Abstract
Limited evidence exists on the combined and mediating effects of systemic inflammation on the association between insulin resistance and cardiovascular events in patients with diabetes and chronic coronary syndrome (CCS). This secondary analysis of a multicenter prospective cohort included 4419 diabetic CCS patients. Triglyceride–glucose index (TyG) and high-sensitivity C-reactive protein (hsCRP) were applied to evaluate insulin resistance and systemic inflammation, respectively. The primary endpoint was major adverse cardiac event (MACE). Associations of TyG and hsCRP with cardiovascular events were estimated using Cox regression. A mediation analysis was performed to assess whether hsCRP mediates the relationship between TyG and cardiovascular events. Within a median 2.1-year follow-up period, 405 MACEs occurred. Patients with high levels of TyG and hsCRP experienced the highest MACE risk (hazard ratio = 1.82, 95% confidence interval: 1.24–2.70, p = 0.002) compared to individuals with low levels of both markers. HsCRP significantly mediated 14.37% of the relationship between TyG and MACE (p < 0.001). In diabetic CCS patients, insulin resistance and systemic inflammation synergically increased the risk of cardiovascular events, and systemic inflammation partially mediated the association between insulin resistance and clinical outcomes. Combining TyG and hsCRP can help identify high-risk patients. Controlling inflammation in patients with insulin resistance may bring added benefits.
1. Introduction
Coronary artery disease (CAD) reigns as the foremost single cause of mortality and the loss of disability-adjusted life years across low-income, middle-income, and high-income countries [1]. Diabetes stands as the primary complication of CAD, with cardiovascular causes emerging as the leading contributors to mortality among diabetic patients [2]. By 2020, the prevalence of diabetes among Chinese adults had reached 12.8%, affecting a population of 130 million individuals [3]. Globally, it is estimated that the number of diabetic patients will be 700 million by 2045 [4]. Despite the advances in secondary prevention strategies and invasive treatment techniques, patients with comorbid traits continue to face a high risk of recurrent cardiovascular events [5,6,7]. Therefore, identifying individuals with modifiable nontraditional cardiovascular risk factors is crucial to implementing effective comprehensive risk reduction and improving their prognosis.
Insulin resistance is the metabolic characteristic of type 2 diabetes and a common manifestation of type 1 diabetes. Insulin resistance contributes to the development of atherosclerosis by causing glucose and lipid metabolism disorder, endothelial dysfunction, coagulation disorders, and smooth muscle cell dysfunction [8], and it has been established as an independent cardiovascular risk factor in diabetic patients [9,10,11]. Triglyceride–glucose index (TyG), derived from fasting triglyceride and blood glucose levels, has emerged as a reliable measure of insulin resistance. It offers the advantages of being readily available, inexpensive, and independent of insulin treatment status. Recent research supports that TyG is independently associated with cardiovascular outcomes in diabetic and non-diabetic patients with different CAD phenotypes [8]. However, the underlying mechanism remains unclear.
As a chronic condition associated with systemic low-grade inflammation, diabetes promotes vascular inflammation by upregulating the expression of pro-inflammatory genes [12]. Inflammation is widely acknowledged as an etiological factor in atherosclerosis and has emerged as another well-established independent cardiovascular risk factor in general and diabetic patients [13,14,15]. High-sensitivity C reactive protein (hsCRP) is a widely used biomarker of systemic inflammation, and its predictive significance for cardiovascular events has been extensively validated in various CAD settings [14,15,16,17].
Both insulin resistance and inflammation play causal roles in atherosclerosis. Their proatherogenic processes share some cells and cytokines but also involve different signaling pathways. Moreover, insulin resistance and inflammation initiate and aggravate each other in a vicious cycle [18]. Additionally, insulin resistance exacerbates atherosclerosis by causing glucose and lipid metabolism disorders and endothelial and smooth muscle dysfunction. These processes often involve the release of pro-inflammatory cytokines [8]. The aforementioned biological findings have given rise to two hypotheses for this study. Firstly, the combined effect of insulin resistance, as measured by TyG, and systemic inflammation, as assessed by hsCRP, may worsen cardiovascular outcomes. Secondly, hsCRP may serve as a mediator between TyG and cardiovascular events.
This study aimed to investigate the combined and mediating effects of hsCRP-reflected inflammation on the association between TyG-reflected insulin resistance and cardiovascular events in diabetic patients with comorbid chronic coronary syndrome (CCS). For this paper, patients were first categorized into four groups based on high and low levels of TyG and hsCRP to evaluate the combined effect of TyG and hsCRP on multiple cardiovascular events. Subsequently, after assessing the prerequisites for mediation analysis, a mediation analysis was performed on eligible events to examine the role of hsCRP in the impact of TyG on these events.
