Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Outcome
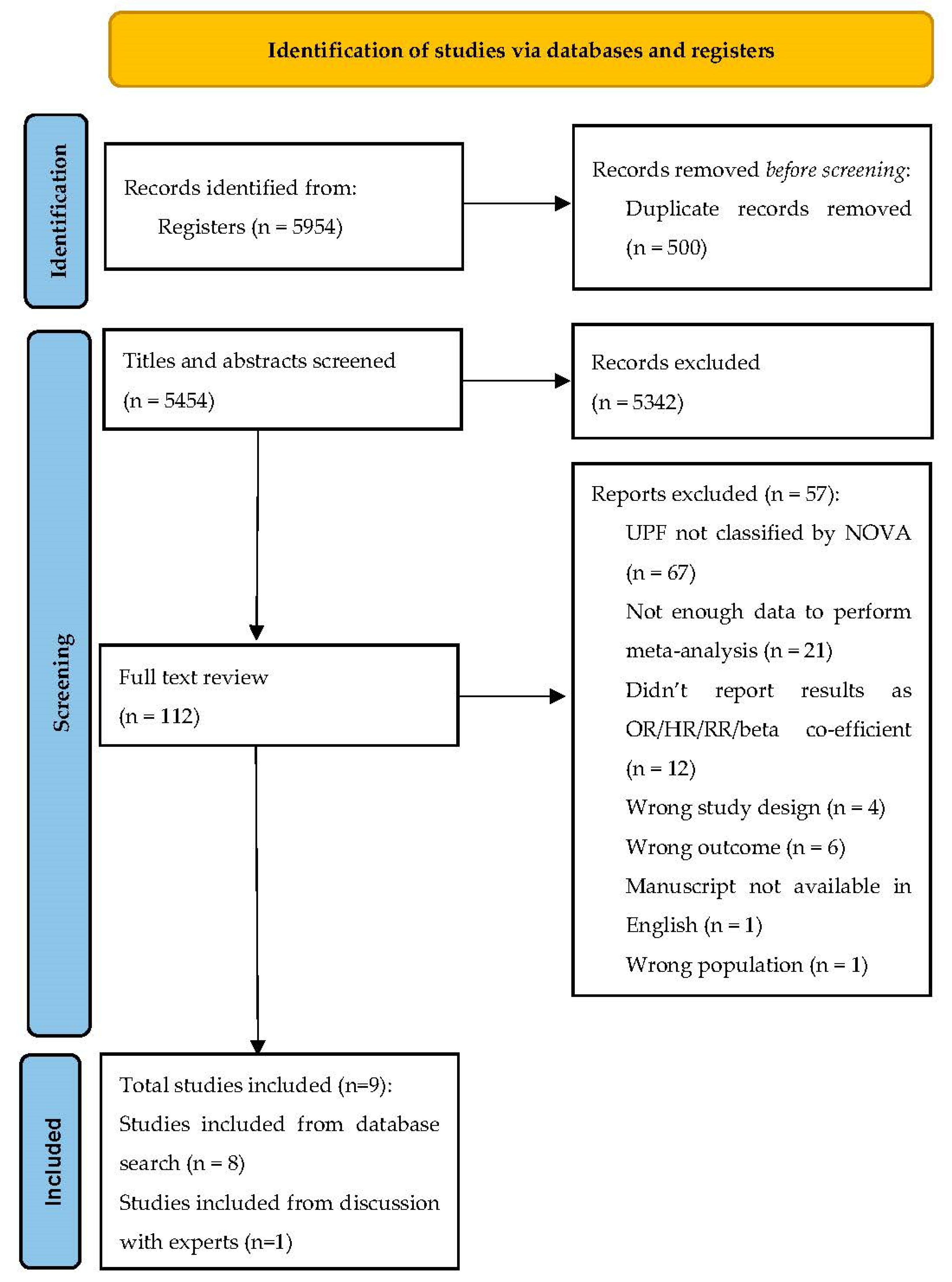
2.5. Study Selection
2.6. Data Extraction
2.7. Quality of Evidence
2.8. Meta-Analysis
3. Results
3.1. Study Characteristics
3.2. Systematic Review
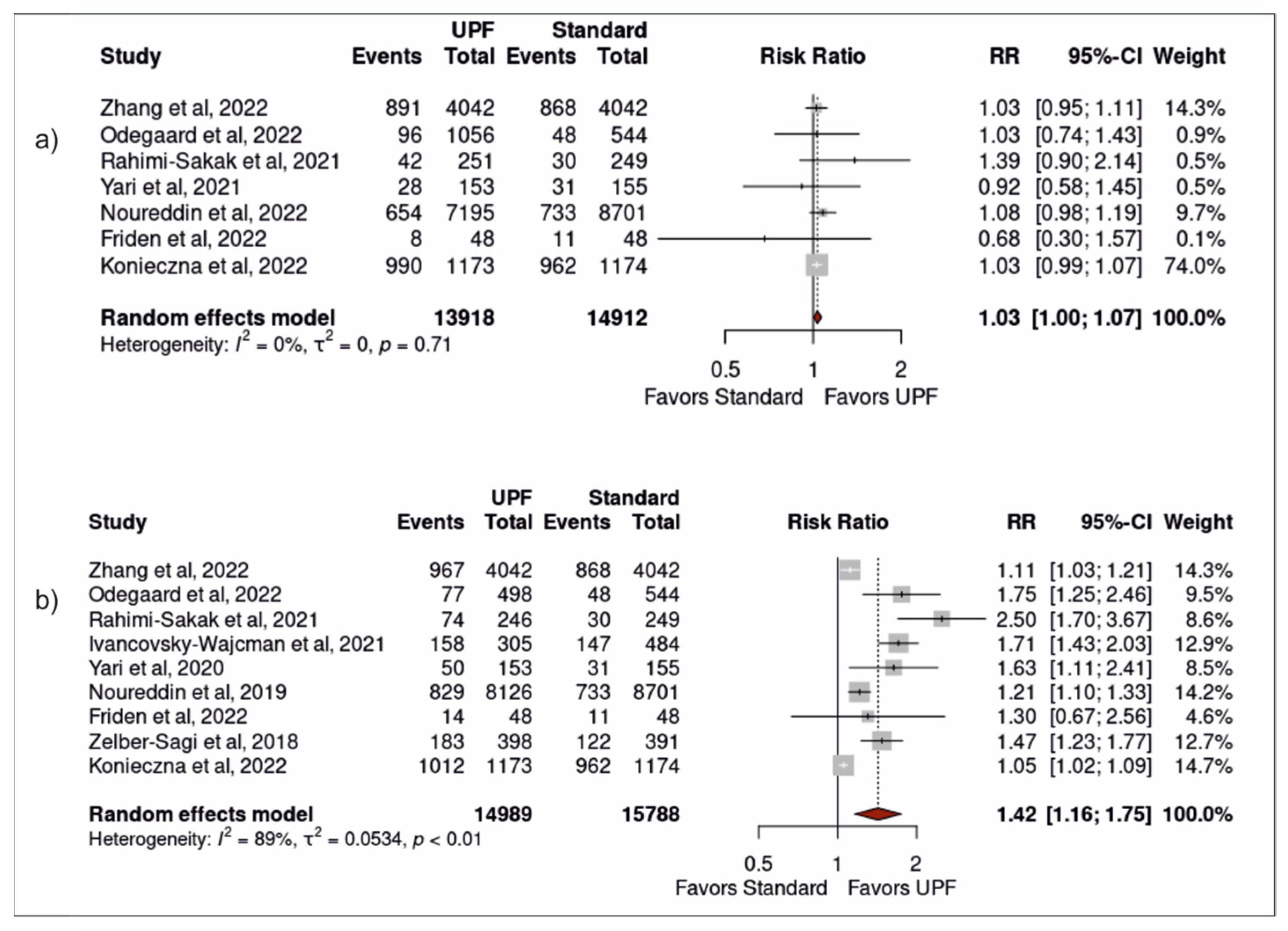
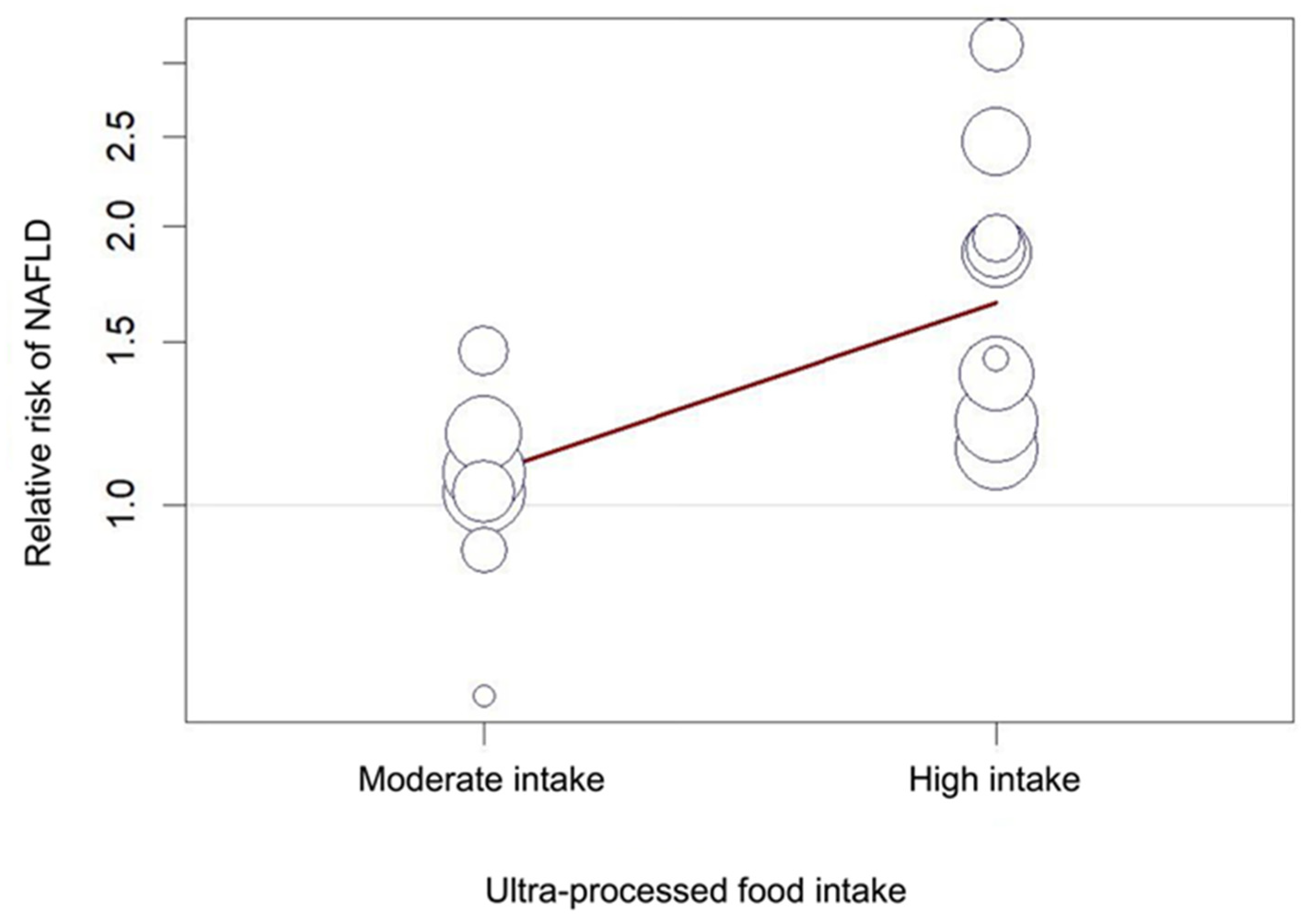
3.3. Association between Ultra-Processed Food Intake and Non-Alcoholic Fatty Liver Disease
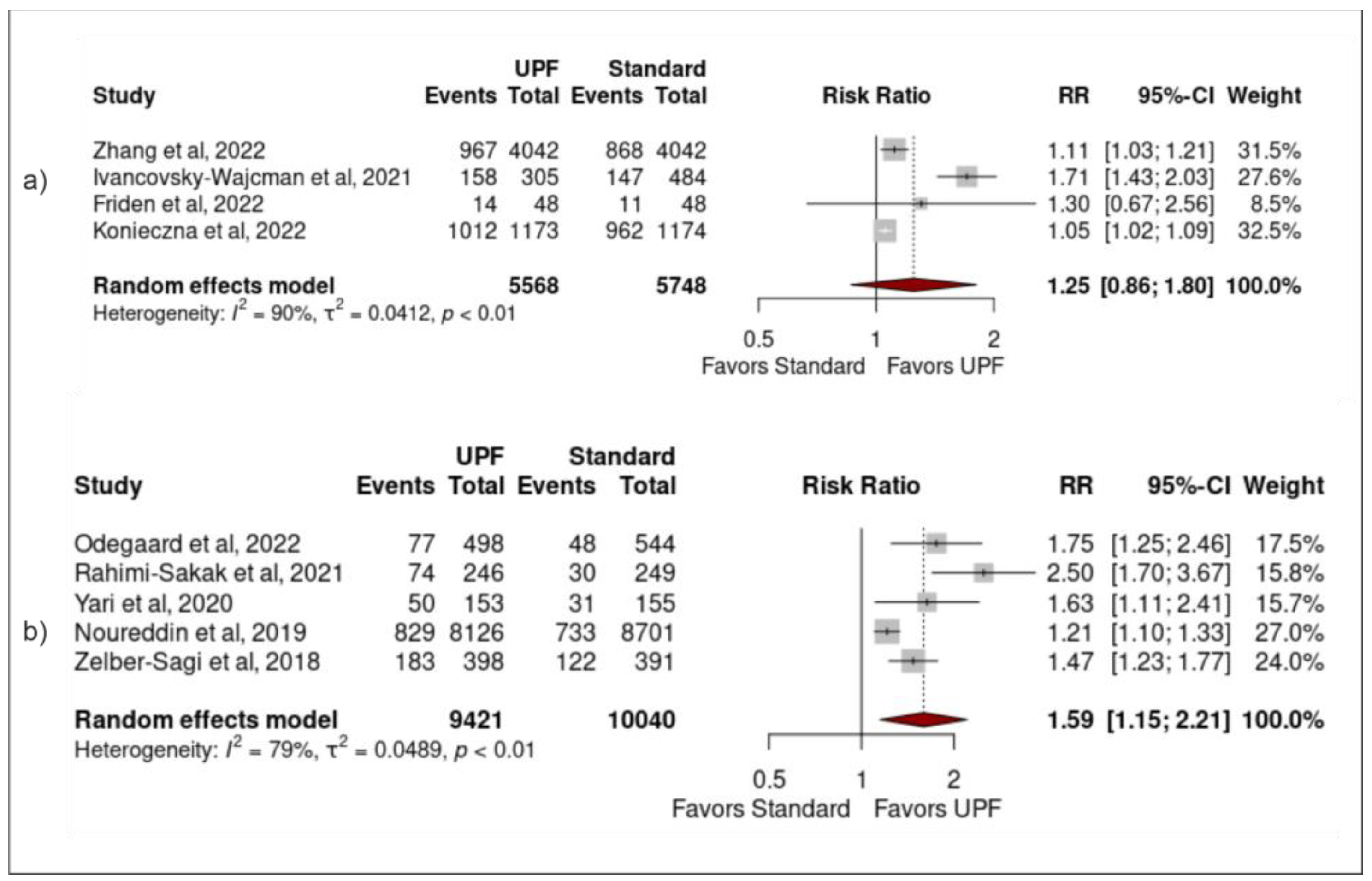
3.4. Sensitivity Analysis
3.5. Publication Bias
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: An updated meta-analysis of 501 022 adult individuals. Gut 2021, 70, 962. [Google Scholar] [CrossRef]
- Lu, F.B.; Hu, E.D.; Xu, L.M.; Chen, L.; Wu, J.L.; Li, H.; Chen, D.Z.; Chen, Y.P. The relationship between obesity and the severity of non-alcoholic fatty liver disease: Systematic review and meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Koskinen, J.; Brown, E.; Magnussen, C.G.; Hutri-Kähönen, N.; Sabin, M.; Tossavainen, P.; Jokinen, E.; Laitinen, T.; Viikari, J.; et al. Fatty liver index predicts incident risk of prediabetes, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). Ann. Med. 2021, 53, 1256–1264. [Google Scholar] [CrossRef]
- Sattar, N.; McConnachie, A.; Ford, I.; Gaw, A.; Cleland, S.J.; Forouhi, N.G.; McFarlane, P.; Shepherd, J.; Cobbe, S.; Packard, C. Serial metabolic measurements and conversion to type 2 diabetes in the west of Scotland coronary prevention study: Specific elevations in alanine aminotransferase and triglycerides suggest hepatic fat accumulation as a potential contributing factor. Diabetes 2007, 56, 984–991. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, K.M.; Kahn, S.E. The Role of Insulin Resistance in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef]
- Stine, J.G.; Long, M.T.; Corey, K.E.; Sallis, R.E.; Allen, A.M.; Armstrong, M.J.; Conroy, D.E.; Cuthbertson, D.J.; Duarte-Rojo, A.; Hallsworth, K.; et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable report on physical activity and nonalcoholic fatty liver disease. Hepatol. Commun. 2023, 7, e0108. [Google Scholar] [CrossRef]
- Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [CrossRef] [PubMed]
- Mantovani, A.; Byrne, C.D.; Targher, G. Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: A systematic review. Lancet Gastroenterol. Hepatol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14 (Suppl. S2), 21–28. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef] [PubMed]
