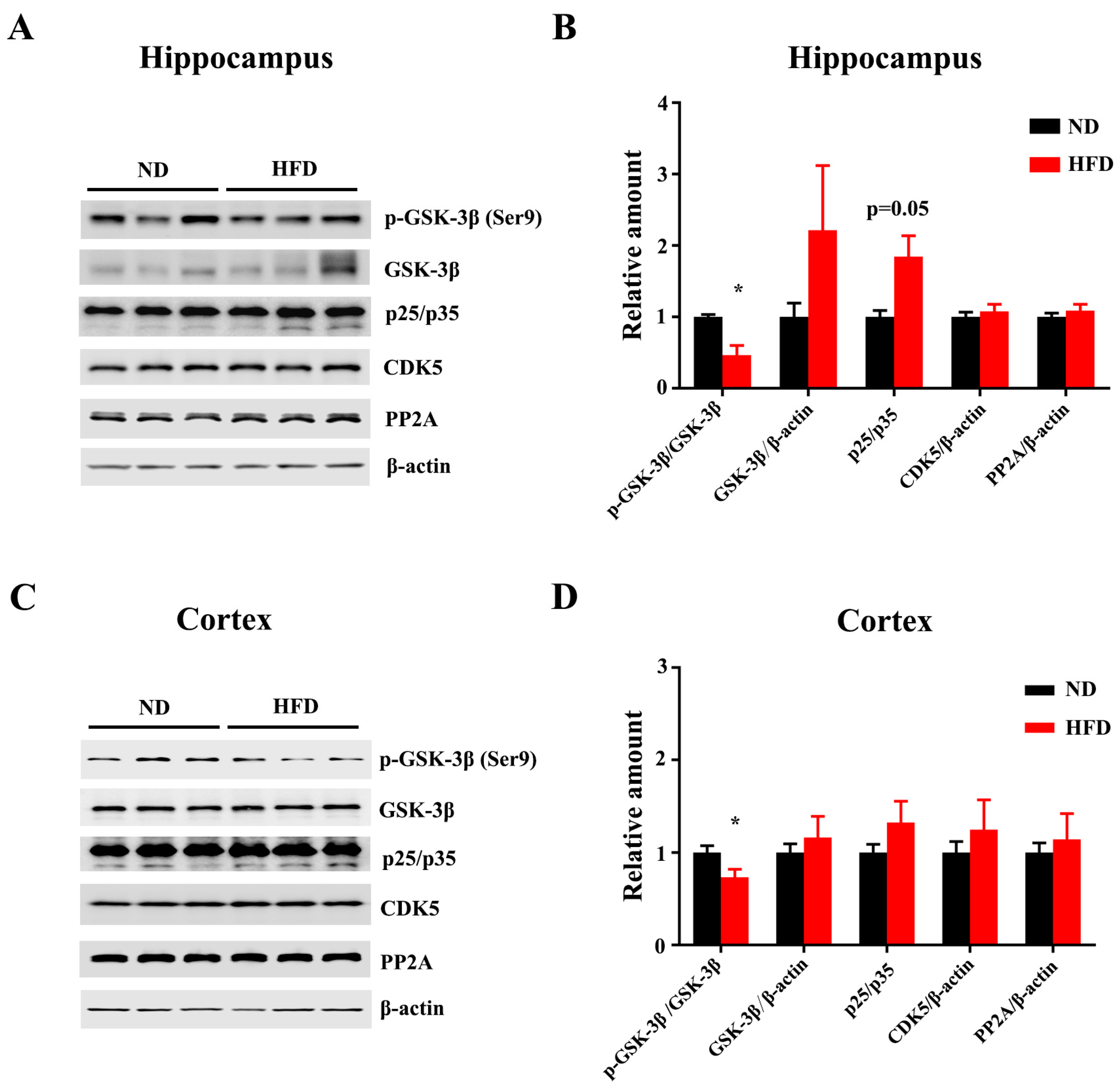
3.2. Long-Term HFD Induces Tau Hyperphosphorylation in Aged Mice
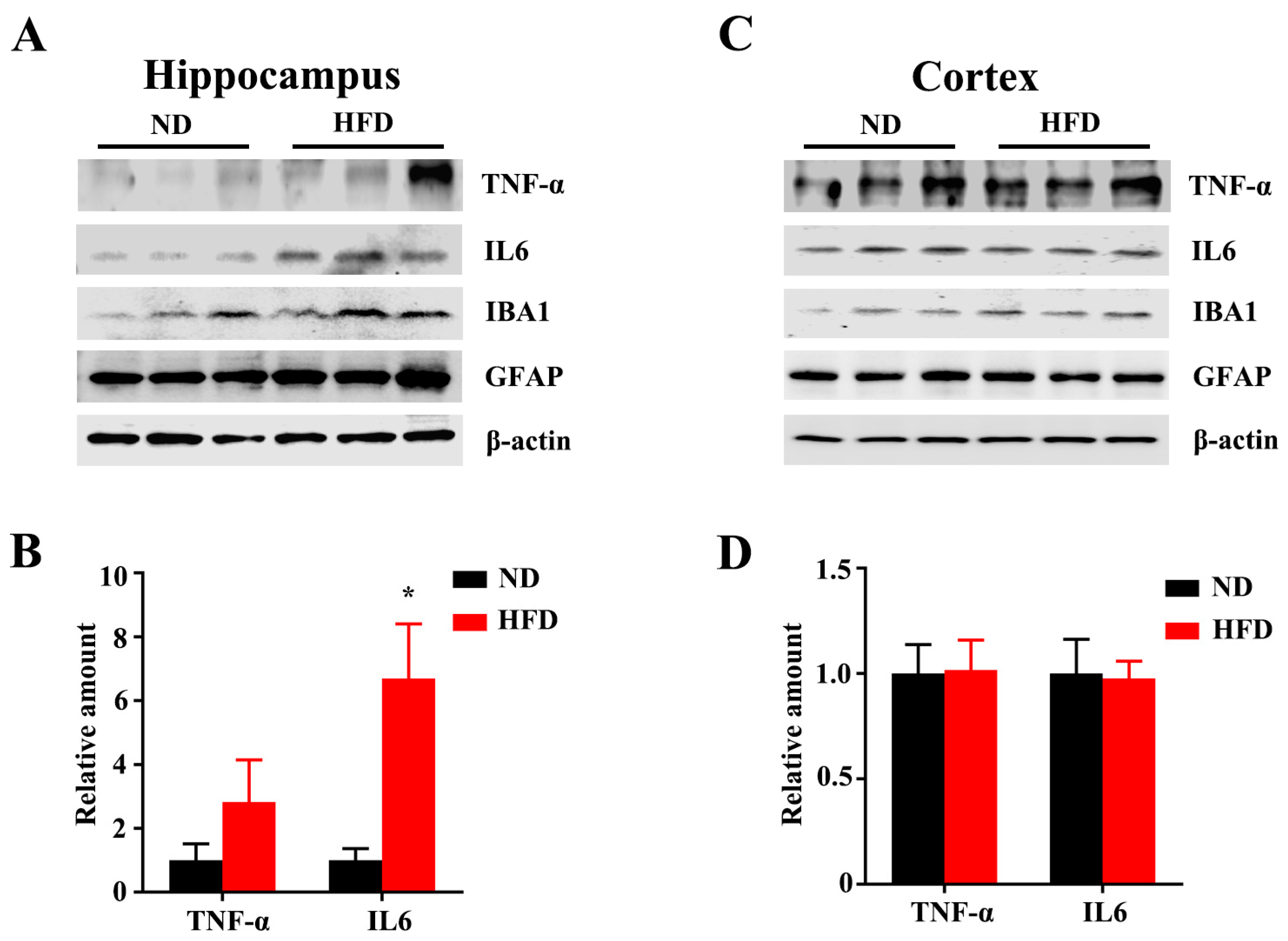
3.3. Long-Term HFD Alters Microglial Morphology and Promotes Inflammation in Aged Mice
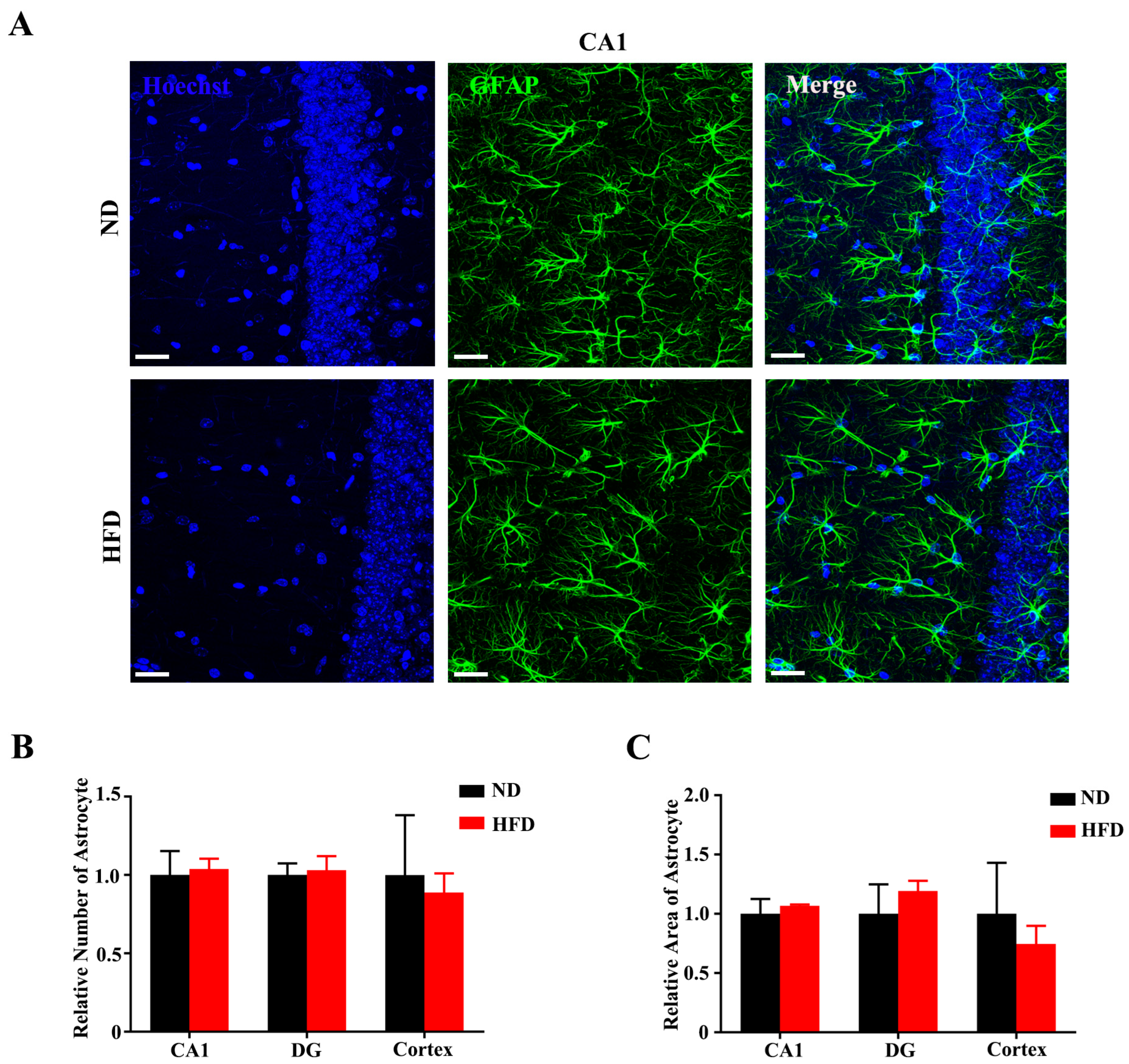
3.4. Long-Term High-Fat Diet Does Not Activate Astrogliosis in Aged Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ataey, A.; Jafarvand, E.; Adham, D.; Moradi-Asl, E. The Relationship Between Obesity, Overweight, and the Human Development Index in World Health Organization Eastern Mediterranean Region Countries. J. Prev. Med. Public Health 2020, 53, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Lartey, S.T.; Si, L.; Otahal, P.; de Graaff, B.; Boateng, G.O.; Biritwum, R.B.; Minicuci, N.; Kowal, P.; Magnussen, C.G.; Palmer, A.J. Annual transition probabilities of overweight and obesity in older adults: Evidence from World Health Organization Study on global AGEing and adult health. Soc. Sci. Med. 2020, 247, 112821. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, J.; Liang, Z.; Gong, X.; Yu, J.; Xiao, Y.; Liu, W.; Wang, X.; Wang, J.Z.; Shu, X. High Fat Diet Mediates Amyloid-beta Cleaving Enzyme 1 Phosphorylation and SUMOylation, Enhancing Cognitive Impairment in APP/PS1 Mice. J. Alzheimers Dis. 2022, 85, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Cordner, Z.A.; Tamashiro, K.L. Effects of high-fat diet exposure on learning & memory. Physiol. Behav. 2015, 152 Pt B, 363–371. [Google Scholar]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Schneider, J.; Wilson, R.S. Dietary fats and the risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 194–200. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Yin, X.; Chen, K.; Hu, Z.; Zhou, Q.; Cao, X.; Chen, Z.; Liu, D. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl. Neurodegener. 2021, 10, 45. [Google Scholar] [CrossRef]