2. Materials and Methods
2.1. Study Design and Participants
The present study utilized data from the PRospective Observational Multi-center cohort for ISchemic and hEmorrhage risk in coronary artery disease patients (PROMISE), designed to develop ischemic and bleeding risk scores specifically for Chinese populations. The PROMISE study recruited 18,701 hospitalized CAD patients from nine centers across China between January 2015 and May 2019. Inclusion criteria included patients of at least 18 years old, diagnosis of CAD, indication of at least one antiplatelet drug, and willingness to participate in the study by providing informed consent. Exclusion criteria were a life expectancy of fewer than six months and current participation in another interventional clinical trial. The decision to initiate invasive treatment was made by the heart team according to current guidelines, taking into account the patient’s preferences. Guideline-recommended secondary prevention medications were prescribed for all patients without documented contraindications. The PROMISE study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Fuwai Hospital (protocol code: 2013-449, date of approval: 4 September 2013; protocol code: No. 2017-860, date of approval: 10 January 2017). All participants provided written informed consent.
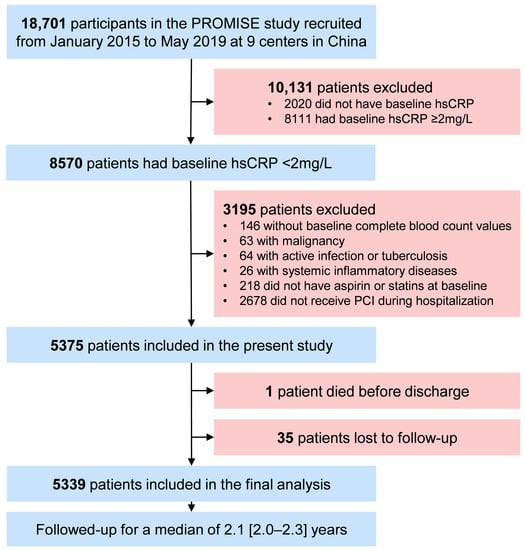
The participant selection process for the present study is outlined in Figure 1. Patients diagnosed with diabetes were included. Patients with acute conditions that may lead to stress hyperglycemia or alter systemic inflammation status, including acute coronary syndrome, active infection or tuberculosis, systemic inflammatory diseases, and malignancy, were excluded from this study. Participants without fasting blood glucose, triglyceride, and hsCRP values and those not having aspirin or statins at baseline were also excluded. A total of 4419 patients were eligible for this study, of whom 1228 (27.79%) were female. They were divided into four groups based on TyG and hsCRP levels: low TyG and hsCRP (L-TyG/L-hsCRP), low TyG with high hsCRP (L-TyG/H-hsCRP), high TyG with low hsCRP (H-TyG/L-hsCRP), and high TyG and hsCRP (H-TyG/H-hsCRP).
2.2. Measures of Insulin Resistance and Systemic Inflammation
Insulin resistance was evaluated using TyG, calculated as Ln (fasting triglyceride (mg/dL) × fasting blood glucose (mg/dL) ÷ 2) [19], with triglyceride converted from mmol/L to mg/dL by multiplying by 88.50. Systemic inflammation was evaluated using hsCRP. Higher TyG and hsCRP levels indicate increased insulin resistance and systemic inflammation, respectively. TyG was categorized at the optimal cut-off value of 8.46. HsCRP was categorized at 2.00 mg/L, aligning with the definition of residual inflammatory risk [20].
2.3. Blood Sampling and Laboratory Testing
Fasting blood samples were drawn in the morning as part of routine clinical practice. Blood glucose was assayed using an enzymatic hexokinase method. Glycated hemoglobin (HbA1c) was measured with automated glycohemoglobin analyzers (Tosoh HLC-723G8, Tokyo, Japan). Lipid profiles were measured with automatic biochemistry analyzers (Hitachi 7150, Tokyo, Japan). HsCRP was measured by rate turbidimetry with immunoassay analyzers (Beckman Assay, Brea, CA, USA).
2.4. Endpoints and Follow-Up
The primary endpoint was major adverse cardiac event (MACE), a composite of cardiac death, non-fatal myocardial infarction, and any revascularization. Secondary endpoints included all-cause death and each component of the composite endpoint. Deaths without an unequivocal non-cardiac cause were presumed to be cardiac. Myocardial infarction was diagnosed following the Fourth Universal Definition of Myocardial Infarction. Any revascularization was defined as percutaneous coronary intervention (PCI) or coronary artery bypass grafting for any lesion driven by ischemic symptoms or events. Outcome data and medication usage were followed up through clinic visits, telephone calls, text messages, and letters by an independent group of clinical research coordinators one and two years after discharge. Two independent cardiologists adjudicated endpoint events, and disagreements were resolved through consensus.