- Newton, S.; Braithwaite, D.; Akinyemiju, T.F. Socio-economic status over the life course and obesity: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0177151. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef] [PubMed]
- Moubarac, J.C.; Parra, D.C.; Cannon, G.; Monteiro, C.A. Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr. Obes. Rep. 2014, 3, 256–272. [Google Scholar] [CrossRef]
- Askari, M.; Heshmati, J.; Shahinfar, H.; Tripathi, N.; Daneshzad, E. Ultra-processed food and the risk of overweight and obesity: A systematic review and meta-analysis of observational studies. Int. J. Obes. 2020, 44, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M.; Bielemann, R.M.; da Silva, B.G.C.; dos Santos, F.S.; Mintem, G.C.; Flores, T.R.; Arcêncio, R.A.; Nunes, B.P. Ultra-processed food and risk of type 2 diabetes: A systematic review and meta-analysis of longitudinal studies. Int. J. Epidemiol. 2022, 51, 1120–1141. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Yang, H.; Qiu, P.; Wang, H.; Wang, F.; Zhao, Q.; Fang, J.; Nie, J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020, 19, 86. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, S.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; Gu, Y.; Wang, Y.; Zhang, T.; et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study. Int. J. Epidemiol. 2022, 51, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Webb, M.; Bentov, I.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int. 2021, 41, 2635–2645. [Google Scholar] [CrossRef]
- Konieczna, J.; Fiol, M.; Colom, A.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Soria-Florido, M.T.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Does Consumption of Ultra-Processed Foods Matter for Liver Health? Prospective Analysis among Older Adults with Metabolic Syndrome. Nutrients 2022, 14, 4142. [Google Scholar] [CrossRef] [PubMed]
- Fridén, M.; Kullberg, J.; Ahlström, H.; Lind, L.; Rosqvist, F. Intake of Ultra-Processed Food and Ectopic-, Visceral- and Other Fat Depots: A Cross-Sectional Study. Front. Nutr. 2022, 9, 774718. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Knüppel, S.; Schwedhelm, C.; Hoffmann, G.; Missbach, B.; Stelmach-Mardas, M.; Dietrich, S.; Eichelmann, F.; Kontopantelis, E.; Iqbal, K.; et al. Perspective: NutriGrade: A Scoring System to Assess and Judge the Meta-Evidence of Randomized Controlled Trials and Cohort Studies in Nutrition Research. Adv. Nutr. 2016, 7, 994–1004. [Google Scholar] [CrossRef]