- Wang, J.Z.; Gao, X.; Wang, Z.H. The physiology and pathology of microtubule-associated protein tau. Essays Biochem. 2014, 56, 111–123. [Google Scholar]
- Alonso, A.C.; Zaidi, T.; Grundke-Iqbal, I.; Iqbal, K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 5562–5566. [Google Scholar] [CrossRef] [Green Version]
- Sen, T.; Saha, P.; Jiang, T.; Sen, N. Correction for Sen et al., Sulfhydration of AKT triggers Tau-phosphorylation by activating glycogen synthase kinase 3beta in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2021, 117, 4418–4427. [Google Scholar] [CrossRef]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.-L.; et al. Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.L.; Li, L. Microglia Regulate Neuronal Circuits in Homeostatic and High-Fat Diet-Induced Inflammatory Conditions. Front. Cell. Neurosci. 2021, 15, 722028. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Vidal-Itriago, A.; Milanova, I.; Korpel, N.L.; Kalsbeek, M.J.; Tom, R.Z.; Kalsbeek, A.; Hofmann, S.M.; Yi, C. Deficiency of leptin receptor in myeloid cells disrupts hypothalamic metabolic circuits and causes body weight increase. Mol. Metab. 2018, 7, 155–160. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [Green Version]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Miron, V.E.; Priller, J. Investigating Microglia in Health and Disease: Challenges and Opportunities. Trends Immunol. 2020, 41, 785–793. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Vidal-Itriago, A.; Kalsbeek, M.J.; Layritz, C.; Garcia-Caceres, C.; Tom, R.Z.; Eichmann, T.O.; Vaz, F.M.; Houtkooper, R.H.; van der Wel, N.; et al. Lipoprotein Lipase Maintains Microglial Innate Immunity in Obesity. Cell Rep. 2017, 20, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, H.L.; Tian, N.; Liu, F.; Wang, L.; Yin, Y.; Yue, L.; Ma, L.; Wan, Y.; Wang, J.Z. Interneuron Accumulation of Phosphorylated tau Impairs Adult Hippocampal Neurogenesis by Suppressing GABAergic Transmission. Cell Stem Cell 2020, 26, 331–345.e6. [Google Scholar] [CrossRef] [PubMed]