2.5. Definition of Variables
Diabetes was defined as fasting blood glucose ≥7.0 mmol/L (126.00 mg/dL), HbA1c ≥ 6.50% (48 mmol/mol), 2 h blood glucose of oral glucose tolerance test ≥11.1 mmol/L, oral antidiabetic medication or insulin use, or self-reported diabetes. Chronic kidney disease was defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or self-reported chronic kidney disease. Hypertension was defined as a blood pressure ≥ 140/90 mmHg on repeated measurements, antihypertensive medication use, or self-reported history of hypertension. Dyslipidemia was defined when at least one of the following criteria was met: total cholesterol ≥ 6.22 mmol/L, triglyceride ≥ 2.26 mmol/L, low-density lipoprotein cholesterol ≥ 4.14 mmol/L, high-density lipoprotein cholesterol < 1.04 mmol/L, lipid-lowering medication use, or self-reported history of dyslipidemia. Three-vessel disease was defined as angiographically significant stenosis (≥50%) of all three main coronary arteries. Postprocedural Thrombolysis in Myocardial Infarction (TIMI) flow grade 3 was considered successful PCI; otherwise, it was considered unsuccessful. The medication adherence for aspirin and statins was categorized as follows: 2 years of regular use, >1 to <2 years of regular use, and <1 year of regular use or irregular use.
2.6. Statistical Analysis
Given the low rate of missing data in the study dataset, missing values were imputed using the median or mode as appropriate for the variable type (Supplementary Materials: Table S1). The correlation between TyG and hsCRP was assessed using Spearman’s rank correlation analysis. Categorical variables were expressed as numbers (percentages) and compared using the χ2 test. Non-normally distributed continuous variables (by the Kolmogorov–Smirnov test) were expressed as medians (interquartile ranges) and compared using the Kruskal–Wallis H test.
Kaplan–Meier survival curves were compared using the log-rank test. Associations of insulin resistance and/or systemic inflammation with cardiovascular events were reported as hazard ratios (HRs) and 95% confidence intervals (CIs) estimated using Cox proportional-hazards regression. The optimal cut-off value for TyG was determined using the maximum Youden index of the receiver operating characteristic curve for predicting MACE. Time-dependent receiver operating characteristic analysis was performed using the survivalROC Package. Restricted cubic splines with 4 knots were fitted using the rms Package when TyG and hsCRP were analyzed continuously. The knot locations were set at the 5th, 35th, 65th, and 95th percentiles of TyG and hsCRP levels.
Covariables for adjustment included age (continuous), sex, body mass index (BMI) (continuous), smoking history, insulin use, peripheral artery disease, left ventricular ejection fraction <40%, left main stem/three-vessel disease, synergy between percutaneous coronary intervention with Taxus and cardiac surgery (SYNTAX) score (categorical), PCI status, aspirin adherence, and statins adherence.
Pre-specified subgroup analyses were performed based on five variables: sex, age (≥65 years vs. <65 years, according to the definition of elderly for the Chinese populations), BMI (≥28 kg/m2 vs. <28 kg/m2, according to the definition of obesity for the Chinese populations), insulin use, and PCI status. Three pre-specified sensitivity analyses were performed to examine the robustness of the results: dividing patients into nine groups by employing cut-off values of 8.46 and 9.31 for TyG and 1.00 and 3.00 mg/L for hsCRP, excluding patients who lost to 2-year follow-up, and excluding those with hsCRP > 10 mg/L. One post-hoc sensitivity analysis was performed by excluding patients with imputed values.
A causal mediation analysis was performed using the mediation Package developed by Imai et al. [21] based on the counterfactual-based framework to assess whether systemic inflammation mediates the association between insulin resistance and cardiovascular events. A directed acyclic graph was used to visualize the assumed causal model, with TyG (continuous) as the exposure, hsCRP (continuous) as the mediator, and endpoint events as the outcome variables. Confounders identified through the directed acyclic graph were adjusted, including age, sex, BMI, smoking history, insulin use, hypertension, dyslipidemia, chronic kidney disease, left ventricular ejection fraction < 40%, HbA1c, aspirin adherence, and statin adherence. Given the time-to-event nature of the outcomes, the Cox proportional-hazards regression model was chosen for mediation analysis. The significance of the mediating effect was examined through 1000 bootstrap samples.
