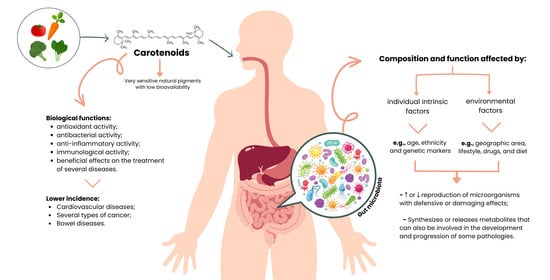
Carotenoids Diet: Digestion, Gut Microbiota Modulation, and Inflammatory Diseases
Abstract
:1. Introduction
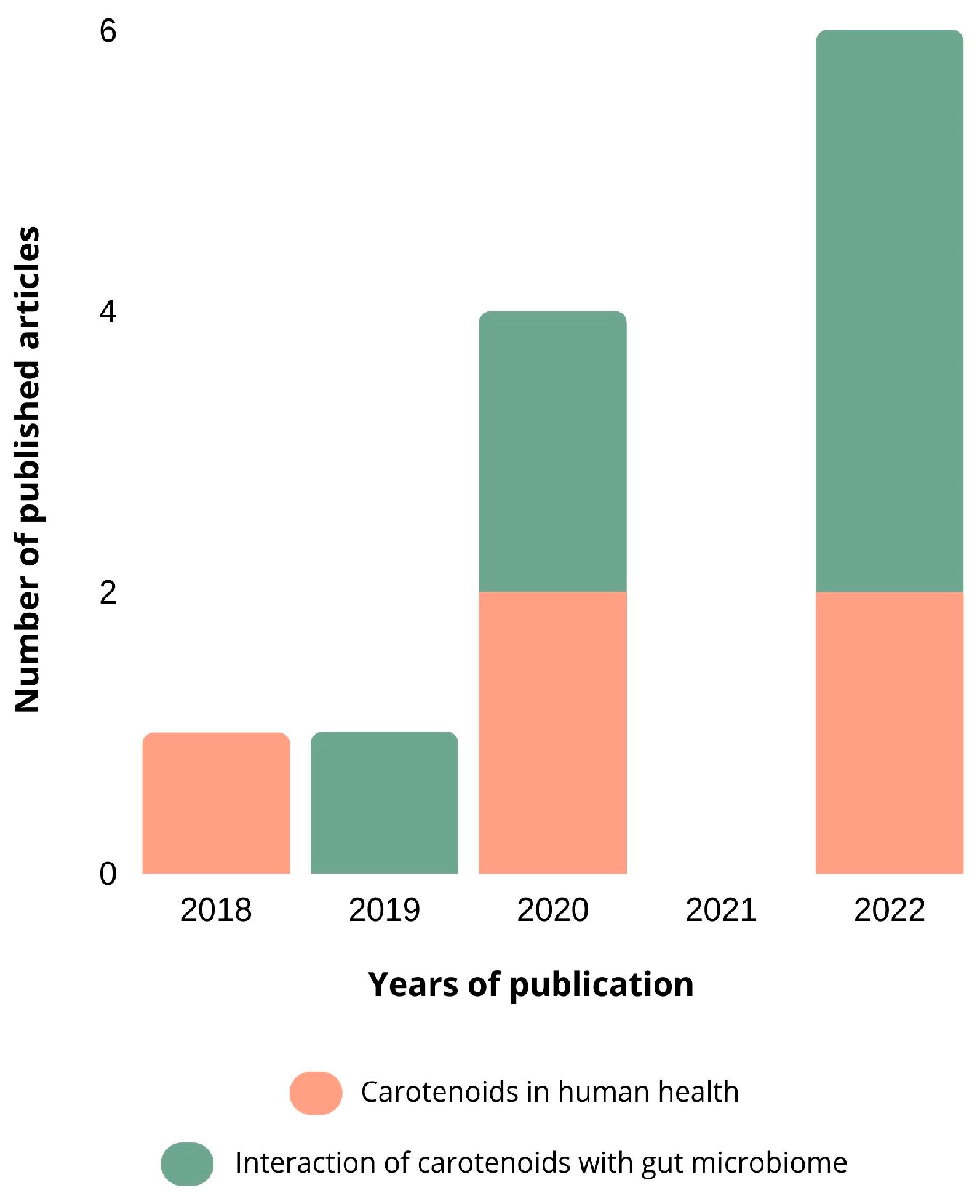
2. Overview of the Publications
2.1. Methodology of Research
2.2. Results
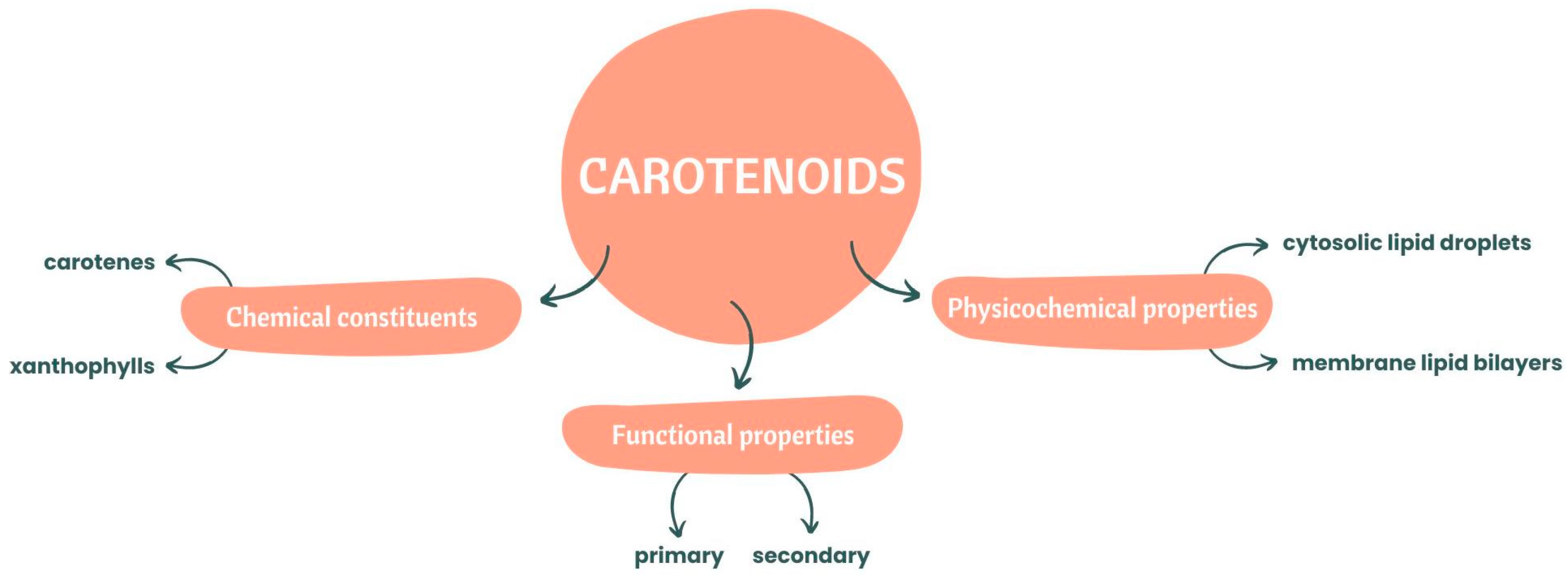
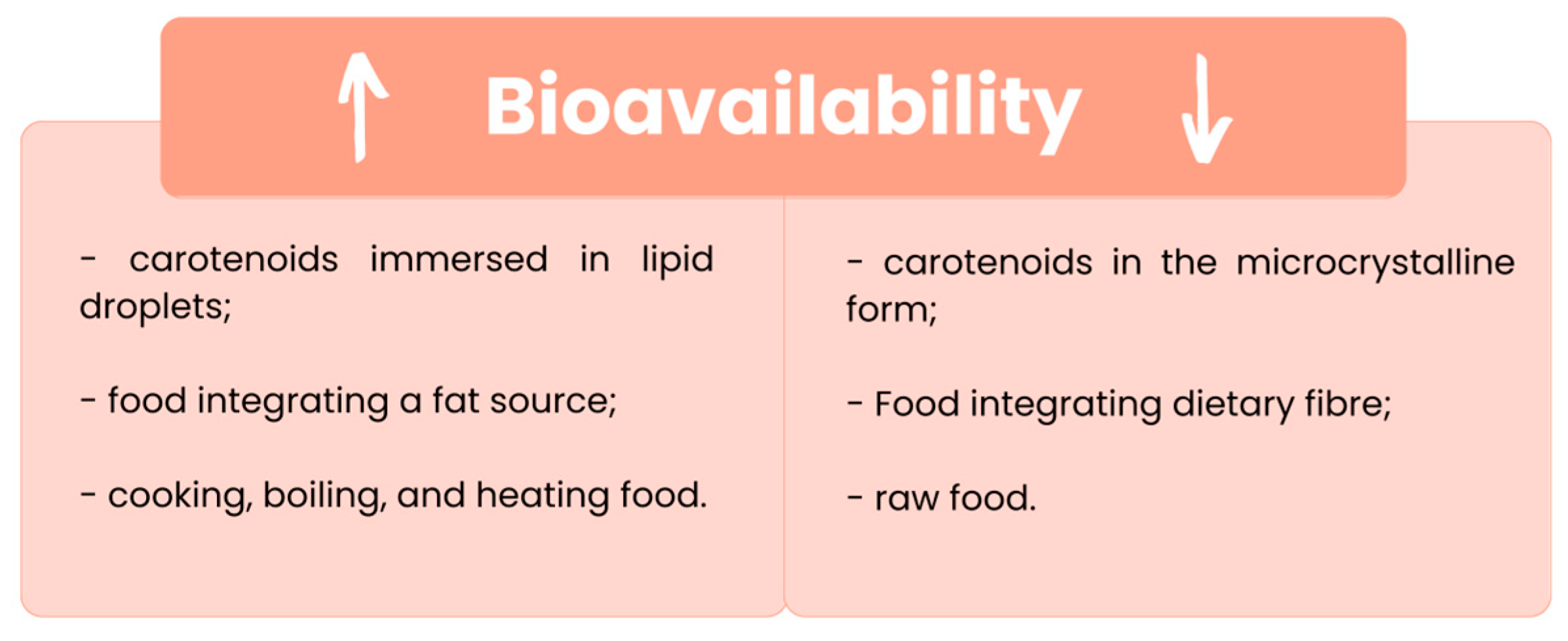
3. Bioaccessibility and Bioavailability of Carotenoids
4. Carotenoid Absorption Mechanism
4.1. Release from the Food Matrix
4.2. Transfer to the Oil Phase
4.3. Micelle Formation
4.4. Absorption
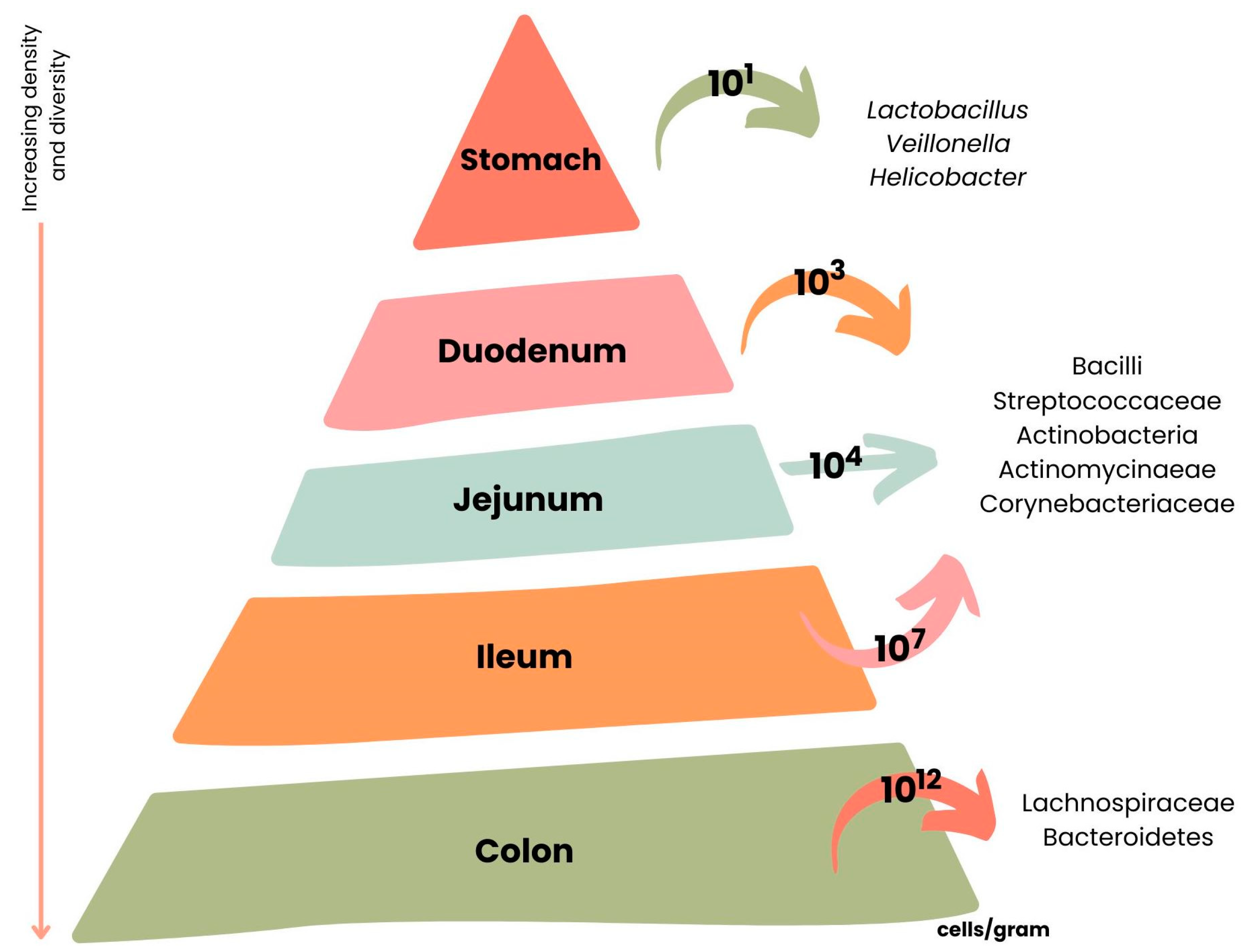
5. Intestinal Microbiota
6. Intestinal Microbiota Metabolites
7. Interaction between Carotenoids and the Intestinal Microbiota
8. Carotenoid Metabolites from Microbiota and Activation/Deactivation of Gene Potentiation in Bowel Diseases
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bolhassani, A.; Milani, A.; Basirnejad, M.; Shahbazi, S. Carotenoids: Biochemistry, Pharmacology and treatment. Br. J. Pharm. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.; Ye, R.; Liu, J. Health Benefits of Carotenoids and Potential Application in Poultry Industry: A Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda-Muñoz, M.; Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M. A Review of Factors That Affect Carotenoid Concentrations in Human Plasma: Differences between Mediterranean and Northern Diets. Eur. J. Clin. Nutr. 2019, 72, 18–25. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.M.; Blaner, W.S. Retinol and Retinyl Esters: Biochemistry and Physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ahmad, S.; Ahmad, A. Green Extraction Methods and Environmental Applications of Carotenoids-a Review. RSC Adv. 2015, 5, 62358–62393. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Health Protective Effects of Carotenoids and Their Interactions with Other Biological Antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of Microparticles of Carotenoids from Natural and Synthetic Sources for Applications in Food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- Yaroshevich, I.A.; Krasilnikov, P.M.; Rubin, A.B. Functional Interpretation of the Role of Cyclic Carotenoids in Photosynthetic Antennas via Quantum Chemical Calculations. Comput. Theor. Chem. 2015, 1070, 27–32. [Google Scholar] [CrossRef]
- Coelho, M.C.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Integral Valorisation of Tomato By-Products towards Bioactive Compounds Recovery: Human Health Benefits. Food Chem. 2023, 410, 135319. [Google Scholar] [CrossRef] [PubMed]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of Methods for Analysis of Carotenoids. TrAC—Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, Inflammation, and Oxidative Stress-Implications of Cellular Signaling Pathways and Relation to Chronic Disease Prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and Beta-Carotene Induce Cell-Cycle Arrest and Apoptosis in Human Breast Cancer Cell Lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar] [PubMed]
- Meurer, M.C.; Mees, M.; Mariano, L.N.B.; Boeing, T.; Somensi, L.B.; Mariott, M.; da Silva, R.d.C.M.V.d.A.F.; dos Santos, A.C.; Longo, B.; Santos França, T.C.; et al. Hydroalcoholic Extract of Tagetes Erecta L. Flowers, Rich in the Carotenoid Lutein, Attenuates Inflammatory Cytokine Secretion and Improves the Oxidative Stress in an Animal Model of Ulcerative Colitis. Nutr. Res. 2019, 66, 95–106. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. What Is Inflammatory Bowel Disease (IBD)? Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022.