- Ayata, P.; Badimon, A.; Strasburger, H.J.; Duff, M.K.; Montgomery, S.E.; Loh, Y.E.; Ebert, A.; Pimenova, A.A.; Ramirez, B.R.; Chan, A.T.; et al. Epigenetic regulation of brain region-specific microglia clearance activity. Nat. Neurosci. 2018, 21, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Walter, S.A.; Pennell, N.A. Reactive microgliosis. Prog. Neurobiol. 1999, 57, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Schram, M.T.; Euser, S.M.; de Craen, A.J.; Witteman, J.C.; Frolich, M.; Hofman, A.; Jolles, J.; Breteler, M.M.; Westendorp, R.G. Systemic markers of inflammation and cognitive decline in old age. J. Am. Geriatr. Soc. 2007, 55, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Dziedzic, T. Systemic inflammatory markers and risk of dementia. Am. J. Alzheimers Dis. Other Demen. 2006, 21, 258–262. [Google Scholar] [CrossRef]
- Nguyen, J.C.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef] [Green Version]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef]
- Zenaro, E.; Pietronigro, E.; della Bianca, V.; Piacentino, G.; Marongiu, L.; Budui, E.T.S.; Rossi, B.; Angiari, S.; Dusi, S.; Montreso, A.; et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The origins of age-related proinflammatory state. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piber, D.; Olmstead, R.; Cho, J.H.; Witarama, T.; Perez, C.; Dietz, N.; Seeman, T.E.; Breen, E.C.; Cole, S.W.; Irwin, M.R. Inflammaging: Age and Systemic, Cellular, and Nuclear Inflammatory Biology in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Ishida, Y.; Nonaka, H.; Iwasaki, T. Functional difference between rat perirhinal cortex and hippocampus in object and place discrimination tasks. Behav. Brain Res. 2009, 197, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Polyanskii, V.B.; Evtikhin, D.V.; Sokolov, E.N.; Kryuchkova, A.V. Limited plasticity of difference neurons in the visual cortex and hippocampus in rabbits during the oddball (random substitutions) test. Neurosci. Behav. Physiol. 