- Lachin, J.M. Power of the Mantel-Haenszel and other tests for discrete or grouped time-to-event data under a chained binomial model. Stat. Med. 2013, 32, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Veroniki, A.A.; Law, M.; Tricco, A.C.; Baker, R. Paule-Mandel estimators for network meta-analysis with random inconsistency effects. Res. Synth. Methods 2017, 8, 416–434. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Van Wagner, L.B.; Pereira, M.A. Levels of abdominal adipose tissue and metabolic-associated fatty liver disease (MAFLD) in middle age according to average fast-food intake over the preceding 25 years: The CARDIA Study. Am. J. Clin. Nutr. 2022, 116, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Zelber-Sagi, S.; Wilkens, L.R.; Porcel, J.; Boushey, C.J.; Le Marchand, L.; Rosen, H.R.; Setiawan, V.W. Diet Associations With Nonalcoholic Fatty Liver Disease in an Ethnically Diverse Population: The Multiethnic Cohort. Hepatology 2020, 71, 1940–1952. [Google Scholar] [CrossRef]
- Yari, Z.; Cheraghpour, M.; Aghamohammadi, V.; Alipour, M.; Ghanei, N.; Hekmatdoost, A. Energy-dense nutrient-poor snacks and risk of non-alcoholic fattyliver disease: A case–control study in Iran. BMC Res. Notes 2020, 13, 221. [Google Scholar] [CrossRef]
- Rahimi-Sakak, F.; Maroofi, M.; Emamat, H.; Hekmatdoost, A. Red and Processed Meat Intake in Relation to Non-Alcoholic Fatty Liver Disease Risk: Results from a Case-Control Study. Clin. Nutr. Res. 2022, 11, 42–49. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef]
- Costa Louzada, M.L.; Martins, A.P.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.C.; Cannon, G.; Monteiro, C.A. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev. Saude Publica 2015, 49, 38. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Wang, J.; Du, J. Association between body mass index and fatty liver risk: A dose-response analysis. Sci. Rep. 2018, 8, 15273. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Tsompanaki, E.; Thanapirom, K.; Papatheodoridi, M.; Parikh, P.; Chotai de Lima, Y.; Tsochatzis, E.A. Systematic Review and Meta-analysis: The Role of Diet in the Development of Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Benini, L.; Castellani, G.; Brighenti, F.; Heaton, K.W.; Brentegani, M.T.; Casiraghi, M.C.; Sembenini, C.; Pellegrini, N.; Fioretta, A.; Minniti, G.; et al. Gastric emptying of a solid meal is accelerated by the removal of dietary fibre naturally present in food. Gut 1995, 36, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Godos, J.; Bonaccio, M.; Vitaglione, P.; Grosso, G. Ultra-Processed Foods and Nutritional Dietary Profile: A Meta-Analysis of Nationally Representative Samples. Nutrients 2021, 13, 3390. [Google Scholar] [CrossRef]