- Hu, F.; Wang Yi, B.; Zhang, W.; Liang, J.; Lin, C.; Li, D.; Wang, F.; Pang, D.; Zhao, Y. Carotenoids and Breast Cancer Risk: A Meta-Analysis and Meta-Regression. Breast Cancer Res. Treat. 2012, 131, 239–253. [Google Scholar] [CrossRef]
- Arain, M.A.; Mei, Z.; Hassan, F.U.; Saeed, M.; Alagawany, M.; Shar, A.H.; Rajput, I.R. Lycopene: A Natural Antioxidant for Prevention of Heat-Induced Oxidative Stress in Poultry. Worlds Poult. Sci. J. 2017, 74, 89–100. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Donhowe, E.G.; Kong, F. Beta-Carotene: Digestion, Microencapsulation, and In Vitro Bioavailability. Food Bioproc. Tech. 2014, 7, 338–354. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential Allies of Cardiovascular Health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T.J.M. Lutein: More than Just a Filter for Blue Light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Yu, R.-B.; Liu, R.; Hao, Z.-X.; Han, C.-C.; Zhu, Z.-H.; Ma, L. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients 2014, 6, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhang, Y.; Li, Y.; Lu, K.; Shen, Y.; Guo, Y.; Qi, Q.; Wang, M.; Zhang, S. NrF2/ARE and NF-ΚB Pathway Regulation May Be the Mechanism for Lutein Inhibition of Human Breast Cancer Cell. Future Oncol. 2018, 14, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing By-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, Medicinal Properties and Their Application in Food and Nutraceutical Industry. J. Med. Plant Res. 2011, 5, 7119–7131. [Google Scholar] [CrossRef]
- Dai, Z.; Li, Z.; Shi, E.; Nie, M.; Feng, L.; Chen, G.; Gao, R.; Zeng, X.; Li, D. Study on the Interaction between Four Typical Carotenoids and Human Gut Microflora Using an in Vitro Fermentation Model. J. Agric. Food Chem. 2022, 70, 13592–13601. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Feng, W.; Liu, J.; Cheng, H.; Zhang, D.; Tan, Y.; Peng, C. Dietary Compounds in Modulation of Gut Microbiota-Derived Metabolites. Front. Nutr. 2022, 9, 939571. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.; Ding, Y.; Wan, P.; Peng, Y.; Chen, C.; Ye, H.; Zeng, X.; Ran, L. Polysaccharides from the Flowers of Tea (Camellia sinensis L.) Modulate Gut Health and Ameliorate Cyclophosphamide-Induced Immunosuppression. J. Funct. Foods 2019, 61, 103470. [Google Scholar] [CrossRef]
- Ekesa, B.; Poulaert, M.; Davey, M.W.; Kimiywe, J.; van den Bergh, I.; Blomme, G.; Dhuique-Mayer, C. Bioaccessibility of Provitamin A Carotenoids in Bananas (Musa Spp.) and Derived Dishes in African Countries. Food Chem. 2012, 133, 1471–1477. [Google Scholar] [CrossRef]
- Sy, C.; Gleize, B.; Dangles, O.; Landrier, J.F.; Veyrat, C.C.; Borel, P. Effects of Physicochemical Properties of Carotenoids on Their Bioaccessibility, Intestinal Cell Uptake, and Blood and Tissue Concentrations. Mol. Nutr. Food Res. 2012, 56, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Haskell, M.J. The Challenge to Reach Nutritional Adequacy for Vitamin A: β-Carotene Bioavailability and Conversion—Evidence in Humans. Am. J. Clin. Nutr. 2012, 96, 1193S–1203S. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Lima, M.J.R.; Oliveira, J.; Teixeira-Lemos, E. Tomato Lycopene: Functional Proprieties and Health. Int. J. Agric. Biosyst. Eng. 2015, 9, 1089–1099. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Kotake-Nara, E.; Hase, M. Effects of Fats and Oils on the Bioaccessibility of Carotenoids and Vitamin E in Vegetables. Biosci. Biotechnol. Biochem. 2013, 77, 1055–1060. [Google Scholar] [CrossRef]
- Molteni, C.; la Motta, C.; Valoppi, F. Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants 2022, 11, 1931. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in Human Health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Arab, M.; Bahramian, B.; Schindeler, A.; Valtchev, P.; Dehghani, F.; McConchie, R. Extraction of Phytochemicals from Tomato Leaf Waste Using Subcritical Carbon Dioxide. Innov. Food Sci. Emerg. Technol. 2019, 57, 102204. [Google Scholar] [CrossRef]
- Manna, L.; Bugnone, C.A.; Banchero, M. Valorization of Hazelnut, Coffee and Grape Wastes through Supercritical Fluid Extraction of Triglycerides and Polyphenols. J. Supercrit. Fluids 2015, 104, 204–211. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial Activity and Composition Profile of Grape (Vitis Vinifera) Pomace Extracts Obtained by Supercritical Fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef] [PubMed]
- de Campos, L.M.A.S.; Leimann, F.V.; Pedrosa, R.C.; Ferreira, S.R.S. Free Radical Scavenging of Grape Pomace Extracts from Cabernet Sauvingnon (Vitis Vinifera). Bioresour. Technol. 2008, 99, 8413–8420. [Google Scholar] [CrossRef]