2006, 36, 441–448. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, J.; Mostert, J.P.; Koch, M.W. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J. Neurol. Sci. 2008, 267, 3–16. [Google Scholar] [CrossRef]
- Seifert, G.; Schilling, K.; Steinhauser, C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat. Rev. Neurosci. 2006, 7, 194–206. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Small, S.A.; Schobel, S.A.; Buxton, R.B.; Witter, M.P.; Barnes, C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 2011, 12, 585–601. [Google Scholar] [CrossRef] [Green Version]
- Pedditzi, E.; Peters, R.; Beckett, N. The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age Ageing 2016, 45, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.M.; Dixit, S.; Saulsberry, A.C.; May, J.M.; Harrison, F.E. Reversal of high fat diet-induced obesity improves glucose tolerance, inflammatory response, beta-amyloid accumulation and cognitive decline in the APP/PSEN1 mouse model of Alzheimer’s disease. Neurobiol. Dis. 2017, 100, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Liang, Z.; Liu, W.; Zhao, Y.; Yang, Y.; Wu, M.; Shang, J.; Xiao, Y.; Mei, Y.; Su, Q.; et al. High Fat Diet Aggravates AD-Related Pathogenic Processes in APP/PS1 Mice. Curr. Alzheimer Res. 2021, 18, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Credle, J.J.; George, J.L.; Wills, J.; Duka, V.; Shah, K.; Lee, Y.C.; Rodriguez, O.; Simkins, T.; Winter, M.; Moechars, D.; et al. GSK-3beta dysregulation contributes to parkinson’s-like pathophysiology with associated region-specific phosphorylation and accumulation of tau and alpha-synuclein. Cell Death Differ. 