Statistical analyses were conducted with R version 4.2.0 (R Core Team 2022, Vienna, Austria). Two-tailed p values < 0.05 were considered statistically significant. Figures were prepared with GraphPad Prism version 9.0.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Study Population and Baseline Characteristics
Of the 4419 participants, the median age was 62 years (interquartile range: 55–68 years), and 1228 (27.79%) were female. Insulin use was reported in 635 (14.37%) patients. Nearly two-thirds of the participants underwent PCI during hospitalization, with a success rate of 95.46%. The median TyG value was 8.96 (interquartile range: 8.59–9.38), while the median hsCRP value was 1.46 mg/L (interquartile range: 0.70–2.92 mg/L).
Table 1 shows baseline characteristics across patient groups categorized by TyG and hsCRP levels. The percentage of patients with high hsCRP levels was higher in the H-TyG group (37.61%) than in the L-TyG group (25.50%). The proportions of female, obese patients, current smokers, and patients undergoing PCI increased sequentially from the L-TyG/L-hsCRP group to the H-TyG/H-hsCRP group, as did the levels of HbA1c and low-density lipoprotein cholesterol. Regardless of their TyG levels, patients with H-hsCRP were more likely to present a higher lesion severity, as reflected by the presence of left main/three-vessel disease and a higher SYNTAX score. Insulin use, comorbidities other than dyslipidemia, reduced cardiac function, and medication adherence were comparable among the four groups.
3.2. Association of TyG and hsCRP with Cardiovascular Events
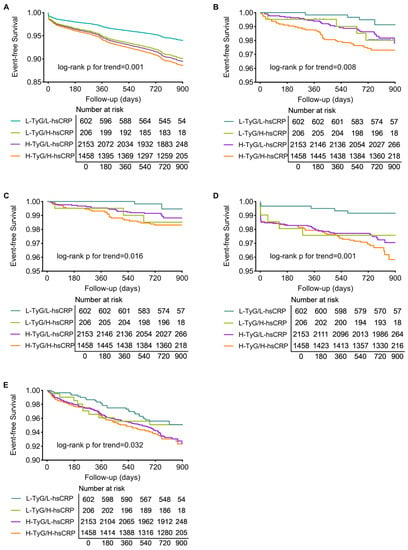
Of the total 4419 participants, 4362 (98.71%) completed a median follow-up of 2.1 years (interquartile range: 2.0–2.3 years), with 405 MACEs, 85 all-cause deaths, 51 cardiac deaths, 118 myocardial infarctions, and 282 cases of any revascularization occurring. The MACE-free survival curves illustrate that patients in the H-TyG/H-hsCRP group had the highest incidence of MACE. Patients with isolated high TyG or high hsCRP levels also experienced more MACE than those in the L-TyG/L-hsCRP group (p for trend = 0.001, Figure 2A). Event-free survival curves for secondary endpoints revealed similar trends (all p for trend < 0.05, Figure 2B–E).
Figure 2.
Kaplan–Meier analysis of patients grouped by TyG and hsCRP levels. Survival curves for MACE (A), all-cause death (B), cardiac death (C), myocardial infarction (D), and any revascularization (E). Abbreviations: H, high; hsCRP, high-sensitivity C-reactive protein; L, low; MACE, major adverse cardiac event; TyG, triglyceride–glucose index.
Univariate Cox analysis for clinical outcomes is shown in Supplementary Materials Table S2. Cox analyses before and after adjustment consistently yielded that the H-TyG/H-hsCRP group had a significantly higher MACE risk compared to the L-TyG/L-hsCRP group (adjusted HR: 1.83, 95% CI: 1.24–2.70, p = 0.002), followed by the H-TyG/L-hsCRP group (adjusted HR: 1.78, 95% CI: 1.22–2.60, p = 0.003). However, the MACE risk did not significantly increase for the L-TyG/H-hsCRP group. The risks of all secondary endpoints were also significantly higher in patients with high levels of both TyG and hsCRP than in those with low levels of both (Table 2).
When patients were grouped solely by TyG or hsCRP levels, high levels of either biomarker were still significantly associated with a higher MACE risk. However, there was no significant difference in the risk of any revascularization between the H-hsCRP and L-hsCRP groups. Additionally, no interaction was observed between TyG and hsCRP categories (Supplementary Materials: Figures S1 and S2, Table S3). The relationship between cardiovascular events and TyG or hsCRP on a continuous scale is plotted in Figure S3.