- Coelho, M.C.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. The Use of Emergent Technologies to Extract Added Value Compounds from Grape By-Products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of Tomato By-Products’ Bioactive Compounds Using Ohmic Technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Campos, D.; Nunes, J.; Vicente, A.A.; Pintado, M. Simulated Digestion of an Olive Pomace Water-Soluble Ingredient: Relationship between the Bioaccessibility of Compounds and Their Potential Health Benefits. Food Funct. 2020, 11, 2238–2254. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of Nutraceuticals: Role of the Food Matrix, Processing Conditions, the Gastrointestinal Tract, and Nanodelivery Systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of Chromoplast Morphology on Carotenoid Bioaccessibility of Carrot, Mango, Papaya, and Tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, M.; Decker, E.A.; McClements, D.J. Influence of Protein Type on Oxidation and Digestibility of Fish Oil-in-Water Emulsions: Gliadin, Caseinate, and Whey Protein. Food Chem. 2015, 175, 249–257. [Google Scholar] [CrossRef]
- Iddir, M.; Degerli, C.; Dingeo, G.; Desmarchelier, C.; Schleeh, T.; Borel, P.; Larondelle, Y.; Bohn, T. Whey Protein Isolate Modulates Beta-Carotene Bioaccessibility Depending on Gastro-Intestinal Digestion Conditions. Food Chem. 2019, 291, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids Bioavailability from Foods: From Plant Pigments to Efficient Biological Activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Roodenburg, A.J.; Leenen, R.; van het Hof, K.H.; Weststrate, J.A.; Tijburg, L.B. Amount of Fat in the Diet Affects Bioavailability of Lutein Esters but Not of Alpha-Carotene, Beta-Carotene, and Vitamin E in Humans. Am. J. Clin. Nutr. 2000, 71, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Niranjana, R.; Gayathri, R.; Nimish Mol, S.; Sugawara, T.; Hirata, T.; Miyashita, K.; Ganesan, P. Carotenoids Modulate the Hallmarks of Cancer Cells. J. Funct. Foods 2015, 18, 968–985. [Google Scholar] [CrossRef]
- Borel, P.; Lietz, G.; Goncalves, A.; Szabo de Edelenyi, F.; Lecompte, S.; Curtis, P.; Goumidi, L.; Caslake, M.J.; Miles, E.A.; Packard, C.; et al. CD36 and SR-BI Are Involved in Cellular Uptake of Provitamin A Carotenoids by Caco-2 and HEK Cells, and Some of Their Genetic Variants Are Associated with Plasma Concentrations of These Micronutrients in Humans. J. Nutr. 2013, 143, 448–456. [Google Scholar] [CrossRef]
- Reboul, E.; Abou, L.; Mikail, C.; Ghiringhelli, O.; André, M.; Portugal, H.; Jourdheuil-Rahmani, D.; Amiot, M.-J.; Lairon, D.; Borel, P. Lutein Transport by Caco-2 TC-7 Cells Occurs Partly by a Facilitated Process Involving the Scavenger Receptor Class B Type I (SR-BI). Biochem. J. 2005, 387, 455–461. [Google Scholar] [CrossRef]
- Moussa, M.; Landrier, J.-F.; Reboul, E.; Ghiringhelli, O.; Coméra, C.; Collet, X.; Fröhlich, K.; Böhm, V.; Borel, P. Lycopene Absorption in Human Intestinal Cells and in Mice Involves Scavenger Receptor Class B Type I but Not Niemann-Pick C1-like 1. J. Nutr. 2008, 138, 1432–1436. [Google Scholar] [CrossRef]
- von Lintig, J.; Moon, J.; Lee, J.; Ramkumar, S. Carotenoid Metabolism at the Intestinal Barrier. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2020, 1865, 158580. [Google Scholar] [CrossRef]
- Yu, M.; Lau, T.Y.; Carr, S.A.; Krieger, M. Contributions of a Disulfide Bond and a Reduced Cysteine Side Chain to the Intrinsic Activity of the High-Density Lipoprotein Receptor SR-BI. Biochemistry 2012, 51, 10044–10055. [Google Scholar] [CrossRef]
- Rodrigueza, W.V.; Thuahnai, S.T.; Temel, R.E.; Lund-Katz, S.; Phillips, M.C.; Williams, D.L. Mechanism of Scavenger Receptor Class B Type I-Mediated Selective Uptake of Cholesteryl Esters from High Density Lipoprotein to Adrenal Cells. J. Biol. Chem. 1999, 274, 20344–20350. [Google Scholar] [CrossRef]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.-F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D Intestinal Absorption Is Not a Simple Passive Diffusion: Evidences for Involvement of Cholesterol Transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.R.; Altmann, S.W. Niemann–Pick C1 Like 1 (NPC1L1) an Intestinal Sterol Transporter. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2009, 1791, 679–683. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid Transport Is Decreased and Expression of the Lipid Transporters SR-BI, NPC1L1, and ABCA1 Is Downregulated in Caco-2 Cells Treated with Ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Suzuki, R.; Kobayashi, M.; Itagaki, S.; Hirano, T.; Noda, T.; Mizuno, S.; Sugawara, M.; Iseki, K. Involvement of Cholesterol Membrane Transporter Niemann-Pick C1-Like 1 in the Intestinal Absorption of Lutein. J. Pharm. Pharm. Sci. 2012, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Gut Bacteria in Health and Disease. Gastroenterol. Hepatol. 2013, 9, 560–569. [Google Scholar]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef]