2015, 22, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Bocarsly, M.E.; Fasolino, M.; Kane, G.A.; LaMarca, E.A.; Kirschen, G.W.; Karatsoreos, I.N.; McEwen, B.S.; Gould, E. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl. Acad. Sci. USA 2015, 112, 15731–15736. [Google Scholar] [CrossRef] [Green Version]
- Cope, E.C.; LaMarca, E.A.; Monari, P.K.; Olson, L.B.; Martinez, S.; Zych, A.D.; Katchur, N.J.; Gould, E. Microglia Play an Active Role in Obesity-Associated Cognitive Decline. J. Neurosci. 2018, 38, 8889–8904. [Google Scholar] [CrossRef] [Green Version]
- Maldonado-Ruiz, R.; Montalvo-Martinez, L.; Fuentes-Mera, L.; Camacho, A. Microglia activation due to obesity programs metabolic failure leading to type two diabetes. Nutr. Diabetes 2017, 7, e254. [Google Scholar] [CrossRef] [Green Version]
- Shu, C.J.; Benoist, C.; Mathis, D. The immune system’s involvement in obesity-driven type 2 diabetes. Semin. Immunol. 2012, 24, 436–442. [Google Scholar] [CrossRef]


| Antibody | Specificity | Type | Species | Source (Catalog Number) |
|---|---|---|---|---|
| Anti-tau (pS214) | Tau phosphorylated at Ser214 | pAb | Rabbit | Immunoway (YP0259) |
| Anti-tau (pT231) | Tau phosphorylated at Thr231 | pAb | Rabbit | Immunoway (YP0267) |
| Anti-tau (pS396) | Tau phosphorylated at Ser396 | pAb | Rabbit | Immunoway (YP0263) |
| Anti-tau (pS422) | Tau phosphorylated at Ser422 | pAb | Rabbit | Immunoway (YP0845) |
| Anti-tau (pS199) | Tau phosphorylated at Ser199 | pAb | Rabbit | GeneTex (GTX24749) |
| Anti-Tau (Tau-5) | Total Tau | mAb | Mouse | Abcam (ab80579) |
| Anti-GSK3β (pS9) | GSK3β phosphorylated at Ser9 | mAb | Rabbit | Cell Signaling (5558S) |
| Anti-GSK3β | Total GSK3β | mAb | Mouse | Immunoway (YM3633) |
| Anti-p25/p35 | Total p25 and p35 | mAb | Rabbit | Cell Signaling (2680S) |
| Anti-CDK5 | Total CDK5 | mAb | Rabbit | Cell Signaling (14145S) |
| Anti-PP2Ac | Total PP2A c subunit | mAb | Rabbit | Cell Signaling (2038S) |
