Exercise Is Medicine for Nonalcoholic Fatty Liver Disease: Exploration of Putative Mechanisms
Abstract
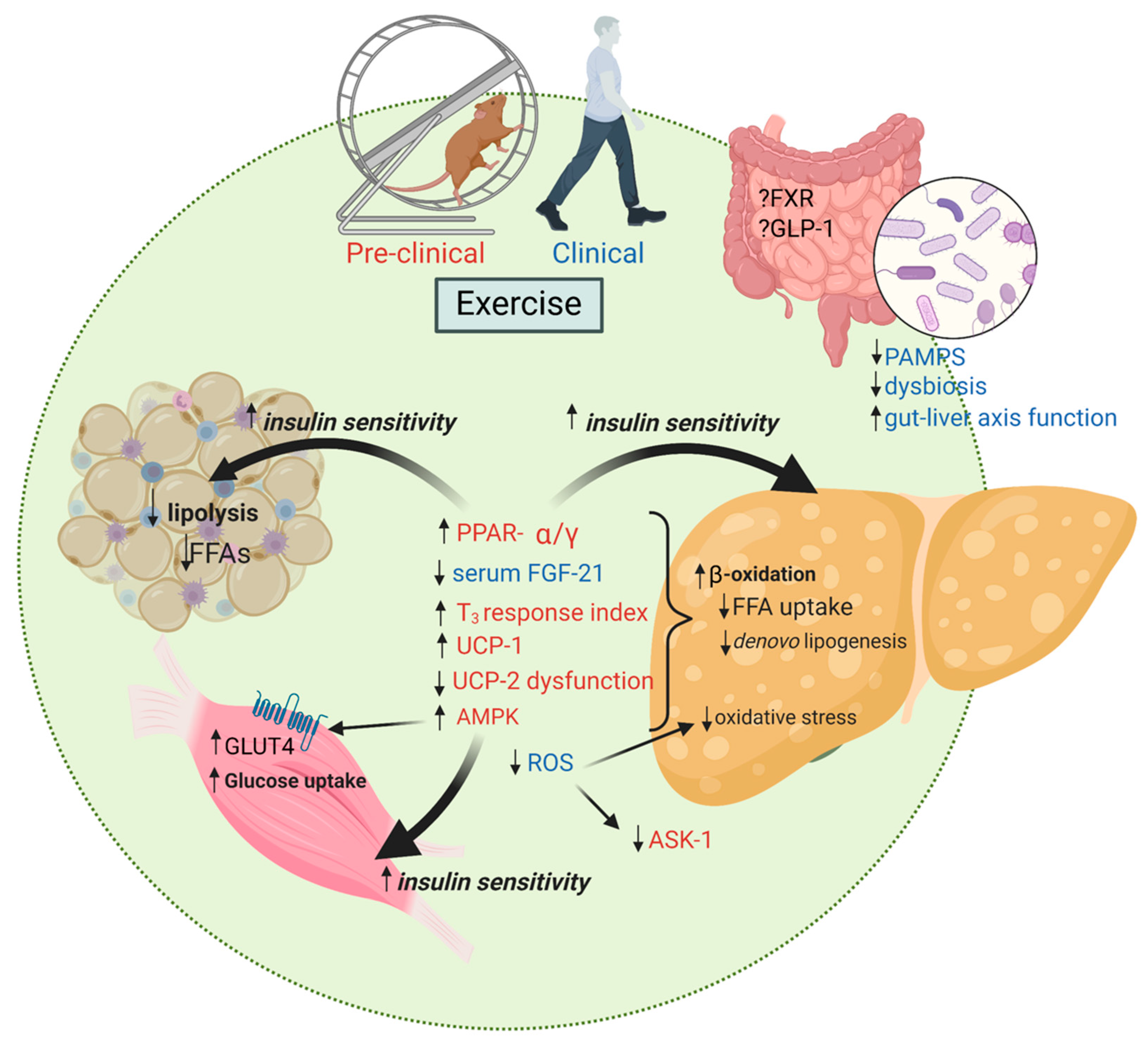
:1. Introduction
2. Exercise Training and Mechanistic Pathways Involved in Hepatic Steatosis
2.1. AMP-Activated Protein Kinase (AMPK)
2.2. Fibroblast Growth Factor (FGF)-19 and -21
2.3. Glucagon-Like Peptide-1 (GLP-1)
2.4. Mitochondrial Function and Beta Oxidation
2.5. Mitochondrial Uncoupling Proteins (UCP)
2.6. Peroxisome Proliferator-Activated Receptor (PPAR)-α/γ
2.7. Thyroid Receptor (THR)-β
3. Exercise Training and Mechanistic Pathways Involved in the Development of Liver Inflammation and Fibrosis
3.1. Apoptosis Signal-Regulating Kinase 1 (ASK-1) and Endoplasmic Reticulum Stress (ERS)
3.2. Farnesoid X Receptor (FXR), Bile Acids and the Microbiome
3.3. Reactive Oxygen Species (ROS)
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Soriano, C.; Schreibman, I.; Rivas, G.; Hummer, B.; Yoo, E.; Schmitz, K.; Sciamanna, C. Breaking Down Barriers to Physical Activity in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2021, 66, 3604–3611. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Thorp, A.; Stine, J.G. Exercise as Medicine: The Impact of Exercise Training on Nonalcoholic Fatty Liver Disease. Curr. Hepatol. Rep. 2020, 19, 402–411. [Google Scholar] [CrossRef]
- Stine, J.G.; Long, M.T.; Corey, K.E.; Sallis, R.E.; Allen, A.M.; Armstrong, M.J.; Conroy, D.E.; Cuthbertson, D.J.; Duarte-Rojo, A.; Hallsworth, K.; et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable Report on Physical Activity and Nonalcoholic Fatty Liver Disease. Hepatol. Commun. 2023, 7, e0108. [Google Scholar] [CrossRef]
- Stine, J.G.; DiJoseph, K.; Pattison, Z.; Harrington, A.; Chinchilli, V.M.; Schmitz, K.H.; Loomba, R. Exercise Training Is Associated with Treatment Response in Liver Fat Content by Magnetic Resonance Imaging Independent of Clinically Significant Body Weight Loss in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Lopez, P.; Lawitz, E.J.; Lucas, K.J.; Loeffler, J.; Kim, W.; Goh, G.B.B.; Huang, J.F.; Serra, C.; Andreone, P.; et al. Tropifexor for nonalcoholic steatohepatitis: An adaptive, randomized, placebo-controlled phase 2a/b trial. Nat. Med. 2023, 29, 392–400. [Google Scholar] [CrossRef]
- Patel, K.; Harrison, S.A.; Elkashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.; Kayali, Z.; Wong, V.W.; Greenbloom, S.; Jayakumar, S.; et al. Cilofexor, a Nonsteroidal FXR Agonist, in Non-Cirrhotic Patients with Nonalcoholic Steatohepatitis: A Phase 2 Randomized Controlled Trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Harrison, S.A.; Abdelmalek, M.F.; Neff, G.; Gunn, N.; Guy, C.D.; Alkhouri, N.; Bashir, M.R.; Freilich, B.; Kohli, A.; Khazanchi, A.; et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 2022, 7, 603–616. [Google Scholar] [CrossRef]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J.; et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021, 27, 1262–1271. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef]
- Cusi, K.; Alkhouri, N.; Harrison, S.A.; Fouqueray, P.; Moller, D.E.; Hallakou-Bozec, S.; Bolze, S.; Grouin, J.M.; Megnien, S.J.; Dubourg, J.; et al. Efficacy and safety of PXL770, a direct AMP kinase activator, for the treatment of non-alcoholic fatty liver disease (STAMP-NAFLD): A randomised, double-blind, placebo-controlled, phase 2a study. Lancet Gastroenterol. Hepatol. 2021, 6, 889–902. [Google Scholar] [CrossRef]
- Han, T.R.; Yang, W.J.; Tan, Q.H.; Bai, S.; Zhong, H.; Tai, Y.; Tong, H. Gut microbiota therapy for nonalcoholic fatty liver disease: Evidence from randomized clinical trials. Front. Microbiol. 2022, 13, 1004911. [Google Scholar] [CrossRef]
- Richter, E.A.; Ruderman, N.B. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem. J. 2009, 418, 261–275. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef]
- Gehrke, N.; Biedenbach, J.; Huber, Y.; Straub, B.K.; Galle, P.R.; Simon, P.; Schattenberg, J.M. Voluntary exercise in mice fed an obesogenic diet alters the hepatic immune phenotype and improves metabolic parameters—An animal model of life style intervention in NAFLD. Sci. Rep. 2019, 9, 4007. [Google Scholar] [CrossRef]
- Alex, S.; Boss, A.; Heerschap, A.; Kersten, S. Exercise training improves liver steatosis in mice. Nutr. Metab. 2015, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Rector, R.S.; Uptergrove, G.M.; Morris, E.M.; Borengasser, S.J.; Laughlin, M.H.; Booth, F.W.; Thyfault, J.P.; Ibdah, J.A. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G874–G883. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, I.; Kim, D.; Koh, Y.; Kong, J.; Lee, S.; Kang, H. Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J. Exerc. Nutr. Biochem. 2014, 18, 339–346. [Google Scholar] [CrossRef]