3.3. Subgroup Analyses and Sensitivity Analyses
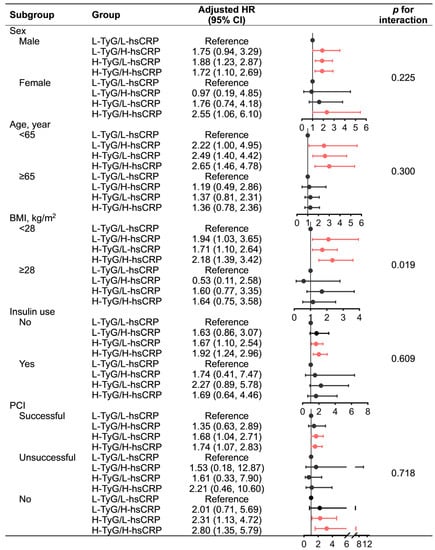
Subgroup analyses by sex and PCI status yielded consistent results with the primary analysis that the H-TyG/H-hsCRP group had the highest MACE risk, followed by the H-TyG/L-hsCRP group. However, it should be noted that the small sample size of the unsuccessful PCI subgroup (n = 129) may have limited the statistical power. Similar results were only observed in non-elderly, non-obese, and insulin-naïve patients, whereas none of the groups in their counterpart subpopulations were significantly associated with MACE risk. Nevertheless, no significant interactions were detected in the subgroup analysis for any subgroup variables, except for BMI (Figure 3).
Figure 3.
Subgroup analysis for the association of TyG and hsCRP with MACE. Red indicates statistical significance, while black indicates statistical non-significance. Adjusted for age (continuous), sex, BMI (continuous), smoking history, peripheral artery disease, chronic kidney disease, prior myocardial infarction, prior stroke, prior revascularization, low-density lipoprotein cholesterol ≤ 1.8 mmol/L, left ventricular ejection fraction < 40%, left main stem/three-vessel disease, synergy between percutaneous coronary intervention with Taxus and cardiac surgery score (categorical), percutaneous coronary intervention status, aspirin adherence, statin adherence. Abbreviations: BMI, body mass index; CI, confidence interval; H, high; HR, hazard ratio; hsCRP, high-sensitivity C-reactive protein; L, low; MACE, major adverse cardiac event; PCI, percutaneous coronary intervention; TyG, triglyceride–glucose index.
Sensitivity analyses supported the robustness of the main results (Supplementary Materials: Table S4). When patients were divided into nine groups, the MACE risk gradually increased across the low, moderate, and high TyG categories for each given hsCRP level. Likewise, the MACE risk also increased as hsCRP levels increased for each given TyG category. Patients in the H-TyG/H-hsCRP group experienced the highest MACE risk. Robust results in agreement with the primary analysis were observed after excluding 57 patients who did not complete the 2-year follow-up, 229 with very high hsCRP levels (>10 mg/L), or 197 with imputed values.
3.4. Mediation Analysis
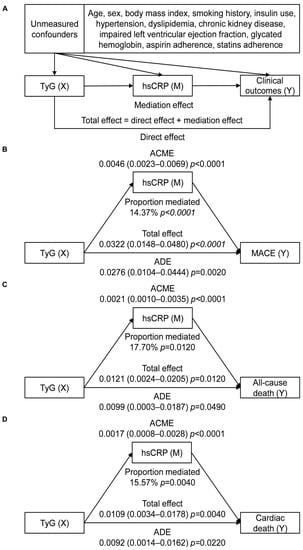
According to the prerequisites for conducting a mediation analysis, TyG should be correlated with hsCRP, both TyG and hsCRP should be associated with cardiovascular events, and the association between TyG and cardiovascular events should be attenuated when adjusting for hsCRP. Figure S4 shows a weak but significant correlation between TyG and hsCRP (Spearman correlation r = 0.196, p < 0.001). When analyzed as continuous variables, the risk of any revascularization increased with elevated TyG rather than hsCRP levels, whereas the risk of myocardial infarction increased with elevated hsCRP but not TyG levels. Both TyG and hsCRP levels were associated with the risks of MACE, all-cause death, and cardiac death regardless of adjustment, with the estimates of HRs of TyG being reduced when additionally adjusted for hsCRP (Supplementary Materials: Table S3). Therefore, mediation analysis was restricted to MACE, all-cause death, and cardiac death. The analysis demonstrated a significant partial mediating effect of systemic inflammation on the association of insulin resistance with MACE, all-cause death, and cardiac death after adjustment for measured confounding. Specifically, hsCRP was found to mediate 14.37% of the association between TyG and MACE, 17.70% of the association between TyG and all-cause death, and 15.57% of the association between TyG and cardiac death. TyG remained directly associated with MACE, all-cause death, and cardiac death (Figure 4).
Figure 4.