- Wiley, N.C.; Dinan, T.G.; Ross, R.P.; Stanton, C.; Clarke, G.; Cryan, J.F. The Microbiota-Gut-Brain Axis as a Key Regulator of Neural Function and the Stress Response: Implications for Human and Animal Health. J. Anim. Sci. 2017, 95, 3225–3246. [Google Scholar] [CrossRef]
- Fine, R.L.; Manfredo Vieira, S.; Gilmore, M.S.; Kriegel, M.A. Mechanisms and Consequences of Gut Commensal Translocation in Chronic Diseases. Gut Microbes 2020, 11, 217–230. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Interactions between Gut Microbiota and Berberine, a Necessary Procedure to Understand the Mechanisms of Berberine. J. Pharm. Anal. 2022, 12, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, N.M.; Teixeira, F.; Silva, S.; Madureira, A.R.; Pintado, M.E. Potential Prebiotic Activity of Tenebrio Molitor Insect Flour Using an Optimized In Vitro Gut Microbiota Model. Food Funct. 2019, 10, 3909–3922. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; He, F.; Li, L.; Guo, L.; Zhang, B.; Yu, S.; Zhao, W. Bioavailability Based on the Gut Microbiota: A New Perspective. Microbiol. Mol. Biol. Rev. 2020, 84, e00072-19. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef]
- von Lintig, J.; Hessel, S.; Isken, A.; Kiefer, C.; Lampert, J.M.; Voolstra, O.; Vogt, K. Towards a Better Understanding of Carotenoid Metabolism in Animals. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2005, 1740, 122–131. [Google Scholar] [CrossRef]
- Giuliano, G.; Al-Babili, S.; von Lintig, J. Carotenoid Oxygenases: Cleave It or Leave It. Trends Plant Sci. 2003, 8, 145–149. [Google Scholar] [CrossRef]
- Floss, D.S.; Walter, M.H. Role of Carotenoid Cleavage Dioxygenase 1 (CCD1) in Apocarotenoid Biogenesis Revisited. Plant Signal. Behav. 2009, 4, 172–175. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shui, X.; Liang, Z.; Huang, Z.; Qi, Y.; He, Y.; Chen, C.; Luo, H.; Lei, W. Gut Microbiota Metabolites as Integral Mediators in Cardiovascular Diseases (Review). Int. J. Mol. Med. 2020, 46, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Waclawiková, B.; el Aidy, S. Role of Microbiota and Tryptophan Metabolites in the Remote Effect of Intestinal Inflammation on Brain and Depression. Pharmaceuticals 2018, 11, 63. [Google Scholar] [CrossRef]
- McCarville, J.L.; Chen, G.Y.; Cuevas, V.D.; Troha, K.; Ayres, J.S. Microbiota Metabolites in Health and Disease. Annu. Rev. Immunol. 2020, 38, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liu, J.; Ao, H.; Yue, S.; Peng, C. Targeting Gut Microbiota for Precision Medicine: Focusing on the Efficacy and Toxicity of Drugs. Theranostics 2020, 10, 11278–11301. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Dalile, B.; van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Felizardo, R.J.F.; Watanabe, I.K.M.; Dardi, P.; Rossoni, L.V.; Câmara, N.O.S. The Interplay among Gut Microbiota, Hypertension and Kidney Diseases: The Role of Short-Chain Fatty Acids. Pharm. Res. 2019, 141, 366–377. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharm. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Pan, L.-L.; Li, B.-B.; Pan, X.-H.; Sun, J. Gut Microbiota in Pancreatic Diseases: Possible New Therapeutic Strategies. Acta Pharm. Sin. 2021, 42, 1027–1039. [Google Scholar] [CrossRef]
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Haraguchi, A.; Ikeda, Y.; Fukuda, S.; Shibata, S. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.C.; Flynn, C.R. Bile Acids, Their Receptors, and the Gut Microbiota. Physiology 2021, 36, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, K.; MacSharry, J.; Casey, P.G.; Shanahan, F.; Joyce, S.A.; Gahan, C.G.M. Unconjugated Bile Acids Influence Expression of Circadian Genes: A Potential Mechanism for Microbe-Host Crosstalk. PLoS ONE 2016, 11, e0167319. [Google Scholar] [CrossRef]
- McMillin, M.; DeMorrow, S. Effects of Bile Acids on Neurological Function and Disease. FASEB J. 2016, 30, 3658–3668. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Ostojic, S.M. Inadequate Production of H2 by Gut Microbiota and Parkinson Disease. Trends Endocrinol. Metab. 2018, 29, 286–288. [Google Scholar] [CrossRef]
- Sen, N. Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration. J. Mol. Biol. 2017, 429, 543–561. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Berean, K.J.; Burgell, R.E.; Muir, J.G.; Gibson, P.R. Intestinal Gases: Influence on Gut Disorders and the Role of Dietary Manipulations. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 733–747. [Google Scholar] [CrossRef]