- Cintra, D.E.; Ropelle, E.R.; Vitto, M.F.; Luciano, T.F.; Souza, D.R.; Engelmann, J.; Marques, S.O.; Lira, F.S.; de Pinho, R.A.; Pauli, J.R.; et al. RETRACTED: Reversion of hepatic steatosis by exercise training in obese mice: The role of sterol regulatory element-binding protein-1c. Life Sci. 2012, 91, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Apro, W.; Wang, L.; Ponten, M.; Blomstrand, E.; Sahlin, K. Resistance exercise induced mTORC1 signaling is not impaired by subsequent endurance exercise in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E22–E32. [Google Scholar] [CrossRef]
- Chen, Z.P.; Stephens, T.J.; Murthy, S.; Canny, B.J.; Hargreaves, M.; Witters, L.A.; Kemp, B.E.; McConell, G.K. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 2003, 52, 2205–2212. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Watt, M.J.; McGee, S.L.; Chan, S.; Hargreaves, M.; Febbraio, M.A.; Stapleton, D.; Kemp, B.E. Reduced glycogen availability is associated with increased AMPKalpha2 activity, nuclear AMPKalpha2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2006, 31, 302–312. [Google Scholar] [CrossRef]
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.-C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011, 2, 112. [Google Scholar] [CrossRef]
- Casuso, R.A.; Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Aragón-Vela, J.; Robles-Sanchez, C.; Nordsborg, N.B.; Hebberecht, M.; Salmeron, L.M.; Huertas, J.R. High-intensity high-volume swimming induces more robust signaling through PGC-1α and AMPK activation than sprint interval swimming in m. triceps brachii. PLoS ONE 2017, 12, e0185494. [Google Scholar] [CrossRef]
- Stine, J.G.; Welles, J.E.; Keating, S.; Hussaini, Z.; Soriano, C.; Heinle, J.W.; Geyer, N.; Chinchilli, V.M.; Loomba, R.; Kimball, S.R. Serum Fibroblast Growth Factor 21 Is Markedly Decreased following Exercise Training in Patients with Biopsy-Proven Nonalcoholic Steatohepatitis. Nutrients 2023, 15, 1481. [Google Scholar] [CrossRef]
- Stine, J.G.; Xu, D.; Schmitz, K.; Sciamanna, C.; Kimball, S.R. Exercise Attenuates Ribosomal Protein Six Phosphorylation in Fatty Liver Disease. Dig. Dis. Sci. 2020, 65, 3238–3243. [Google Scholar] [CrossRef]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef]
- Kurosu, H.; Choi, M.; Ogawa, Y.; Dickson, A.S.; Goetz, R.; Eliseenkova, A.V.; Mohammadi, M.; Rosenblatt, K.P.; Kliewer, S.A.; Kuro, O.M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 2007, 282, 26687–26695. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Charles, E.D.; Sanyal, A.J.; Harrison, S.A.; Neuschwander-Tetri, B.A.; Goodman, Z.; Ehman, R.A.; Karsdal, M.; Nakajima, A.; Du, S.; et al. The FALCON program: Two phase 2b randomized, double-blind, placebo-controlled studies to assess the efficacy and safety of pegbelfermin in the treatment of patients with nonalcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Contemp. Clin. Trials 2021, 104, 106335. [Google Scholar] [CrossRef]
- Brown, E.A.; Minnich, A.; Sanyal, A.J.; Loomba, R.; Du, S.; Schwarz, J.; Ehman, R.L.; Karsdal, M.; Leeming, D.J.; Cizza, G.; et al. Effect of pegbelfermin on NASH and fibrosis-related biomarkers and correlation with histological response in the FALCON 1 trial. JHEP Rep. Innov. Hepatol. 2023, 5, 100661. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S. Actions and mode of actions of FGF19 subfamily members. Endocr. J. 2008, 55, 23–31. [Google Scholar] [CrossRef]
- Lin, B.C.; Wang, M.; Blackmore, C.; Desnoyers, L.R. Liver-specific activities of FGF19 require Klotho beta. J. Biol. Chem. 2007, 282, 27277–27284. [Google Scholar] [CrossRef]
- Suzuki, M.; Uehara, Y.; Motomura-Matsuzaka, K.; Oki, J.; Koyama, Y.; Kimura, M.; Asada, M.; Komi-Kuramochi, A.; Oka, S.; Imamura, T. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol. 2008, 22, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Moure, R.; Cairó, M.; Morón-Ros, S.; Quesada-López, T.; Campderrós, L.; Cereijo, R.; Hernáez, A.; Villarroya, F.; Giralt, M. Levels of β-klotho determine the thermogenic responsiveness of adipose tissues: Involvement of the autocrine action of FGF21. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E822–E834. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Y.; Hu, Y.; Wang, G. The role of fibroblast growth factor 21 in the pathogenesis of non-alcoholic fatty liver disease and implications for therapy. Metabolism 2015, 64, 380–390. [Google Scholar] [CrossRef]
- Falamarzi, K.; Malekpour, M.; Tafti, M.F.; Azarpira, N.; Behboodi, M.; Zarei, M. The role of FGF21 and its analogs on liver associated diseases. Front. Med. 2022, 9, 967375. [Google Scholar] [CrossRef]
