Glycocalyx–Sodium Interaction in Vascular Endothelium
Abstract
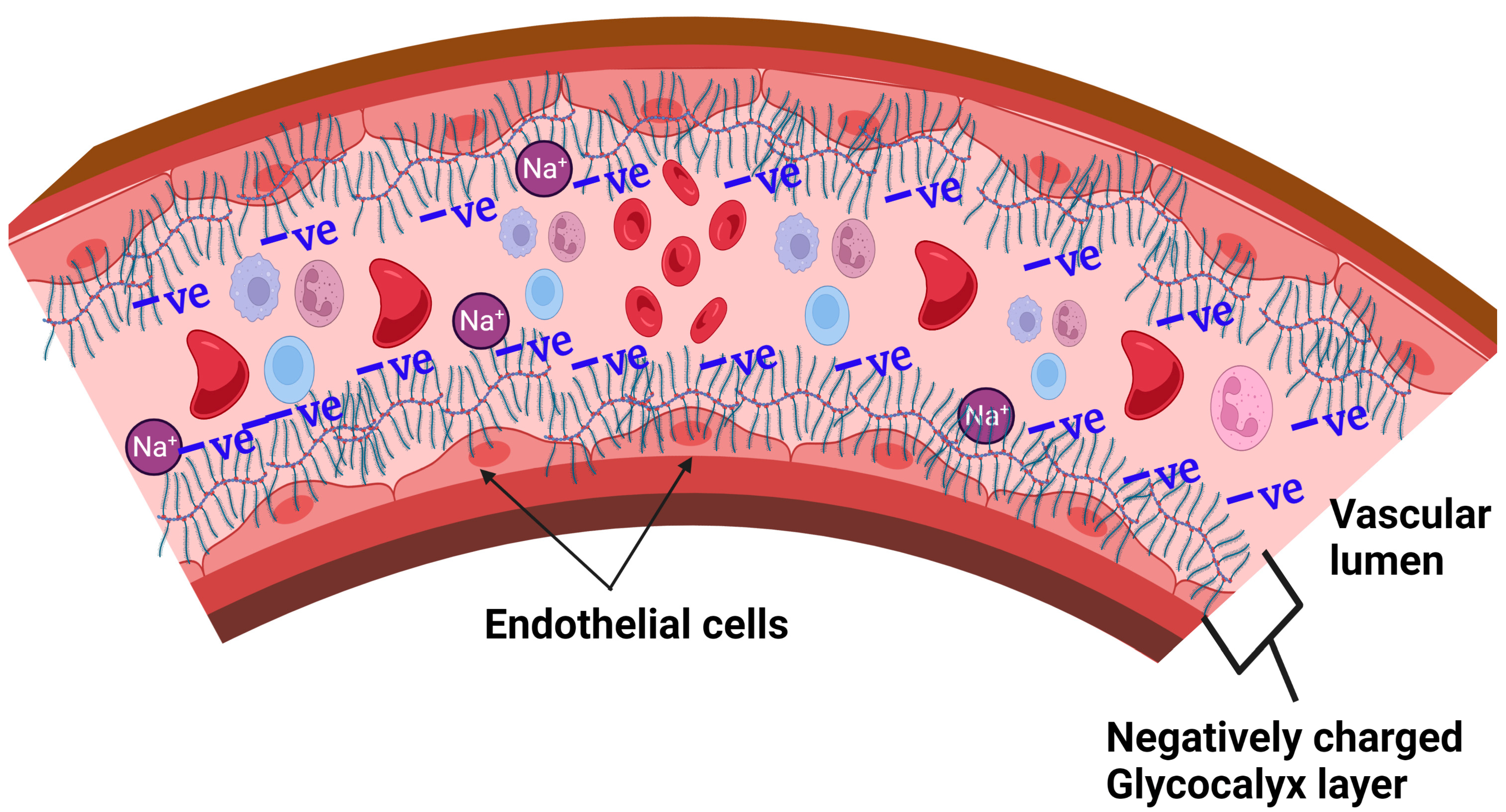
1. Background
2. Major Components of the Glycocalyx in CVD Pathophysiology
2.1. Heparan Sulfate Proteoglycans (Syndecans and Glypicans)
2.1.1. Syndecans
2.1.2. Glypicans
2.2. Hyaluronan
3. Glycocalyx and Salt Interactions
3.1. Proposed Glycocalyx-Salt Interaction Mechanisms Contributing to Hypertension and Cardiovascular Disease
3.2. Possible Strategies for Reducing the Damaging Effects of NaCl-Salt Overload on Vascular Endothelium
4. Current Diagnostic Tools to Determine Glycocalyx and Endothelial Health Status
Erythrocyte Salt Sensitivity Test
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandrycky, C.J.; Howard, C.C.; Rayner, S.G.; Shin, Y.J.; Zheng, Y. Organ-on-a-chip systems for vascular biology. J. Mol. Cell. Cardiol. 2021, 159, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.J. Examining Vascular Structure and Function Using Confocal Microscopy and 3D Imaging Techniques. Adv. Exp. Med. Biol. 2019, 1120, 97–106. [Google Scholar]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Clough, G. Relationship between microvascular permeability and ultrastructure. Prog. Biophys. Mol. Biol. 1991, 55, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Stan, R.V.; Tse, D.; Deharvengt, S.J.; Smits, N.C.; Xu, Y.; Luciano, M.R.; McGarry, C.L.; Buitendijk, M.; Nemani, K.V.; Elgueta, R.; et al. The diaphragms of fenestrated endothelia: Gatekeepers of vascular permeability and blood composition. Dev. Cell 2012, 23, 1203–1218. [Google Scholar] [CrossRef]
- Dull, R.O.; Hahn, R.G. The glycocalyx as a permeability barrier: Basic science and clinical evidence. Crit. Care 2022, 26, 273. [Google Scholar] [CrossRef]
- Jin, J.; Fang, F.; Gao, W.; Chen, H.; Wen, J.; Wen, X.; Chen, J. The Structure and Function of the Glycocalyx and Its Connection with Blood-Brain Barrier. Front. Cell. Neurosci. 2021, 15, 739699. [Google Scholar] [CrossRef]
- Haymet, A.B.; Bartnikowski, N.; Wood, E.S.; Vallely, M.P.; McBride, A.; Yacoub, S.; Biering, S.B.; Harris, E.; Suen, J.Y.; Fraser, J.F. Studying the Endothelial Glycocalyx in vitro: What Is Missing? Front. Cardiovasc. Med. 2021, 8, 647086. [Google Scholar] [CrossRef]
- Wang, G.; Tiemeier, G.L.; van den Berg, B.M.; Rabelink, T.J. Endothelial Glycocalyx Hyaluronan: Regulation and Role in Prevention of Diabetic Complications. Am. J. Pathol. 2020, 190, 781–790. [Google Scholar] [CrossRef]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef]
- Melrose, J. Perlecan, a modular instructive proteoglycan with diverse functional properties. Int. J. Biochem. Cell Biol. 2020, 128, 105849. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H. Vascular endothelium: A vulnerable transit zone for mercilesssodium. Nephrol. Dial. Transplant. 2014, 29, 240–246. [Google Scholar] [CrossRef]