- Singh, S.B.; Lin, H.C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Stone, T.W.; Maes, M.; Misiak, B.; Samochowiec, J.; Szulc, A. Gut Microbiota-Derived Vitamins—Underrated Powers of a Multipotent Ally in Psychiatric Health and Disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 107, 110240. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, V.; Rezzi, S.; Eggersdorfer, M.; Galli, F. Metabolic and Functional Interplay between Gut Microbiota and Fat-Soluble Vitamins. Crit. Rev. Food Sci. Nutr. 2021, 61, 3211–3232. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic Adaptation to a High-Fat Diet Is Associated with a Change in the Gut Microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota-Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.-J.; Zhao, D.-M.; Chen, B.; Zhang, G.-Q.; Chen, S.; Cao, R.-F.; Yu, H.; Zhao, C.-Y.; et al. Probiotics and Fructo-Oligosaccharide Intervention Modulate the Microbiota-Gut Brain Axis to Improve Autism Spectrum Reducing Also the Hyper-Serotonergic State and the Dopamine Metabolism Disorder. Pharm. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Wang, Z.; Cui, Y.; Tan, X.; Yuan, T.; Liu, Q.; Liu, Z.; Liu, X. Supplementation of Lycopene Attenuates Lipopolysaccharide-Induced Amyloidogenesis and Cognitive Impairments via Mediating Neuroinflammation and Oxidative Stress. J. Nutr. Biochem. 2018, 56, 16–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How Gut Microbiota Contributes to Heart Failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharm. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharm. Sci 2021, 42, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, S.; Ma, N.; Johnston, L.J.; Wu, C.; Ma, X. Metabolites of Microbiota Response to Tryptophan and Intestinal Mucosal Immunity: A Therapeutic Target to Control Intestinal Inflammation. Med. Res. Rev. 2021, 41, 1061–1088. [Google Scholar] [CrossRef]
- Donia, M.S.; Fischbach, M.A. Small Molecules from the Human Microbiota. Science 2015, 349, 1254766. [Google Scholar] [CrossRef]
- Mousa, W.K.; Athar, B.; Merwin, N.J.; Magarvey, N.A. Antibiotics and Specialized Metabolites from the Human Microbiota. Nat. Prod. Rep. 2017, 34, 1302–1331. [Google Scholar] [CrossRef]
- Perruzza, L.; Gargari, G.; Proietti, M.; Fosso, B.; D’Erchia, A.M.; Faliti, C.E.; Rezzonico-Jost, T.; Scribano, D.; Mauri, L.; Colombo, D.; et al. T Follicular Helper Cells Promote a Beneficial Gut Ecosystem for Host Metabolic Homeostasis by Sensing Microbiota-Derived Extracellular ATP. Cell Rep. 2017, 18, 2566–2575. [Google Scholar] [CrossRef]
- Li, Z.; Dai, Z.; Shi, E.; Wan, P.; Chen, G.; Zhang, Z.; Xu, Y.; Gao, R.; Zeng, X.; Li, D. Study on the Interaction between β-Carotene and Gut Microflora Using an in Vitro Fermentation Model. Food Sci. Hum. Wellness 2023, 12, 1369–1378. [Google Scholar] [CrossRef]
- Jalal, F.; Nesheim, M.C.; Agus, Z.; Sanjur, D.; Habicht, J.P. Serum Retinol Concentrations in Children Are Affected by Food Sources of Beta-Carotene, Fat Intake, and Anthelmintic Drug Treatment. Am. J. Clin. Nutr. 1998, 68, 623–629. [Google Scholar] [CrossRef]
- Kaulmann, A.; André, C.M.; Schneider, Y.-J.; Hoffmann, L.; Bohn, T. Carotenoid and Polyphenol Bioaccessibility and Cellular Uptake from Plum and Cabbage Varieties. Food Chem. 2016, 197, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-Related Factors Explaining Interindividual Variability of Carotenoid Bioavailability and Tissue Concentrations in Humans. Mol. Nutr. Food Res. 2017, 61, 1600685. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Bassis, C.M.; Plegue, M.A.; Ren, J.; Chan, R.; Sidahmed, E.; Turgeon, D.K.; Ruffin, M.T.; Kato, I.; Sen, A. Colonic Mucosal Bacteria Are Associated with Inter-Individual Variability in Serum Carotenoid Concentrations. J. Acad. Nutr. Diet 2018, 118, 606–616.e3. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, M.; Fu, X.; Zhang, Z.; Zhu, L.; Zheng, X.; Liu, J. Astaxanthin Prevents Alcoholic Fatty Liver Disease by Modulating Mouse Gut Microbiota. Nutrients 2018, 10, 1298. [Google Scholar] [CrossRef]
- Yin, R.; Kuo, H.-C.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R.; Kong, A.-N. Gut Microbiota, Dietary Phytochemicals, and Benefits to Human Health. Curr. Pharm. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef]
- Eroglu, A.; Al’Abri, I.; Kopec, R.E.; Crook, N.; Bohn, T. Carotenoids and Their Health Benefits as Derived via Their Interactions with Gut Microbiota. Adv. Nutr. 2022, 14, 238–255. [Google Scholar] [CrossRef]
- Linnewiel-Hermoni, K.; Motro, Y.; Miller, Y.; Levy, J.; Sharoni, Y. Carotenoid Derivatives Inhibit Nuclear Factor Kappa B Activity in Bone and Cancer Cells by Targeting Key Thiol Groups. Free Radic. Biol. Med. 2014, 75, 105–120. [Google Scholar] [CrossRef]
- Li, R.; Hong, P.; Zheng, X. β-Carotene Attenuates Lipopolysaccharide-Induced Inflammation via Inhibition of the NF-ΚB, JAK2/STAT3 and JNK/P38 MAPK Signaling Pathways in Macrophages. Anim. Sci. J. 2019, 90, 140–148. [Google Scholar] [CrossRef]