- Slusher, A.L.; Whitehurst, M.; Zoeller, R.F.; Mock, J.T.; Maharaj, M.; Huang, C.J. Attenuated fibroblast growth factor 21 response to acute aerobic exercise in obese individuals. Nutr. Metab. Cardiovasc. Dis. NMCD 2015, 25, 839–845. [Google Scholar] [CrossRef]
- Banitalebi, E.; Kazemi, A.; Faramarzi, M.; Nasiri, S.; Haghighi, M.M. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. 2019, 217, 101–109. [Google Scholar] [CrossRef]
- Rad, M.M.; Bijeh, N.; Hosseini, S.R.A.; Saeb, A.R. The effect of two concurrent exercise modalities on serum concentrations of FGF21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2020, 129, 424–433. [Google Scholar] [CrossRef]
- Kong, Z.; Sun, S.; Liu, M.; Shi, Q. Short-Term High-Intensity Interval Training on Body Composition and Blood Glucose in Overweight and Obese Young Women. J. Diabetes Res. 2016, 2016, 4073618. [Google Scholar] [CrossRef]
- Takahashi, A.; Abe, K.; Fujita, M.; Hayashi, M.; Okai, K.; Ohira, H. Simple resistance exercise decreases cytokeratin 18 and fibroblast growth factor 21 levels in patients with nonalcoholic fatty liver disease: A retrospective clinical study. Medicine 2020, 99, e20399. [Google Scholar] [CrossRef]
- Porflitt-Rodríguez, M.; Guzmán-Arriagada, V.; Sandoval-Valderrama, R.; Tam, C.S.; Pavicic, F.; Ehrenfeld, P.; Martínez-Huenchullán, S. Effects of aerobic exercise on fibroblast growth factor 21 in overweight and obesity. A systematic review. Metabolism 2022, 129, 155137. [Google Scholar] [CrossRef]
- Morville, T.; Sahl, R.E.; Trammell, S.A.; Svenningsen, J.S.; Gillum, M.P.; Helge, J.W.; Clemmensen, C. Divergent effects of resistance and endurance exercise on plasma bile acids, FGF19, and FGF21 in humans. JCI Insight 2018, 3, e122737. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Bensila, M.; Bettahi, I.; Jerobin, J.; Samra, T.A.; Aye, M.M.; Alkasem, M.; Siveen, K.S.; Sathyapalan, T.; Skarulis, M.; et al. Dynamic Changes in Circulating Endocrine FGF19 Subfamily and Fetuin-A in Response to Intralipid and Insulin Infusions in Healthy and PCOS Women. Front. Endocrinol. 2020, 11, 568500. [Google Scholar] [CrossRef]
- Mercer, K.E.; Maurer, A.; Pack, L.M.; Ono-Moore, K.; Spray, B.J.; Campbell, C.; Chandler, C.J.; Burnett, D.; Souza, E.; Casazza, G.; et al. Exercise training and diet-induced weight loss increase markers of hepatic bile acid (BA) synthesis and reduce serum total BA concentrations in obese women. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E864–E873. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989. [Google Scholar] [CrossRef]
- Tchang, B.G.; Aras, M.; Kumar, R.B.; Aronne, L.J. Pharmacologic Treatment of Overweight and Obesity in Adults. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ueda, S.Y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Fujimoto, S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J. Endocrinol. 2009, 203, 357–364. [Google Scholar] [CrossRef]
- Martins, C.; Stensvold, D.; Finlayson, G.; Holst, J.; Wisloff, U.; Kulseng, B.; Morgan, L.; King, N.A. Effect of moderate- and high-intensity acute exercise on appetite in obese individuals. Med. Sci. Sport. Exerc. 2015, 47, 40–48. [Google Scholar] [CrossRef]
- Kullman, E.L.; Kelly, K.R.; Haus, J.M.; Fealy, C.E.; Scelsi, A.R.; Pagadala, M.R.; Flask, C.A.; McCullough, A.J.; Kirwan, J.P. Short-term aerobic exercise training improves gut peptide regulation in nonalcoholic fatty liver disease. J. Appl. Physiol. 2016, 120, 1159–1164. [Google Scholar] [CrossRef]
- Hari, A.; Fealy, C.E.; Axelrod, C.L.; Haus, J.M.; Flask, C.A.; McCullough, A.J.; Kirwan, J.P. Exercise Training Rapidly Increases Hepatic Insulin Extraction in NAFLD. Med. Sci. Sport. Exerc. 2020, 52, 1449–1455. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Stevanović, J.; Beleza, J.; Coxito, P.; Ascensão, A.; Magalhães, J. Physical exercise and liver “fitness”: Role of mitochondrial function and epigenetics-related mechanisms in non-alcoholic fatty liver disease. Mol. Metab. 2020, 32, 1–14. [Google Scholar] [CrossRef]
- Selen, E.S.; Choi, J.; Wolfgang, M.J. Discordant hepatic fatty acid oxidation and triglyceride hydrolysis leads to liver disease. JCI Insight 2021, 6, e135626. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef]
- Morris, E.M.; McCoin, C.S.; Allen, J.A.; Gastecki, M.L.; Koch, L.G.; Britton, S.L.; Fletcher, J.A.; Fu, X.; Ding, W.X.; Burgess, S.C.; et al. Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis. J. Physiol. 2017, 595, 4909–4926. [Google Scholar] [CrossRef]