- Hydes, T.; Alam, U.; Cuthbertson, D.J. The Impact of Macronutrient Intake on Non-alcoholic Fatty Liver Disease (NAFLD): Too Much Fat, Too Much Carbohydrate, or Just Too Many Calories? Front. Nutr. 2021, 8, 640557. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Steele, T.; Wilding, J.P.; Halford, J.C.; Harrold, J.A.; Hamer, M.; Karpe, F. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int. J. Obes. 2017, 41, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Walhin, J.P.; Gonzalez, J.T.; Betts, J.A. Physiological responses to carbohydrate overfeeding. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 379–384. [Google Scholar] [CrossRef]
- Pérez-Montes de Oca, A.; Julián, M.T.; Ramos, A.; Puig-Domingo, M.; Alonso, N. Microbiota, Fiber, and NAFLD: Is There Any Connection? Nutrients 2020, 12, 3100. [Google Scholar] [CrossRef] [PubMed]
- Park, W.Y.; Yiannakou, I.; Petersen, J.M.; Hoffmann, U.; Ma, J.; Long, M.T. Sugar-Sweetened Beverage, Diet Soda, and Nonalcoholic Fatty Liver Disease Over 6 Years: The Framingham Heart Study. Clin. Gastroenterol. Hepatol. 2022, 20, 2524–2532.e2. [Google Scholar] [CrossRef]
- Qin, P.; Li, Q.; Zhao, Y.; Chen, Q.; Sun, X.; Liu, Y.; Li, H.; Wang, T.; Chen, X.; Zhou, Q.; et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Samuel, V.T. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends Endocrinol. Metab. 2016, 27, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Eweis, D.S.; Abed, F.; Stiban, J. Carbon dioxide in carbonated beverages induces ghrelin release and increased food consumption in male rats: Implications on the onset of obesity. Obes. Res. Clin. Pract. 2017, 11, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Shojaei-Zarghani, S.; Safarpour, A.R.; Fattahi, M.R.; Keshtkar, A. Sodium in relation with nonalcoholic fatty liver disease: A systematic review and meta-analysis of observational studies. Food Sci. Nutr. 2022, 10, 1579–1591. [Google Scholar] [CrossRef]
- Tong, M.; Neusner, A.; Longato, L.; Lawton, M.; Wands, J.R.; de la Monte, S.M. Nitrosamine exposure causes insulin resistance diseases: Relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 827–844. [Google Scholar] [PubMed]
- Collison, K.S.; Maqbool, Z.; Saleh, S.M.; Inglis, A.; Makhoul, N.J.; Bakheet, R.; Al-Johi, M.; Al-Rabiah, R.; Zaidi, M.Z.; Al-Mohanna, F.A. Effect of dietary monosodium glutamate on trans fat-induced nonalcoholic fatty liver disease. J. Lipid Res. 2009, 50, 1521–1537. [Google Scholar] [CrossRef]