Mediation effect of hsCRP on the association between TyG and cardiovascular events. (A) Directed acyclic graph; (B) mediation analysis for MACE; (C) mediation analysis for all-cause death; (D) mediation analysis for cardiac death. Abbreviations: ACME, average causal mediation effect; ADE, average direct effect; hsCRP, high-sensitivity C-reactive protein; MACE, major adverse cardiac event; TyG, triglyceride–glucose index.
4. Discussion
This study showed that the combination of TyG-measured insulin resistance and hsCRP-measured systemic inflammation significantly increased the 2-year risk of multiple cardiovascular events in diabetic CCS patients, including MACE, all-cause death, cardiac death, myocardial infarction, and any revascularization. HsCRP partially mediated the impact of TyG on MACE, all-cause death, and cardiac death.
Insulin resistance causes hyperglycemia and hypertriglyceridemia, enhances oxidative stress and vascular inflammation, induces endothelial dysfunction characterized by a pro-inflammatory, pro-thrombotic, vasoconstrictive phenotype, and promotes smooth muscle cell proliferation and collagen deposition, which lead to atherosclerosis and cardiovascular events [8]. Tao et al. demonstrated the predictive value of TyG for cardiovascular events [8]. This study confirmed a significant association between TyG-assessed insulin resistance and MACE in diabetic patients with CCS. Likewise, this study also revealed that hsCRP-assessed inflammation was an independent cardiovascular risk factor for diabetic patients with CCS. Both insulin resistance and systemic inflammation are metabolic characteristics of diabetes and independent risk factors for cardiovascular events. However, few epidemiological studies have investigated the causal pathways through which these factors contribute to cardiovascular events.
This study found that high TyG and hsCRP levels identified individuals at the highest cardiovascular risk, with significantly increased risks of MACE and secondary outcome events compared with those with lower levels of both markers. These findings may be attributed to the combined effects of insulin resistance and inflammation in promoting atherosclerosis. Insulin resistance and inflammation contribute strongly to endothelial dysfunction [8,10,13], and hyperglycemia can also damage the endothelium, albeit to a lesser extent [22]. HsCRP appears to have the potential to damage the endothelium directly as well [16,17,23]. Then, increased triglyceride-rich lipoproteins pass through the compromised endothelial barrier [24]. In an insulin-resistant and hyperglycemic environment, oxidized or glycated lipoproteins trigger a heightened inflammatory response beneath the endothelium [22], ultimately exacerbating atherosclerosis and increasing plaque instability. Furthermore, insulin resistance and systemic inflammation mutually amplify each other in a vicious cycle [10,18]. Tissues may become more sensitive to pro-inflammatory stimuli in the presence of hyperinsulinemia [18].
Another finding of this study was that hsCRP partially mediated the impact of TyG on MACE, all-cause death, and cardiac death in diabetic patients with CCS. Similarly, a previous study revealed that hsCRP partially mediated the association between insulin resistance and poor clinical outcomes in non-diabetic patients with ischemic stroke [25]. These observations provide epidemiological evidence supporting the biologically plausible hypothesis that inflammation plays a role in the link between insulin resistance and cardiovascular events. Insulin resistance and hyperglycemia can activate the NLRP3 (NOD (nucleotide oligomerization domain)-, LRR (leucine-rich repeat)-, and PYD (pyrin domain)-containing protein 3) inflammasome, leading to the release of cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor-α. The NLRP3 pathway is a critical element in atherosclerotic pathogenesis, and hsCRP is a downstream marker of these cytokines. Therefore, elevated TyG levels can exacerbate atherosclerosis through an upregulated NLRP3 signaling pathway represented by hsCRP [8,10,22]. However, the link between TyG and cardiovascular events may involve other mediators, such as traditional cardiovascular risks (hypertension and dyslipidemia) and unidentified factors. In addition, hsCRP does not capture other pro-atherogenic inflammatory pathways, such as Toll-like receptors, proprotein convertase subtilisin/kexin type 9, Notch, and Wnt pathways [13]. Further studies are required to explore the mechanisms underlying the causal relationship between TyG and cardiovascular events.
Due to the lower likelihood of females being admitted to PCI-capable hospitals than males [26], the proportion of females in this study may be slightly lower than the actual representation in the CAD population. However, this does not impact the finding of a consistent combined effect of insulin resistance and inflammation on cardiovascular outcomes in both sexes. In elderly and insulin-using patients, the prognostic significance of TyG/hsCRP levels was not observed. However, no significant interaction between TyG/hsCRP levels and age or insulin use was detected, indicating that this observation may be attributed to the small sample sizes and low statistical power of these subpopulations. A meta-analysis has demonstrated that TyG stably predicts mortality in the elderly [27], and its predictive ability is not affected by insulin treatment status [28]. In addition, the observed interaction between BMI and TyG/hsCRP levels was likely due to chance and cannot be interpreted as evidence of the “obesity paradox”, as the median hsCRP value was lower in obese patients with hsCRP ≥ 2.00 mg/L than non-obese patients, despite a higher proportion of individuals with hsCRP ≥ 2.00 mg/L in the obese group than the non-obese group.