- Fletcher, J.A.; Meers, G.M.; Linden, M.A.; Kearney, M.L.; Morris, E.M.; Thyfault, J.P.; Rector, R.S. Impact of various exercise modalities on hepatic mitochondrial function. Med. Sci. Sport. Exerc. 2014, 46, 1089–1097. [Google Scholar] [CrossRef]
- Sabag, A.; Keating, S.E.; Way, K.L.; Sultana, R.N.; Lanting, S.M.; Twigg, S.M.; Johnson, N.A. The association between cardiorespiratory fitness, liver fat and insulin resistance in adults with or without type 2 diabetes: A cross-sectional analysis. BMC Sport. Sci. Med. Rehabil. 2021, 13, 40. [Google Scholar] [CrossRef]
- Dahmus, J.; Hummer, B.; Rivas, G.; Schmitz, K.; Caldwell, S.H.; Argo, C.K.; Schreibman, I.; Stine, J.G. Patients with Nonalcoholic Steatohepatitis and Advanced Liver Disease Have the Lowest Cardiorespiratory Fitness. Dig. Dis. Sci. 2023, 68, 2695–2703. [Google Scholar] [CrossRef]
- Sabag, A.; Way, K.L.; Sultana, R.N.; Keating, S.E.; Gerofi, J.A.; Chuter, V.H.; Byrne, N.M.; Baker, M.K.; George, J.; Caterson, I.D.; et al. The Effect of a Novel Low-Volume Aerobic Exercise Intervention on Liver Fat in Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2020, 43, 2371–2378. [Google Scholar] [CrossRef]
- Sultana, R.N.; Sabag, A.; Keating, S.E.; Johnson, N.A. The Effect of Low-Volume High-Intensity Interval Training on Body Composition and Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis. Sport. Med. 2019, 49, 1687–1721. [Google Scholar] [CrossRef]
- Sabag, A.; Barr, L.; Armour, M.; Armstrong, A.; Baker, C.J.; Twigg, S.M.; Chang, D.; Hackett, D.A.; Keating, S.E.; George, J.; et al. The Effect of High-intensity Interval Training vs Moderate-intensity Continuous Training on Liver Fat: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2022, 107, 862–881. [Google Scholar] [CrossRef]
- Ledesma, A.; de Lacoba, M.G.; Rial, E. The mitochondrial uncoupling proteins. Genome Biol. 2002, 3, 1–9. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The ‘novel’ ‘uncoupling’ proteins UCP2 and UCP3: What do they really do? Pros and cons for suggested functions. Exp. Physiol. 2003, 88, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Esteves, T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005, 2, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Echtay, K.S.; Liu, Q.; Caskey, T.; Winkler, E.; Frischmuth, K.; Bienengräber, M.; Klingenberg, M. Regulation of UCP3 by nucleotides is different from regulation of UCP1. FEBS Lett. 1999, 450, 8–12. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Li, W.J.; Wang, C.M. The role of uncoupling proteins in diabetes mellitus. J. Diabetes Res. 2013, 2013, 585897. [Google Scholar] [CrossRef]
- Dalgaard, L.T.; Pedersen, O. Uncoupling proteins: Functional characteristics and role in the pathogenesis of obesity and Type II diabetes. Diabetologia 2001, 44, 946–965. [Google Scholar] [CrossRef]
- Chavin, K.D.; Yang, S.; Lin, H.Z.; Chatham, J.; Chacko, V.P.; Hoek, J.B.; Walajtys-Rode, E.; Rashid, A.; Chen, C.H.; Huang, C.C.; et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J. Biol. Chem. 1999, 274, 5692–5700. [Google Scholar] [CrossRef]
- Ribeiro, M.O.; Bianco, S.D.; Kaneshige, M.; Schultz, J.J.; Cheng, S.Y.; Bianco, A.C.; Brent, G.A. Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-beta isoform specific and required for adaptive thermogenesis. Endocrinology 2010, 151, 432–440. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y.; Xie, L.; Yang, R.; Chen, L.; Zhang, L.; Long, T.; Yang, H.; Mao, X.; Fan, Q.; et al. Association of UCP1 polymorphisms with type 2 diabetes mellitus and their interaction with physical activity and sedentary behavior. Gene 2020, 739, 144497. [Google Scholar] [CrossRef]
- Muhammad, H.F.L.; Sulistyoningrum, D.C.; Huriyati, E.; Lee, Y.Y.; Muda, W. The interaction between energy intake, physical activity and UCP2 -866G/A gene variation on weight gain and changes in adiposity: An Indonesian Nutrigenetic Cohort (INDOGENIC). Br. J. Nutr. 2021, 125, 611–617. [Google Scholar] [CrossRef]
- Grundlingh, J.; Dargan, P.I.; El-Zanfaly, M.; Wood, D.M. 2,4-dinitrophenol (DNP): A weight loss agent with significant acute toxicity and risk of death. J. Med. Toxicol. 2011, 7, 205–212. [Google Scholar] [CrossRef]
- Noureddin, M.; Khan, S.; Portell, F.; Jorkasky, D.; Dennis, J.; Khan, O.; Sanyal, A.J. HU6 reduces liver fat in subjects with high BMI NAFLD: Top-line results from a phase 2a trial. Hepatology 2022, 76, S94–S95. [Google Scholar]
- Kim, H.J.; Kim, Y.J.; Seong, J.K. AMP-activated protein kinase activation in skeletal muscle modulates exercise-induced uncoupled protein 1 expression in brown adipocyte in mouse model. J. Physiol. 2022, 600, 2359–2376. [Google Scholar] [CrossRef]