- Emamat, H.; Ghalandari, H.; Tangestani, H.; Abdollahi, A.; Hekmatdoost, A. Artificial sweeteners are related to non-alcoholic fatty liver disease: Microbiota dysbiosis as a novel potential mechanism. EXCLI J. 2020, 19, 620–626. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Lutsey, P.L.; Wang, Y.; Lima, J.A.; Michos, E.D.; Jacobs, D.R., Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009, 32, 688–694. [Google Scholar] [CrossRef]
- Payne, A.N.; Chassard, C.; Lacroix, C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: Implications for host-microbe interactions contributing to obesity. Obes. Rev. 2012, 13, 799–809. [Google Scholar] [CrossRef]
- Dallio, M.; Diano, N.; Masarone, M.; Gravina, A.G.; Patanè, V.; Romeo, M.; Di Sarno, R.; Errico, S.; Nicolucci, C.; Abenavoli, L.; et al. Chemical Effect of Bisphenol A on Non-Alcoholic Fatty Liver Disease. Int. J. Environ. Res. Public Health 2019, 16, 3134. [Google Scholar] [CrossRef]
- Hwang, S.; Lim, J.E.; Choi, Y.; Jee, S.H. Bisphenol A exposure and type 2 diabetes mellitus risk: A meta-analysis. BMC Endocr. Disord. 2018, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Han, T.; Sun, X.; Chen, Y.; Xu, J.; Wang, Y.; Yang, X.; Jiang, W.; Sun, C. Relationship Between Carbohydrate Intake (Quantity, Quality, and Time Eaten) and Mortality (Total, Cardiovascular, and Diabetes): Assessment of 2003–2014 National Health and Nutrition Examination Survey Participants. Diabetes Care 2022, 45, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Boysen, O.; Boysen-Urban, K.; Bradford, H.; Balié, J. Taxing highly processed foods: What could be the impacts on obesity and underweight in sub-Saharan Africa? World Dev. 2019, 119, 55–67. [Google Scholar] [CrossRef]
- Caro, J.C.; Smith-Taillie, L.; Ng, S.W.; Popkin, B. Designing a food tax to impact food-related non-communicable diseases: The case of Chile. Food Policy 2017, 71, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Barquera, S.; Corvalan, C.; Hofman, K.J.; Monteiro, C.; Ng, S.W.; Swart, E.C.; Taillie, L.S. Towards unified and impactful policies to reduce ultra-processed food consumption and promote healthier eating. Lancet Diabetes Endocrinol. 2021, 9, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Colchero, M.A.; Popkin, B.M.; Rivera, J.A.; Ng, S.W. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: Observational study. BMJ 2016, 352, h6704. [Google Scholar] [CrossRef]