- Li, Z.; Dong, X.; Liu, H.; Chen, X.; Shi, H.; Fan, Y.; Hou, D.; Zhang, X. Astaxanthin Protects ARPE-19 Cells from Oxidative Stress via Upregulation of Nrf2-Regulated Phase II Enzymes through Activation of PI3K/Akt. Mol. Vis. 2013, 19, 1656–1666. [Google Scholar]
- Bohn, T. Bioactivity of Carotenoids—Chasms of Knowledge. Int. J. Vitam. Nutr. Res. 2017, 87, 5–9. [Google Scholar] [CrossRef]
- Arathi, B.P.; Sowmya, P.R.-R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Biofunctionality of Carotenoid Metabolites: An Insight into Qualitative and Quantitative Analysis. In Metabolomics—Fundamentals and Applications; InTech: Vienna, Austria, 2016. [Google Scholar]


| Carotenoid | Biological Functions | References |
|---|---|---|
| β-carotene | Stimulates the proliferation of lymphocytes; reduces the low-density lipoprotein (LDL) susceptibility to oxidation; activates cell communication; reduces inflammation; improves cardiovascular health. | [2,20,21] |
| Lutein | Scavenges oxygen intermediates; blue light filter; maintenance of eye health; decreases the proliferation of breast cancer cells; reduces oxidative stress and apoptosis. | [1,21,22,23,24] |
| Lycopene | Inhibits lipid peroxidation; eliminates reactive oxygen species (ROS); reinforces the immune system; free radical quencher; prevents skin damage. | [2,21] |
| Groups | Typical Metabolites | Specific Function | Associated Diseases | References |
|---|---|---|---|---|
| Short-chain fatty acids | Acetate, propionate, butyrate, hexanoate, isovalerate, isobutyrate. | Regulation of intestinal microbiota composition, barrier integrity, and hormone production. | Diabetes, obesity, colorectal cancer, Crohn’s and Parkinson’s diseases. | [87,88,89,90,91,92,93] |
| Bile acids | Cholate, hyocholate, deoxycholate, glycocholate, hyodeoxycholate. | Regulation of intestinal microbiota composition, hormones, immunity, and motility. | Amyotrophic lateral sclerosis, cancer, Alzheimer’s, and Parkinson’s diseases. | [94,95,96,97,98] |
| Gases | H2S, H2, CO2, CH2, CH4, NO. | CH4 slows intestinal motility; H2S regulates intestinal inflammation and motility; NO mediates gastric mucosal protection. | Parkinson’s disease, colitis, ulcer. | [85,99,100,101,102] |
| Vitamins | Vitamins B2, B3, B5, B6, B9, B12, and K. | Involved in cellular metabolism, modulate immune function and cell proliferation, supply vitamins for hosts. | Vitamin-associated diseases such as schizophrenia and dementia. | [103,104] |
| Lipids | Conjugated fatty acids, cholesterol, lipopolysaccharides (LPS). | Conjugated fatty acids regulate the immune system; cholesterol acts as a material base for bile acid synthesis; LPS triggers systemic inflammation. | Non-alcoholic fatty liver disease, hyperinsulinemia, hypercholesterolemia. | [105,106] |
| Neurotransmitters | Dopamine, catecholamines, 5-HT, GABA. | Regulate intestinal motility, memory, and stress responses. | Parkinson’s disease, autism. | [85,107,108] |
| Choline metabolites | Dimethylglycine, methylamine, dimethylamine. | Inhibit bile acid synthesis; promote inflammation; exacerbate mitochondrial dysfunction. | Obesity, diabetes, heart failure, hypertension. | [109,110,111] |
| Tryptophan and indole derivatives | Indole-3-lactic acid, indole acetic acid, indole-3-acetamide, indole, serotonin. | Influence the intestinal microbial drug resistance and virulence; regulate intestinal barrier functions, hormone secretion, and motility. | Ulcerative colitis, Crohn’s, Alzheimer’s, and Parkinson’s diseases, stroke, irritable bowel syndrome. | [112,113,114,115,116] |
| Others | Ethanol, triphosadenine, ruminococcin A, cytolysin, microcin B17, benzoate, hippurate, cadaverine. | Regulate intestinal response, act as antibiotics to modulate intestinal microbiota composition, supply nutrients, toxic to host cells. | C. difficile and H. pylori infections, irritable bowel syndrome, ulcerative colitis. | [105,117,118,119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, H.R.; Coelho, M.C.; Gomes, A.M.; Pintado, M.E. Carotenoids Diet: Digestion, Gut Microbiota Modulation, and Inflammatory Diseases. Nutrients 2023, 15, 2265. https://doi.org/10.3390/nu15102265
Rocha HR, Coelho MC, Gomes AM, Pintado ME. Carotenoids Diet: Digestion, Gut Microbiota Modulation, and Inflammatory Diseases. Nutrients. 2023; 15(10):2265. https://doi.org/10.3390/nu15102265
Chicago/Turabian StyleRocha, Helena R., Marta C. Coelho, Ana M. Gomes, and Manuela E. Pintado. 2023. "Carotenoids Diet: Digestion, Gut Microbiota Modulation, and Inflammatory Diseases" Nutrients 15, no. 10: 2265. https://doi.org/10.3390/nu15102265
APA StyleRocha, H. R., Coelho, M. C., Gomes, A. M., & Pintado, M. E. (2023). Carotenoids Diet: Digestion, Gut Microbiota Modulation, and Inflammatory Diseases. Nutrients, 15(10), 2265. https://doi.org/10.3390/nu15102265