- Yin, R.; Ma, Y.; Zhang, N.; Yang, L.; Zhao, D. Combined effects of voluntary running and liraglutide on glucose homeostasis, fatty acid composition of brown adipose tissue phospholipids, and white adipose tissue browning in db/db mice. Chin. J. Physiol. 2022, 65, 117–124. [Google Scholar] [CrossRef]
- Tanimura, R.; Kobayashi, L.; Shirai, T.; Takemasa, T. Effects of exercise intensity on white adipose tissue browning and its regulatory signals in mice. Physiol. Rep. 2022, 10, e15205. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Liu, S.; Zhang, S.; Mao, Y.; Fang, L. Eight Weeks of High-Intensity Interval Static Strength Training Improves Skeletal Muscle Atrophy and Motor Function in Aged Rats via the PGC-1α/FNDC5/UCP1 Pathway. Clin. Interv. Aging 2021, 16, 811–821. [Google Scholar] [CrossRef]
- Hong, J.; Park, E.; Lee, J.; Lee, Y.; Rooney, B.V.; Park, Y. Exercise training mitigates ER stress and UCP2 deficiency-associated coronary vascular dysfunction in atherosclerosis. Sci. Rep. 2021, 11, 15449. [Google Scholar] [CrossRef]
- Zhou, M.; Lin, B.Z.; Coughlin, S.; Vallega, G.; Pilch, P.F. UCP-3 expression in skeletal muscle: Effects of exercise, hypoxia, and AMP-activated protein kinase. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E622–E629. [Google Scholar] [CrossRef]
- Gonçalves, I.O.; Passos, E.; Rocha-Rodrigues, S.; Diogo, C.V.; Torrella, J.R.; Rizo, D.; Viscor, G.; Santos-Alves, E.; Marques-Aleixo, I.; Oliveira, P.J.; et al. Physical exercise prevents and mitigates non-alcoholic steatohepatitis-induced liver mitochondrial structural and bioenergetics impairments. Mitochondrion 2014, 15, 40–51. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Poulsen, L.; Siersbæk, M.; Mandrup, S. PPARs: Fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 2012, 23, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.; Simões, I.C.M.; Teixeira, J.; Cagide, F.; Potes, Y.; Soares, P.; Carvalho, A.; Tavares, L.C.; Benfeito, S.; Pereira, S.P.; et al. Mitochondria-targeted anti-oxidant AntiOxCIN(4) improved liver steatosis in Western diet-fed mice by preventing lipid accumulation due to upregulation of fatty acid oxidation, quality control mechanism and antioxidant defense systems. Redox Biol. 2022, 55, 102400. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Lustig, D.G.; Baheza, R.A.; Hasenour, C.M.; Lee-Young, R.S.; Donahue, E.P.; Lynes, S.E.; Swift, L.L.; Charron, M.J.; Damon, B.M.; et al. Hepatic glucagon action is essential for exercise-induced reversal of mouse fatty liver. Diabetes 2011, 60, 2720–2729. [Google Scholar] [CrossRef] [PubMed]
- Diniz, T.A.; de Lima Junior, E.A.; Teixeira, A.A.; Biondo, L.A.; da Rocha, L.A.F.; Valadão, I.C.; Silveira, L.S.; Cabral-Santos, C.; de Souza, C.O.; Neto, J.C.R. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 2021, 266, 118868. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Ricquier, D.; Bouillaud, F. Mitochondrial uncoupling proteins: From mitochondria to the regulation of energy balance. J. Physiol. 2000, 529 Pt 1, 3–10. [Google Scholar] [CrossRef]
- Vernia, S.; Cavanagh-Kyros, J.; Garcia-Haro, L.; Sabio, G.; Barrett, T.; Jung, D.Y.; Kim, J.K.; Xu, J.; Shulha, H.P.; Garber, M.; et al. The PPARα-FGF21 hormone axis contributes to metabolic regulation by the hepatic JNK signaling pathway. Cell Metab. 2014, 20, 512–525. [Google Scholar] [CrossRef]
- Li, D.D.; Ma, J.M.; Li, M.J.; Gao, L.L.; Fan, Y.N.; Zhang, Y.N.; Tao, X.J.; Yang, J.J. Supplementation of Lycium barbarum Polysaccharide Combined with Aerobic Exercise Ameliorates High-Fat-Induced Nonalcoholic Steatohepatitis via AMPK/PPARα/PGC-1α Pathway. Nutrients 2022, 14, 3247. [Google Scholar] [CrossRef]
- Maciejewska, A.; Sawczuk, M.; Cięszczyk, P. Variation in the PPARα gene in Polish rowers. J. Sci. Med. Sport 2011, 14, 58–64. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Mozhayskaya, I.A.; Flavell, D.M.; Astratenkova, I.V.; Komkova, A.I.; Lyubaeva, E.V.; Tarakin, P.P.; Shenkman, B.S.; Vdovina, A.B.; Netreba, A.I.; et al. PPARalpha gene variation and physical performance in Russian athletes. Eur. J. Appl. Physiol. 2006, 97, 103–108. [Google Scholar] [CrossRef]
- Nishida, Y.; Iyadomi, M.; Tominaga, H.; Taniguchi, H.; Higaki, Y.; Tanaka, H.; Horita, M.; Shimanoe, C.; Hara, M.; Tanaka, K. Influence of Single-Nucleotide Polymorphisms in PPAR-δ, PPAR-γ, and PRKAA2 on the Changes in Anthropometric Indices and Blood Measurements through Exercise-Centered Lifestyle Intervention in Japanese Middle-Aged Men. Int. J. Mol. Sci. 2018, 19, 703. [Google Scholar] [CrossRef]