- Colchero, M.A.; Rivera-Dommarco, J.; Popkin, B.M.; Ng, S.W. In Mexico, Evidence Of Sustained Consumer Response Two Years After Implementing A Sugar-Sweetened Beverage Tax. Health Aff. 2017, 36, 564–571. [Google Scholar] [CrossRef]
- Pérez Rodrigo, C.; Aranceta, J.; Salvador, G.; Varela-Moreiras, G. Food frequency questionnaires. Nutr. Hosp. 2015, 31 (Suppl. S3), 49–56. [Google Scholar] [CrossRef]
- Gomercić, M.; Duvnjak, M.; Barsić, N. [Ultrasonography in the diagnosis of nonalcoholic fatty liver disease]. Acta Med. Croatica 2009, 63 (Suppl. S3), 1–3. [Google Scholar]
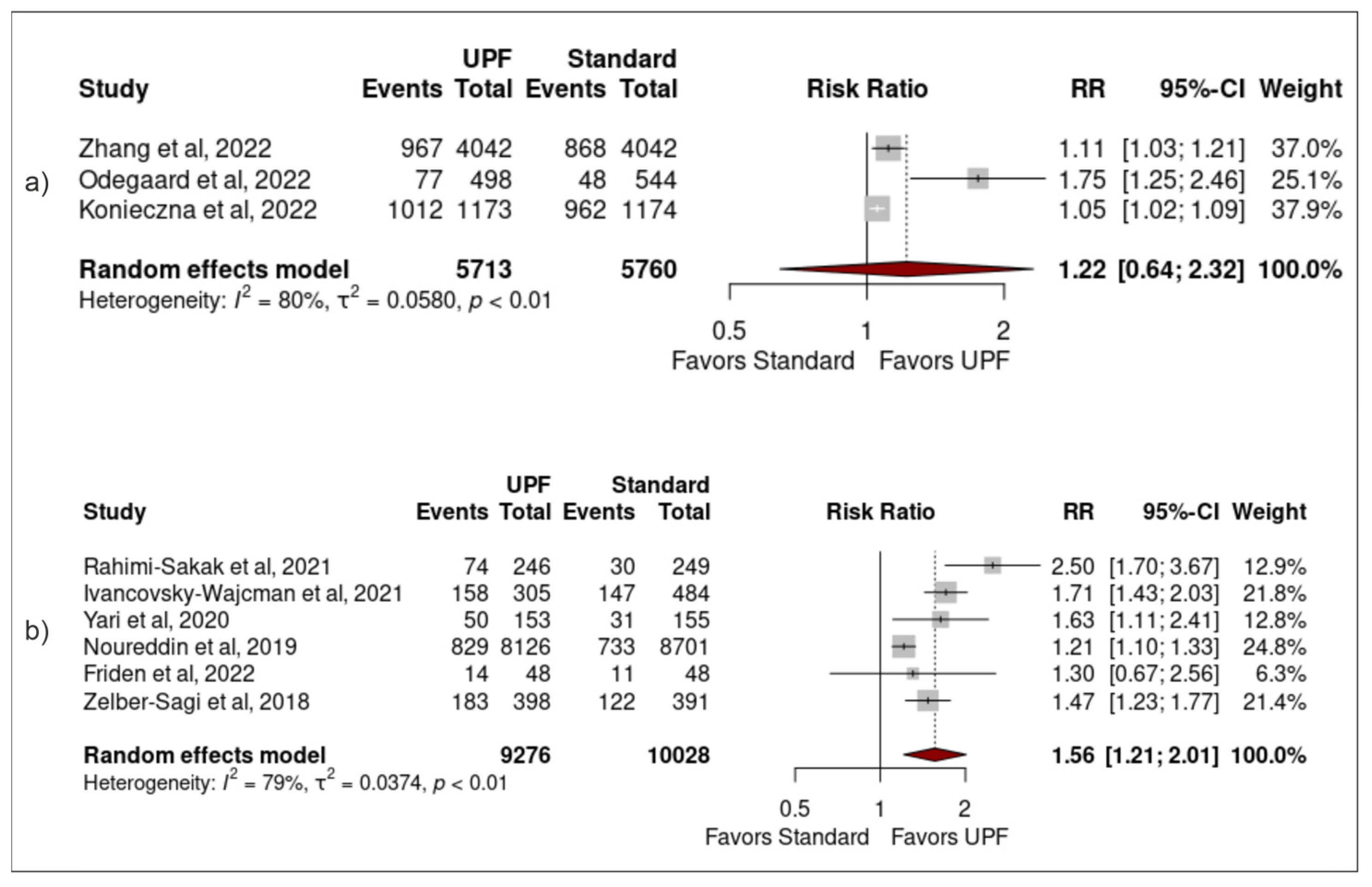
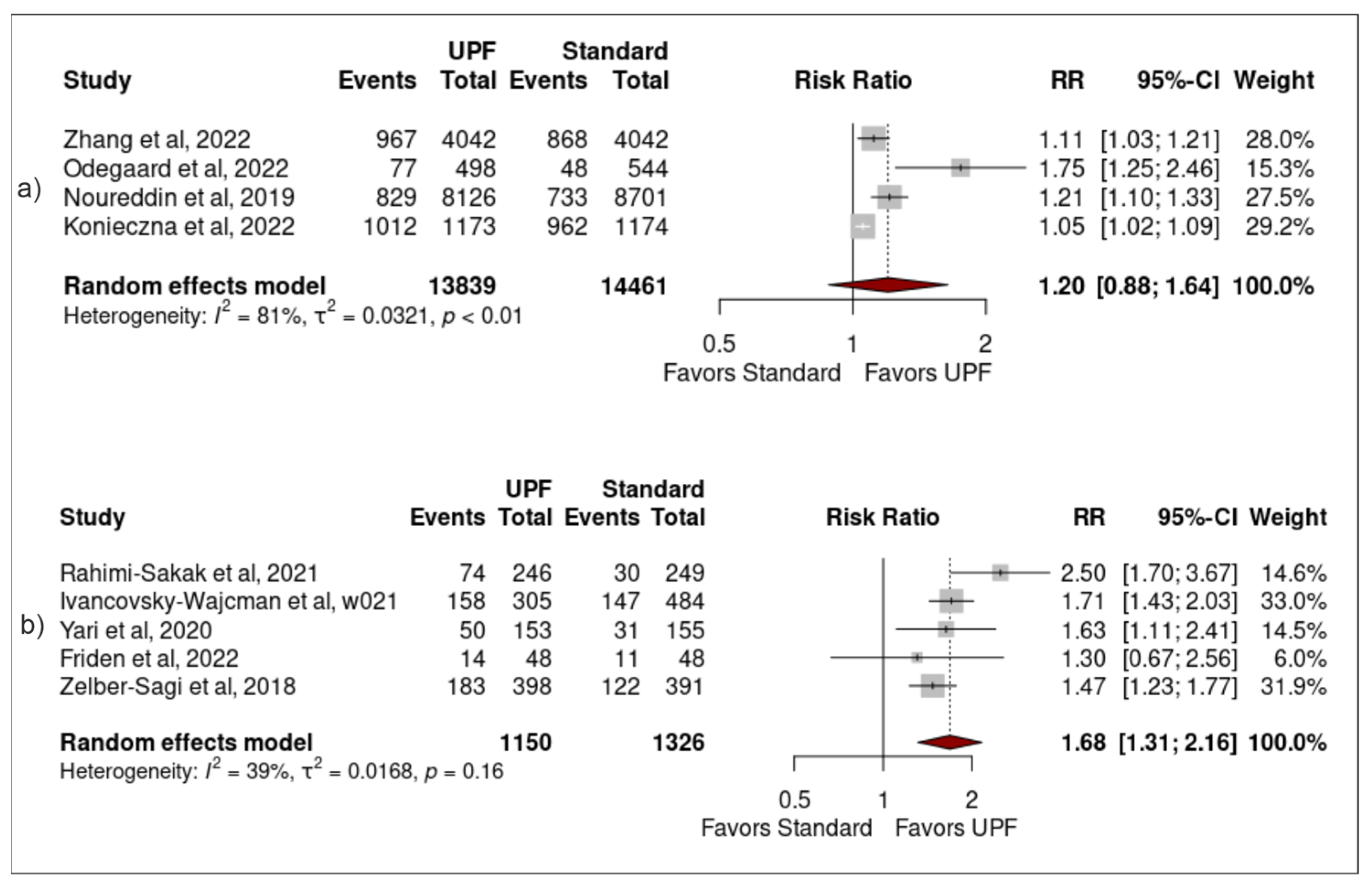
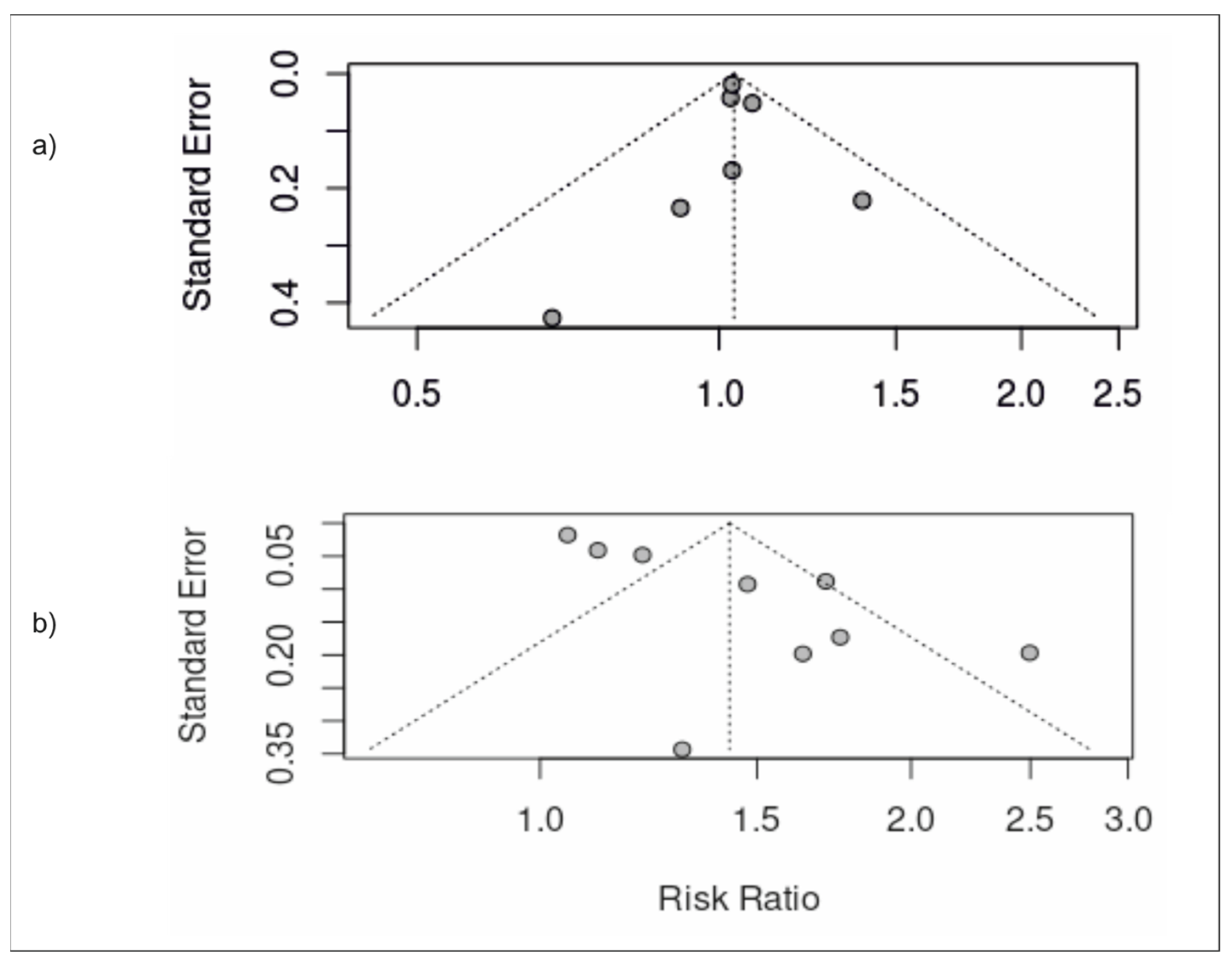
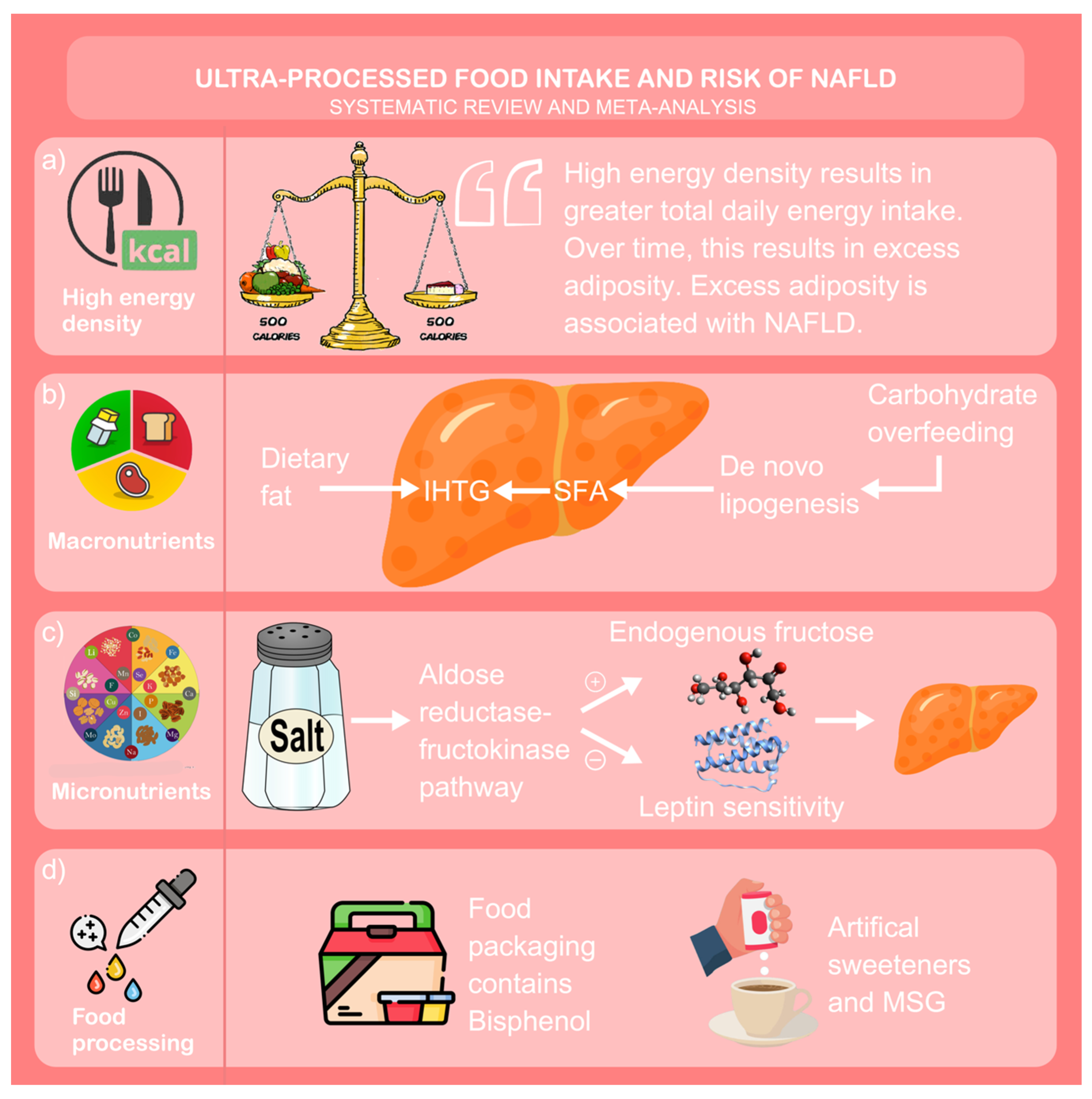
| ID | Sample | Population (SEX/AGE) | Study Design (Follow up) | Exposure (Via NOVA Unless Stated Otherwise) (Moderate vs. High Intake of Upf) | Adjustment | Outcome (Risk of Nafld) |
|---|---|---|---|---|---|---|
| Zhang et al. [24] | Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) Cohort Study | 16,168 males/females aged 18–90 years | Cohort (4.2 years) | Moderate: 2° quartile (30.1g/1000 kcal per day) High: 4° quartile (113.7 g/1000 kcal per day) | Age, sex, BMI, smoking, alcohol, education, occupation, monthly household income, physical activity, family history of cardiometabolic disease, depressive symptoms, total energy intake, healthy diet score, diabetes, hypertension and hyperlipidaemia | Moderate intake increased the risk by 13% High intake increased the risk by 18% |
| Odegaard et al. [34] | Coronary Artery Risk Development in Young Adults (CARDIA) study | 3001 male/females, aged 24–29 years | Cohort (25 years) | Fast-food: Moderate: 3° quintile (1–2x/week) High: 5° quintile (>3x/week) | Age, sex, race, study centre, education, employment history, household income, smoking, alcohol, diet quality, energy intake, physical activity, and prevalence of type 2 diabetes or history of a CVD event at the year 25 exam | Moderate intake increased the risk by over two-fold High intake increased the risk by over five-fold |
| Yari et al. [36] | Iranian males and females | 614 male and females, mean age 38.92 years | Case-control study | Energy-dense nutrient- poor snacks: Moderate: 2° quartile (3.7% total energy intake) High: = 4° quartile (9.7% total energy intake) | Age, sex, BMI, physical activity, alcohol and total energy intake | Moderate intake had no significant association High intake increased the risk by over two-fold |