- Bkaily, G.; Jacques, D. Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 1998. [Google Scholar] [CrossRef]
- Knežević, D.; Ćurko-Cofek, B.; Batinac, T.; Laškarin, G.; Rakić, M.; Šoštarič, M.; Zdravković, M.; Šustić, A.; Sotošek, V.; Batičić, L. Endothelial Dysfunction in Patients Undergoing Cardiac Surgery: A Narrative Review and Clinical Implications. J. Cardiovasc. Dev. Dis. 2023, 10, 213. [Google Scholar] [CrossRef]
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial Glycocalyx. Compr. Physiol. 2022, 12, 3781–3811. [Google Scholar] [PubMed]
- Mortazavi, C.M.; Hoyt, J.M.; Patel, A.; Chignalia, A.Z. The glycocalyx and calcium dynamics in endothelial cells. Curr. Top. Membr. 2023, 91, 21–41. [Google Scholar]
- Askari, H.; Sadeghinejad, M.; Fancher, I.S. Mechanotransduction and the endothelial glycocalyx: Interactions with membrane and cytoskeletal proteins to transduce force. Curr. Top. Membr. 2023, 91, 43–60. [Google Scholar]
- Pot, C.; Chen, A.Y.; Ha, J.N.; Schmid-Schönbein, G.W. Proteolytic Cleavage of the Red Blood Cell Glycocalyx in a Genetic Form of Hypertension. Cell. Mol. Bioeng. 2011, 4, 678–692. [Google Scholar] [CrossRef]
- Salmon, A.H.; Ferguson, J.K.; Burford, J.L.; Gevorgyan, H.; Nakano, D.; Harper, S.J.; Harper, D.O. Bates, and J. Peti-Peterdi, Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J. Am. Soc. Nephrol. 2012, 23, 1339–1350. [Google Scholar] [CrossRef]
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef]
- Esko, J.D.; Rostand, K.S.; Weinke, J.L. Tumor formation dependent on proteoglycan biosynthesis. Science 1988, 241, 1092–1096. [Google Scholar] [CrossRef]
- Llaneza, A.; Vizoso, F.; Rodriguez, J.C.; Raigoso, P.; Garcia-Muniz, J.L.; Allende, M.T.; Garcia-Moran, M. Hyaluronic acid as prognostic marker in resectable colorectal cancer. Br. J. Surg. 2000, 87, 1690–1696. [Google Scholar] [CrossRef]
- Adamia, S.; Maxwell, C.A.; Pilarski, L.M. Hyaluronan and hyaluronan synthases: Potential therapeutic targets in cancer. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 3–14. [Google Scholar] [CrossRef]
- Dogné, S.; Flamion, B. Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am. J. Pathol. 2020, 190, 768–780. [Google Scholar] [CrossRef]
- Csóka, A.B.; Scherer, S.W.; Stern, R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics 1999, 60, 356–361. [Google Scholar] [CrossRef]
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015, 80, 389–402. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Robich, M.; Ryzhov, S.; Kacer, D.; Palmeri, M.; Peterson, S.M.; Quinn, R.D.; Carter, D.; Sheppard, F.; Hayes, T.; Sawyer, D.B.; et al. Prolonged Cardiopulmonary Bypass is Associated with Endothelial Glycocalyx Degradation. J. Surg. Res. 2020, 251, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, D.; Richter, R.P.; Anand, T.; Cardenas, J.C.; Richter, J.R. Alterations in heparan sulfate proteoglycan synthesis and sulfation and the impact on vascular endothelial function. Matrix Biol. Plus 2022, 16, 100121. [Google Scholar] [CrossRef] [PubMed]