- Blond, M.B.; Schnurr, T.M.; Rosenkilde, M.; Quist, J.S.; Gram, A.S.; Reichkendler, M.H.; Auerbach, P.L.; Nordby, P.; Skovgaard, L.T.; Ribel-Madsen, R.; et al. PPARG Pro12Ala Ala carriers exhibit greater improvements in peripheral insulin sensitivity in response to 12 weeks of aerobic exercise training. Physiol. Genom. 2019, 51, 254–260. [Google Scholar] [CrossRef]
- Gu, X.; Ma, X.; Mo, L.; Wang, Q. The role of exercise intensity on fatty liver in rats. Chin. J. Physiol. 2022, 65, 301–310. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Nebot, E.; Camiletti-Moirón, D.; Aparicio, V.A.; Lopez-Jurado, M.; Aranda, P.; Arrebola, F.; Fernandez-Segura, E.; et al. Aerobic interval exercise improves parameters of nonalcoholic fatty liver disease (NAFLD) and other alterations of metabolic syndrome in obese Zucker rats. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 1242–1252. [Google Scholar] [CrossRef]
- Nikroo, H.; Hosseini, S.R.A.; Fathi, M.; Sardar, M.A.; Khazaei, M. The effect of aerobic, resistance, and combined training on PPAR-α, SIRT1 gene expression, and insulin resistance in high-fat diet-induced NAFLD male rats. Physiol. Behav. 2020, 227, 113149. [Google Scholar] [CrossRef]
- Bae-Gartz, I.; Kasper, P.; Großmann, N.; Breuer, S.; Janoschek, R.; Kretschmer, T.; Appel, S.; Schmitz, L.; Vohlen, C.; Quaas, A.; et al. Maternal exercise conveys protection against NAFLD in the offspring via hepatic metabolic programming. Sci. Rep. 2020, 10, 15424. [Google Scholar] [CrossRef]
- Batatinha, H.A.; Lima, E.A.; Teixeira, A.A.; Souza, C.O.; Biondo, L.A.; Silveira, L.S.; Lira, F.S.; Neto, J.C.R. Association between Aerobic Exercise and Rosiglitazone Avoided the NAFLD and Liver Inflammation Exacerbated in PPAR-α Knockout Mice. J. Cell. Physiol. 2017, 232, 1008–1019. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cai, Y. Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR-γ and genes involved in the beta-oxidation of fatty acids in ApoE-KO mice fed a high-fat diet. Lipids Health Dis. 2019, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Ness, G.C. Transcriptional and posttranscriptional regulation of rat hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase by thyroid hormones. J. Biol. Chem. 1988, 263, 12448–12453. [Google Scholar] [CrossRef]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Thyroid Hormone Signaling and the Liver. Hepatology 2020, 72, 742–752. [Google Scholar] [CrossRef]
- Qiu, S.; Cao, P.; Guo, Y.; Lu, H.; Hu, Y. Exploring the Causality between Hypothyroidism and Non-alcoholic Fatty Liver: A Mendelian Randomization Study. Front. Cell Dev. Biol. 2021, 9, 643582. [Google Scholar] [CrossRef]
- Li, L.M.; Song, Y.; Shi, Y.Q.; Sun, L.L. Thyroid hormone receptor-beta agonists in NAFLD therapy: Possibilities and challenges. J. Clin. Endocrinol. Metab. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Ying, H.; Araki, O.; Furuya, F.; Kato, Y.; Cheng, S.Y. Impaired adipogenesis caused by a mutated thyroid hormone alpha1 receptor. Mol. Cell. Biol. 2007, 27, 2359–2371. [Google Scholar] [CrossRef]
- Domouzoglou, E.M.; Fisher, F.M.; Astapova, I.; Fox, E.C.; Kharitonenkov, A.; Flier, J.S.; Hollenberg, A.N.; Maratos-Flier, E. Fibroblast growth factor 21 and thyroid hormone show mutual regulatory dependency but have independent actions in vivo. Endocrinology 2014, 155, 2031–2040. [Google Scholar] [CrossRef]
- Videla, L.A.; Fernández, V.; Vargas, R.; Cornejo, P.; Tapia, G.; Varela, N.; Valenzuela, R.; Arenas, A.; Fernández, J.; Hernández-Rodas, M.C.; et al. Upregulation of rat liver PPARα-FGF21 signaling by a docosahexaenoic acid and thyroid hormone combined protocol. BioFactors 2016, 42, 638–646. [Google Scholar] [CrossRef]
- Klasson, C.L.; Sadhir, S.; Pontzer, H. Daily physical activity is negatively associated with thyroid hormone levels, inflammation, and immune system markers among men and women in the NHANES dataset. PLoS ONE 2022, 17, e0270221. [Google Scholar] [CrossRef]
- Aristizabal, J.C.; Freidenreich, D.J.; Volk, B.M.; Kupchak, B.R.; Saenz, C.; Maresh, C.M.; Kraemer, W.J.; Volek, J.S. Effect of resistance training on resting metabolic rate and its estimation by a dual-energy X-ray absorptiometry metabolic map. Eur. J. Clin. Nutr. 2015, 69, 831–836. [Google Scholar] [CrossRef]
- Berahman, H.; Elmieh, A.; Chafy, M.R.F. The effect of water-based rhythmic exercise training on glucose homeostasis and thyroid hormones in postmenopausal women with metabolic syndrome. Horm. Mol. Biol. Clin. Investig. 2021, 42, 189–193. [Google Scholar] [CrossRef]
- Ciloglu, F.; Peker, I.; Pehlivan, A.; Karacabey, K.; Ilhan, N.; Saygin, O.; Ozmerdivenli, R. Exercise intensity and its effects on thyroid hormones. Neuro Endocrinol. Lett. 2005, 26, 830–834. [Google Scholar] [PubMed]