Clinical trials have demonstrated the potential of anti-inflammatory therapy for improving cardiovascular outcomes in the secondary prevention of CAD. However, there are still challenges in incorporating anti-inflammatory therapy into CAD management. One hurdle is the need for more precise risk stratification to determine patients who would benefit the most, thereby improving cost-effectiveness. This study indicates that combining TyG and hsCRP can help identify patients with extremely high cardiovascular risk. Anti-inflammatory therapy for these individuals may provide additional benefits beyond directly attenuating systemic inflammation, including reducing the synergistic and mediating effects of inflammation on the negative prognostic impact of insulin resistance.
The limitations of this study should be acknowledged. Firstly, while potential confounders were identified and adjusted, unknown or unmeasured confounding cannot be ruled out due to the observational nature of the study. For instance, we were unable to adjust for the types and dosages of statins and aspirin, both of which are known to have anti-inflammatory effects. Secondly, the study cohort primarily consisted of individuals of Han Chinese ethnicity. Caution should be exercised when generalizing the results to other racial and ethical populations. Thirdly, the results of this study may be affected by potential regression dilution bias and temporal changes in TyG and hsCRP levels, given that these biomarkers were only measured once at baseline. Baseline hsCRP values can be influenced by several non-pathological factors, such as age, sex, obesity, hormone replacement therapy, and smoking. Relying on a single measurement of hsCRP may lead to an inaccurate assessment of inflammation. Lastly, since TyG and hsCRP measurements were collected simultaneously, the logical time order between the exposure and the mediator cannot be assured.
5. Conclusions
In diabetic CCS patients, TyG and hsCRP synergically increased the 2-year risk of multiple cardiovascular events, including MACE, all-cause death, cardiac death, myocardial infarction, and any revascularization. HsCRP partially mediated the impact of TyG on MACE, all-cause death, and cardiac death. Combining insulin resistance and systemic inflammation can help identify high-risk patients, and controlling inflammation in patients with insulin resistance may bring added benefits. The study was limited by potential unmeasured confounding, limited generalizability to other ethnic populations, single-time measurement of biomarkers, and the inability to establish the temporal order between the exposure and the mediator, which should be considered when interpreting the findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15122808/s1, Table S1: Number of missing values and corresponding dispositions; Table S2: Univariable Cox proportional-hazards regression analysis for clinical outcomes; Table S3: Association of TyG or hsCRP with cardiovascular events; Table S4: Sensitivity analyses of the associations of TyG and hsCRP with MACE; Figure S1: Kaplan–Meier analysis of patients grouped by TyG levels; Figure S2: Kaplan–Meier analysis of patients grouped by TyG and hsCRP levels; Figure S3: Restricted cubic spline curves of TyG or hsCRP for cardiovascular events; Figure S4: Spearman correlation between TyG and hsCRP.
Author Contributions
Conceptualization, J.Y. and Y.H.; methodology; X.W., Z.L., Z.Z., Y.Z., Z.W., Y.F., Q.W. and X.G.; formal analysis, T.L. and P.W.; validation, X.T.; resources, J.Y. and Y.H.; data curation, X.T., J.X., Y.S., Y.C., N.X., Y.Y., R.L. and P.Z.; writing—original draft preparation, T.L. and P.W.; writing—review and editing, J.Y. and Y.H.; visualization, T.L. and P.W.; supervision, J.Y. and Y.H.; project administration, X.W., Z.L., Z.Z., Y.Z., Z.W., Y.F., Q.W. and X.G.; funding acquisition, J.Y. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the China National Key R&D Program during the 13th Five-year Plan Period, grant numbers 2016YFC1301300, 2016YFC1301301; the CAMS Innovation Fund for Medical Sciences, grant number 2020-I2 M-C&T-B-049; and National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences, grant number NCRC2020013.