| Anti-TNF-α | Total TNF-α | mAb | Mouse | Proteintech (60291-1-Ig) |
| Anti-IL-6 | Total IL-6 | pAb | Rabbit | Proteintech (21865-1-AP) |
| Anti-IBA1 | Total IBA1 | mAb | Rabbit | Abcam (ab178846) |
| Anti-GFAP(GA5) | Total GFAP | mAb | Mouse | Cell Signaling (3670S) |
| Anti-Synapsin-1 | Total Synapsin-1 | mAb | Rabbit | Cell Signaling (5297S) |
| Anti-Synaptotagmin | Total Synaptotagmin | mAb | Rabbit | Cell Signaling (14558S) |
| Anti-PSD93 | Total PSD93 | mAb | Rabbit | Cell Signaling (19046S) |
| Anti-PSD95 | Total PSD95 | mAb | Rabbit | Cell Signaling (3450S) |
| Anti-β-actin | Total β-actin | mAb | Mouse | Cell Signaling (3700S) |
| Anti-Rabbit 488 | Donkey anti-Rabbit Alexa Fluor™ 488 | pAb | Rabbit | Invitrogen (A21206) |
| Anti-Mouse 488 | Donkey anti-Mouse Alexa Fluor™ 488 | pAb | Mouse | Invitrogen (A21202) |
| HRP-Ribbit IgG | HRP Conjugated Goat Anti-Ribbit IgG | pAb | Rabbit | BOSTER (BA1050) |
| HRP-Mouse IgG | HRP Conjugated Goat Anti-mouse IgG | pAb | Mouse | BOSTER (BA1054) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Gong, X.; Ye, R.; Zhao, Y.; Yu, J.; Zhao, Y.; Bao, J. Long-Term High-Fat Diet Consumption Induces Cognitive Decline Accompanied by Tau Hyper-Phosphorylation and Microglial Activation in Aging. Nutrients 2023, 15, 250. https://doi.org/10.3390/nu15010250
Liang Z, Gong X, Ye R, Zhao Y, Yu J, Zhao Y, Bao J. Long-Term High-Fat Diet Consumption Induces Cognitive Decline Accompanied by Tau Hyper-Phosphorylation and Microglial Activation in Aging. Nutrients. 2023; 15(1):250. https://doi.org/10.3390/nu15010250
Chicago/Turabian StyleLiang, Zheng, Xiaokang Gong, Runjia Ye, Yang Zhao, Jin Yu, Yanna Zhao, and Jian Bao. 2023. "Long-Term High-Fat Diet Consumption Induces Cognitive Decline Accompanied by Tau Hyper-Phosphorylation and Microglial Activation in Aging" Nutrients 15, no. 1: 250. https://doi.org/10.3390/nu15010250