- Liu, Q.; Li, H.; He, W.; Zhao, Q.; Huang, C.; Wang, Q.; Zheng, Z.; Zhang, X.; Shi, X.; Li, X. Role of aerobic exercise in ameliorating NASH: Insights into the hepatic thyroid hormone signaling and circulating thyroid hormones. Front. Endocrinol. 2022, 13, 1075986. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Lawitz, E.; Mantry, P.; Jayakumar, S.; Caldwell, S.; Arnold, H.; Diehl, A.; Djedjos, C.; Han, L.; Myers, R.; et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018, 67, 549–559. [Google Scholar] [CrossRef]
- Harrison, S.A.; Wong, V.W.; Okanoue, T.; Bzowej, N.; Vuppalanchi, R.; Younes, Z.; Kohli, A.; Sarin, S.; Caldwell, S.H.; Alkhouri, N.; et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J. Hepatol. 2020, 73, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, T.; Zhou, P.; Li, R.; Liu, Z.; Xie, J.; Hua, T.; Sun, Q. Exercise ameliorates insulin resistance and improves ASK1-mediated insulin signalling in obese rats. J. Cell. Mol. Med. 2021, 25, 10930–10938. [Google Scholar] [CrossRef]
- Zou, Y.; Qi, Z. Understanding the Role of Exercise in Nonalcoholic Fatty Liver Disease: ERS-Linked Molecular Pathways. Mediat. Inflamm. 2020, 2020, 6412916. [Google Scholar] [CrossRef]
- Sun, L.; Cai, J.; Gonzalez, F.J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 335–347. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Jiang, C.; Patterson, A.D. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology 2016, 151, 845–859. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Majzoub, A.M.; Nayfeh, T.; Barnard, A.; Munaganuru, N.; Dave, S.; Singh, S.; Murad, M.H.; Loomba, R. Systematic review with network meta-analysis: Comparative efficacy of pharmacologic therapies for fibrosis improvement and resolution of NASH. Aliment. Pharmacol. Ther. 2021, 54, 880–889. [Google Scholar] [CrossRef]
- Molina-Molina, E.; Baccetto, R.L.; Wang, D.Q.; de Bari, O.; Krawczyk, M.; Portincasa, P. Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur. J. Clin. Investig. 2018, 48, e12958. [Google Scholar] [CrossRef]
- Meissner, M.; Lombardo, E.; Havinga, R.; Tietge, U.J.; Kuipers, F.; Groen, A.K. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 2011, 218, 323–329. [Google Scholar] [CrossRef]
- Wertheim, B.C.; Martínez, M.E.; Ashbeck, E.L.; Roe, D.J.; Jacobs, E.T.; Alberts, D.S.; Thompson, P.A. Physical activity as a determinant of fecal bile acid levels. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1591–1598. [Google Scholar] [CrossRef]
- Danese, E.; Salvagno, G.L.; Tarperi, C.; Negrini, D.; Montagnana, M.; Festa, L.; Sanchis-Gomar, F.; Schena, F.; Lippi, G. Middle-distance running acutely influences the concentration and composition of serum bile acids: Potential implications for cancer risk? Oncotarget 2017, 8, 52775–52782. [Google Scholar] [CrossRef]
- Hughes, A.; Dahmus, J.; Rivas, G.; Hummer, B.; See, J.R.C.; Wright, J.R.; Lamendella, R.; Schmitz, K.H.; Sciamanna, C.; Ruffin, M.; et al. Exercise Training Reverses Gut Dysbiosis in Patients with Biopsy-Proven Nonalcoholic Steatohepatitis: A Proof of Concept Study. Clin. Gastroenterol. Hepatol. 2021, 19, 1723–1725. [Google Scholar] [CrossRef]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, L.; Le, S.; Yang, Y.; Zhao, C.; Zhang, X.; Yang, X.; Xu, T.; Xu, L.; Wiklund, P.; et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat. Commun. 2022, 13, 2555. [Google Scholar] [CrossRef]
- Cheng, S.; Ge, J.; Zhao, C.; Le, S.; Yang, Y.; Ke, D.; Wu, N.; Tan, X.; Zhang, X.; Du, X.; et al. Effect of aerobic exercise and diet on liver fat in pre-diabetic patients with non-alcoholic-fatty-liver-disease: A randomized controlled trial. Sci. Rep. 2017, 7, 15952. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Deeg, M.A.; Crabb, D.W. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2004, 99, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef]
- Ohtani, N.; Kawada, N. Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatol. Commun. 2019, 3, 456–470. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Fang, Z.; Dou, G.; Wang, L. MicroRNAs in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2021, 17, 1851–1863. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Vuppalanchi, R.; Gawrieh, S.; Ghabril, M.; Saxena, R.; Cummings, O.W.; Chalasani, N. Vitamin E Improves Transplant-Free Survival and Hepatic Decompensation among Patients with Nonalcoholic Steatohepatitis and Advanced Fibrosis. Hepatology 2020, 71, 495–509. [Google Scholar] [CrossRef]
- Oh, S.; Tanaka, K.; Warabi, E.; Shoda, J. Exercise reduces inflammation and oxidative stress in obesity-related liver diseases. Med. Sci. Sport. Exerc. 2013, 45, 2214–2222. [Google Scholar] [CrossRef]