Institutional Review Board Statement
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Fuwai Hospital (protocol code: 2013-449, date of approval: 2013-09-04; protocol code: No. 2017-860, date of approval: 2017-01-10). All participants provided written informed consent.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank all the study participants and their families for their cooperation and the staff of all centers for data collection, management, and monitoring.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Cavender, M.A.; Steg, P.G.; Smith, S.C., Jr.; Eagle, K.; Ohman, E.M.; Goto, S.; Kuder, J.; Im, K.; Wilson, P.W.; Bhatt, D.L.; et al. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 2015, 132, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Arnold, S.V.; Bhatt, D.L.; Barsness, G.W.; Beatty, A.L.; Deedwania, P.C.; Inzucchi, S.E.; Kosiborod, M.; Leiter, L.A.; Lipska, K.J.; Newman, J.D.; et al. Clinical Management of Stable Coronary Artery Disease in Patients with Type 2 Diabetes Mellitus: A Scientific Statement from the American Heart Association. Circulation 2020, 141, e779–e806. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef]
- Tao, L.C.; Xu, J.N.; Wang, T.T.; Hua, F.; Li, J.J. Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc. Diabetol. 2022, 21, 68. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuniga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Patel, T.P.; Rawal, K.; Bagchi, A.K.; Akolkar, G.; Bernardes, N.; Dias, D.D.S.; Gupta, S.; Singal, P.K. Insulin resistance: An additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail. Rev. 2016, 21, 11–23. [Google Scholar] [CrossRef]
- Hartge, M.M.; Unger, T.; Kintscher, U. The endothelium and vascular inflammation in diabetes. Diabetes Vasc. Dis. Res. 2007, 4, 84–88. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Lawler, P.R.; Bhatt, D.L.; Godoy, L.C.; Luscher, T.F.; Bonow, R.O.; Verma, S.; Ridker, P.M. Targeting cardiovascular inflammation: Next steps in clinical translation. Eur. Heart J. 2021, 42, 113–131. [Google Scholar] [CrossRef]
- Sharif, S.; Van der Graaf, Y.; Cramer, M.J.; Kapelle, L.J.; de Borst, G.J.; Visseren, F.L.J.; Westerink, J.; on behalf of the SMART study group. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 220. [Google Scholar] [CrossRef]
- Badimon, L.; Pena, E.; Arderiu, G.; Padro, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef]
- Denegri, A.; Boriani, G. High Sensitivity C-reactive Protein (hsCRP) and its Implications in Cardiovascular Outcomes. Curr. Pharm. Des. 2021, 27, 263–275. [Google Scholar] [CrossRef]
- Puschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, 11, 4358. [Google Scholar] [CrossRef]
- Simental-Mendia, L.E.; Rodriguez-Moran, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Everett, B.M. Residual Inflammatory Risk: A Common and Important Risk Factor for Recurrent Cardiovascular Events. J. Am. Coll. Cardiol. 2019, 73, 2410–2412. [Google Scholar] [CrossRef]
- Imai, K.; Keele, L.; Tingley, D. A general approach to causal mediation analysis. Psychol. Methods 2010, 15, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 2014, 10, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Bassuk, S.S.; Rifai, N.; Ridker, P.M. High-sensitivity C-reactive protein: Clinical importance. Curr. Probl. Cardiol. 2004, 29, 439–493. [Google Scholar] [PubMed]
- Boren, J.; Taskinen, M.R.; Bjornson, E.; Packard, C.J. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat. Rev. Cardiol. 2022, 19, 577–592. [Google Scholar] [CrossRef]
- Jin, A.; Wang, S.; Li, J.; Wang, M.; Lin, J.; Li, H.; Meng, X.; Wang, Y.; Pan, Y. Mediation of Systemic Inflammation on Insulin Resistance and Prognosis of Nondiabetic Patients with Ischemic Stroke. Stroke 2023, 54, 759–769. [Google Scholar] [CrossRef]
- Du, X.; Spatz, E.S.; Dreyer, R.P.; Hu, S.; Wu, C.; Li, X.; Li, J.; Wang, S.; Masoudi, F.A.; Spertus, J.A.; et al. Sex Differences in Clinical Profiles and Quality of Care Among Patients with ST-Segment Elevation Myocardial Infarction From 2001 to 2011: Insights From the China Patient-Centered Evaluative Assessment of Cardiac Events (PEACE)-Retrospective Study. J. Am. Heart Assoc. 2016, 5, e002157. [Google Scholar] [CrossRef]
- Luo, J.W.; Duan, W.H.; Yu, Y.Q.; Song, L.; Shi, D.Z. Prognostic Significance of Triglyceride-Glucose Index for Adverse Cardiovascular Events in Patients With Coronary Artery Disease: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 774781. [Google Scholar] [CrossRef]
- Placzkowska, S.; Pawlik-Sobecka, L.; Kokot, I.; Piwowar, A. Indirect insulin resistance detection: Current clinical trends and laboratory limitations. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2019, 163, 187–199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).