| Rahimi-Sakak et al. [37] | Iranian males and females | 999 males/females, mean age of 43.54 years | Case-control study | Processed meat: Moderate: 2° quartile (0.4–2.4/day) High: 4° quartile (>6.6 g/day) | Age, gender, BMI, total energy intake, dietary factors, diabetes, smoking, and physical activity | Moderate intake had no significant association High intake increased risk by over three-fold |
| Noureddin et al. [35] | The Multi-ethnic Cohort (MEC) study | 32,448 males/females, mean age 57.7 years | Nested case-control study | Processed meat: Moderate: 2° quartile (1.6–3.3 g/day) High: 4° quartile (>5.7 g/day) | BMI, alcohol intake, coffee drinking, total sweetened beverage intake, physical activity, total energy intake, education, smoking status and cardiovascular disease | Moderate intake had no significant association High intake increased the risk by 18% |
| Ivancovsky-Wajcman et al. [25] | Israeli males and females | 789 males/females, mean age 58.83 years | Cross-sectional study | Moderate: no data High: >28% total energy intake | Age, gender, BMI, SFA intake, protein intake as a percentage of total energy intake, physical activity, coffee drinking and fibre intake | High intake had no significant association |
| Friden et al. [27] | Prospective investigation of Obesity, Energy and Metabolism (POEM) | 286 males/females, age-matched at 50 years | Cross-sectional study | Moderate: 2° tertile (37.6% total energy intake) High: 3° tertile (49% total energy intake) | Sex, education level, physical activity level, smoking status, dietary factors and BMI | Moderate or high intake had no significant association |
| Zelber-Sagi et al. [38] | Colonoscopy screening at the Department for Gastroenterology and Hepatology at Tel Aviv Medical Centre | 789 males/females, mean age 58.83 years | Cross-sectional study | Processed meat: Moderate: no data available High: >0.33 daily portions | Age, gender, energy intake per day, BMI, weekly hours of physical activity, smoking status, weekly alcohol portions, saturated fat (percent of daily energy) and cholesterol intake | High intake increased the risk by 47% |
| Konieczna et al. [26] | PREDIMED-Plus trial | 5867 males/females, mean age 65.0 years | Cohort (1 year) | Moderate: 3° quintile (6.23% of g/day) High: 5° quintile (19% of g/day) | Age at inclusion, sex, study arm, and follow-up time (months), baseline educational level, smoking habits, alcohol intake | Moderate intake was associated with a two-fold increased likelihood High intake was associated with four-fold increased likelihood |