- Bataille, A.M.; Manautou, J.E. Nrf2: A potential target for new therapeutics in liver disease. Clin. Pharmacol. Ther. 2012, 92, 340–348. [Google Scholar] [CrossRef]
- Safdar, A.; deBeer, J.; Tarnopolsky, M.A. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 2010, 49, 1487–1493. [Google Scholar] [CrossRef]
- Oh, S.; Tsujimoto, T.; Kim, B.; Uchida, F.; Suzuki, H.; Iizumi, S.; Isobe, T.; Sakae, T.; Tanaka, K.; Shoda, J. Weight-loss-independent benefits of exercise on liver steatosis and stiffness in Japanese men with NAFLD. JHEP Rep. 2021, 3, 100253. [Google Scholar] [CrossRef]
- Trinity, J.D.; Broxterman, R.M.; Richardson, R.S. Regulation of exercise blood flow: Role of free radicals. Free Radic. Biol. Med. 2016, 98, 90–102. [Google Scholar] [CrossRef]
- Chun, S.K.; Lee, S.; Yang, M.J.; Leeuwenburgh, C.; Kim, J.S. Exercise-Induced Autophagy in Fatty Liver Disease. Exerc. Sport Sci. Rev. 2017, 45, 181–186. [Google Scholar] [CrossRef]
| Mechanism of Action | Effects on Hepatic Steatosis | Animal Evidence | Human Evidence | Extrahepatic Benefits | Safety |
|---|---|---|---|---|---|
| AMPK | ↓ steatosis | Aerobic and resistance training increases AMPK activation | N.A. | ↑ GLUT-4 expression | Exercise is safe for people with NAFLD/NASH when supervised by an appropriately qualified exercise professional and with adequate screening and monitoring protocols. |
| ↑ glucose uptake | |||||
| FGF-19 and -21 | ↓ steatosis | Aerobic exercise training increases FGF receptor 1 and FGF receptor 2 Chronic exercise training increases β-klotho Chronic exercise training reduces serum FGF-21 | Aerobic and resistance training reduces serum FGF-21 | ↑ hepatic glycogen synthesis | |
| Effect of exercise on FGF-19 is unclear | ↑ hepatic gluconeogenesis | ||||
| GLP-1 | ↓ steatosis | N.A. | Acute aerobic exercise ↑ GLP-1 | ↑ weight loss | |
| Short term high-intensity aerobic exercise reversed GLP-1 resistance | ↑ insulin production | ||||
| N.A. for chronic training adaptation | ↓ appetite | ||||
| Mitochondrial function and β-oxidation | ↓ steatosis | Exercise training improves mitochondrial function, ↑ mitochondrial content and ↑ β-oxidation | Exercise training improves mitochondrial function, ↑ mitochondrial content and ↑ β-oxidation | ↑ insulin sensitivity | |
| UCP | ↓ steatosis | Aerobic exercise upregulates UCP-1 and reverses UCP-2 dysfunction in the liver | N.A. | Prevent oxidative stress | |
| PPAR-α/γ | ↓ steatosis | Exercise training (moderate-intensity running or swimming; high-intensity interval training; resistance training) activates PPAR-α | N.A. | ↑ insulin sensitivity | |
| Maternal aerobic exercise may protect against early life NAFLD in offspring | |||||
| THR-β | ↓ steatosis | Aerobic exercise training increases T3 response index | N.A. | Acute exercise ↑ free T3 and T4 | |
| ↑ mitochondria size and number | |||||
| ↑ glucose uptake | |||||
| ↑ gluconeogenesis | |||||
| Chronic exercise ↑ T3 and T4 turnover at same absolute intensity |
| Mechanism of Action | Effects on Liver Fibrosis | Animal Evidence | Human Evidence | Extrahepatic Benefits | Safety |
|---|---|---|---|---|---|
| ASK-1 | ↓ fibrosis | Aerobic exercise (swimming) regulates ASK-1-mediated insulin signaling in animal models of obesity | N.A. | ↓ ERS | Exercise is safe for people with NAFLD/NASH when supervised by an appropriately qualified exercise professional and with adequate screening and monitoring protocols. |
| ↓ hepatocyte injury | No evidence in NAFLD/NASH | ||||
| FXR | ↓ fibrosis | N.A. | N.A. | ||
| ROS | ↓ fibrosis via a reduction in inflammation | N.A. | Aerobic exercise reduces ROS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinle, J.W.; DiJoseph, K.; Sabag, A.; Oh, S.; Kimball, S.R.; Keating, S.; Stine, J.G. Exercise Is Medicine for Nonalcoholic Fatty Liver Disease: Exploration of Putative Mechanisms. Nutrients 2023, 15, 2452. https://doi.org/10.3390/nu15112452
Heinle JW, DiJoseph K, Sabag A, Oh S, Kimball SR, Keating S, Stine JG. Exercise Is Medicine for Nonalcoholic Fatty Liver Disease: Exploration of Putative Mechanisms. Nutrients. 2023; 15(11):2452. https://doi.org/10.3390/nu15112452
Chicago/Turabian StyleHeinle, James Westley, Kara DiJoseph, Angelo Sabag, Sechang Oh, Scot R. Kimball, Shelley Keating, and Jonathan G. Stine. 2023. "Exercise Is Medicine for Nonalcoholic Fatty Liver Disease: Exploration of Putative Mechanisms" Nutrients 15, no. 11: 2452. https://doi.org/10.3390/nu15112452
APA StyleHeinle, J. W., DiJoseph, K., Sabag, A., Oh, S., Kimball, S. R., Keating, S., & Stine, J. G. (2023). Exercise Is Medicine for Nonalcoholic Fatty Liver Disease: Exploration of Putative Mechanisms. Nutrients, 15(11), 2452. https://doi.org/10.3390/nu15112452