| ID | Dietary Assessment Tool | Nafld Diagnostic Tool (Nafld Criteria) | Odds Ratio (or) or Beta Coefficient (BC) | Effect Size (Relative RISK (RR)) |
|---|---|---|---|---|
| Zhang et al. [24] | FFQ | US abdomen: (any two of: (a) increased echogenicity; (b) deep attenuation of signal; (c) vascular blurring) | Moderate: (OR 1.13 (1.03–1.25) (p = <0.01)) High: (OR 1.18 (1.07–1.30) (p = <0.01)) | Moderate: (RR 1.03 (0.95–1.11)) High: (RR 1.11 (1.03–1.21)) |
| Odegaard et al. [34] | Semi-structured interview | CT abdomen: (liver attenuation < 40 HU) | Moderate: (OR 2.31 (1.34–3.98) (p = <0.01)) High: (OR 5.18 (2.87–9.37) (p = <0.01)) | Moderate: (RR 1.03 (0.74–1.43)) High: (RR 1.75 (1.25–2.46)) |
| Yari et al. [36] | FFQ | Fibroscan: (CAP score > 263) | Moderate: (OR 0.92 (0.48–1.77)) High: (OR 2.27 (1.19-4.31) (p = <0.01)) | Moderate: (RR 0.92 (0.58–1.45)) High: (RR 1.63 (1.11–2.41)) |
| Rahimi-Sakak et al. [37] | FFQ | Fibroscan: (CAP score > 263) | Moderate: (OR 1.72 (0.84–3.52)) High: (OR 3.42 (2.16-5.43) (p = <0.01)) | Moderate: (RR 1.39 (0.90–2.14)) High: (RR 2.50 (1.70–3.67)) |
| Noureddin et al. [35] | FFQ | US abdomen: (standardised criteria) | Moderate: (OR 1.03 (0.02–1.16)) High: (OR 1.18 (1.05–1.32) (p = <0.01)) | Moderate: (RR 1.08 (0.98–1.19)) High: (RR 1.21 (1.10–1.33)) |
| Ivancovsky-Wajcman et al. [25] | FFQ | US abdomen: (standardised criteria) | High: (OR 1.12 (0.78–1.59) (p = 0.55)) | High: (RR 1.71 (1.43–2.03)) |
| Friden et al. [27] | FFQ | MRI: (hepatic fat content > 5.5%) | High: (OR 1.32 (0.84–2.09) (p = 0.23)) | Moderate: (RR 0.68 (0.30–1.57)) High: (RR 1.30 (0.67–2.56)) |
| Zelber-Sagi et al. [38] | FFQ | US abdomen: (standardised criteria) | High: (OR 1.47 (1.04–2.09) (p = 0.031)) | High: (RR 1.47 (1.23–1.77)) |
| Konieczna et al. [26] | FFQ | FLI: (score > 60) | Moderate: (BC 2.01 (1.46–2.55) (p = <0.001)) High: (BC 3.73 (3.10-4.35) (p = <0.001)) | Moderate: (RR 1.03 (0.99–1.07)) High: (RR 1.05 (1.02–1.09)) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henney, A.E.; Gillespie, C.S.; Alam, U.; Hydes, T.J.; Cuthbertson, D.J. Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2266. https://doi.org/10.3390/nu15102266
Henney AE, Gillespie CS, Alam U, Hydes TJ, Cuthbertson DJ. Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(10):2266. https://doi.org/10.3390/nu15102266
Chicago/Turabian StyleHenney, Alex E., Conor S. Gillespie, Uazman Alam, Theresa J. Hydes, and Daniel J. Cuthbertson. 2023. "Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis" Nutrients 15, no. 10: 2266. https://doi.org/10.3390/nu15102266