- Gondelaud, F.; Ricard-Blum, S. Structures and interactions of syndecans. FEBS J. 2019, 286, 2994–3007. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R.; Gopal, S.; Lim, H.C.; Nørgaard, S.; Multhaupt, H.A. Fell-Muir Lecture: Syndecans: From peripheral coreceptors to mainstream regulators of cell behaviour. Int. J. Exp. Pathol. 2015, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deepa, S.S.; Yamada, S.; Zako, M.; Goldberger, O.; Sugahara, K. Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J. Biol. Chem. 2004, 279, 37368–37376. [Google Scholar]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Bollmann, M. Soluble syndecans: Biomarkers for diseases and therapeutic options. Br. J. Pharmacol. 2019, 176, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Pruessmeyer, J.; Martin, C.; Hess, F.M.; Schwarz, N.; Schmidt, S.; Kogel, T.; Hoettecke, N.; Schmidt, B.; Sechi, A.; Uhlig, S.; et al. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J. Biol. Chem. 2010, 285, 555–564. [Google Scholar] [CrossRef]
- Itoh, Y. Modulation of Microenvironment Signals by Proteolytic Shedding of Cell Surface Extracellular Matrix Receptors. Front. Cell Dev. Biol. 2021, 9, 736735. [Google Scholar] [CrossRef]
- Gopal, S.; Arokiasamy, S.; Pataki, C.; Whiteford, J.R.; Couchman, J.R. Syndecan receptors: Pericellular regulators in development and inflammatory disease. Open Biol. 2021, 11, 200377. [Google Scholar] [CrossRef]
- Ha, E.; Kim, M.J.; Choi, B.K.; Rho, J.J.; Oh, D.J.; Rho, T.H.; Kim, K.H.; Lee, H.J.; Shin, D.H.; Yim, S.V.; et al. Positive association of obesity with single nucleotide polymorphisms of syndecan 3 in the Korean population. J. Clin. Endocrinol. Metab. 2006, 91, 5095–5099. [Google Scholar] [CrossRef]
- Chang, B.C.; Hwang, L.C.; Huang, W.H. Positive Association of Metabolic Syndrome with a Single Nucleotide Polymorphism of Syndecan-3 (rs2282440) in the Taiwanese Population. Int. J. Endocrinol. 2018, 2018, 9282598. [Google Scholar] [CrossRef]
- Huang, W.H.; Hwang, L.C.; Chan, H.L.; Lin, H.Y.; Lin, Y.H. Study of seven single-nucleotide polymorphisms identified in East Asians for association with obesity in a Taiwanese population. BMJ Open 2016, 6, e011713. [Google Scholar] [CrossRef] [PubMed]
- Kunnas, T.; Nikkari, S.T. Contribution of syndecan-4 genetic variants to hypertension, the TAMRISK study. BMC Res. Notes 2014, 7, 815. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Banuth, A.M.M.; Nader, H.B.; Dreyfuss, J.L. Altered shear stress on endothelial cells leads to remodeling of extracellular matrix and induction of angiogenesis. PLoS ONE 2020, 15, e0241040. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.G.; Herum, K.M.; Carlson, C.C.; Christensen, G. Syndecans in heart fibrosis. Cell Tissue Res. 2016, 365, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.G.; Aronsen, J.M.; Melleby, A.O.; Strand, M.E.; Skogestad, J.; Bendiksen, B.A.; Ahmed, M.S.; Sjaastad, I.; Attramadal, H.; Carlson, C.R.; et al. Cardiomyocyte-specific overexpression of syndecan-4 in mice results in activation of calcineurin-NFAT signalling and exacerbated cardiac hypertrophy. Mol. Biol. Rep. 2022, 49, 11795–11809. [Google Scholar] [CrossRef]
- Milusev, A.; Rieben, R.; Sorvillo, N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 897087. [Google Scholar] [CrossRef]
- Abassi, Z.; Armaly, Z.; Heyman, S.N. Glycocalyx Degradation in Ischemia-Reperfusion Injury. Am. J. Pathol. 2020, 190, 752–767. [Google Scholar] [CrossRef]
- Kim, Y.H.; Nijst, P.; Kiefer, K.; Tang, W.H. Endothelial Glycocalyx as Biomarker for Cardiovascular Diseases: Mechanistic and Clinical Implications. Curr. Heart Fail Rep. 2017, 14, 117–126. [Google Scholar] [CrossRef]
- Ajaero, C.N.; Procter, N.E.K.; Chirkov, Y.Y.; Heresztyn, T.; Arstall, M.A.; McGavigan, A.D.; Frenneaux, M.P.; Horowitz, J.D. Endothelial dysfunction and glycocalyx shedding in heart failure: Insights from patients receiving cardiac resynchronisation therapy. Heart Vessel. 2020, 35, 197–206. [Google Scholar] [CrossRef]
- Filmus, J.; Capurro, M.; Rast, J. Glypicans. Genome Biol. 2008, 9, 224. [Google Scholar] [CrossRef]
- Filmus, J. The function of glypicans in the mammalian embryo. Am. J. Physiol. Cell Physiol. 2022, 322, C694–C698. [Google Scholar] [CrossRef] [PubMed]
- Filmus, J.; Capurro, M. The role of glypicans in Hedgehog signaling. Matrix Biol. 2014, 35, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Meyer, K.; Nandini, C.D.; Qiao, D.; Salamat, S.; Friedl, A. Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am. J. Pathol. 2006, 168, 2014–2026. [Google Scholar] [CrossRef] [PubMed]
- Traister, A.; Shi, W.; Filmus, J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem. J. 2008, 410, 503–511. [Google Scholar] [CrossRef]
- Zeng, Y. Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J. Cell. Mol. Med. 2017, 21, 1457–1462. [Google Scholar] [CrossRef]
- Bartosch, A.M.W.; Mathews, R.; Mahmoud, M.M.; Cancel, L.M.; Haq, Z.S.; Tarbell, J.M. Heparan sulfate proteoglycan glypican-1 and PECAM-1 cooperate in shear-induced endothelial nitric oxide production. Sci. Rep. 2021, 11, 11386. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef]
- Rilla, K.; Oikari, S.; Jokela, T.A.; Hyttinen, J.M.; Kärnä, R.; Tammi, R.H.; Tammi, M.I. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J. Biol. Chem. 2013, 288, 5973–5983. [Google Scholar] [CrossRef]
- Harada, H.; Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607. [Google Scholar] [CrossRef]
- Kaul, A.; Short, W.D.; Wang, X.; Keswani, S.G. Hyaluronidases in Human Diseases. Int. J. Mol. Sci. 2021, 22, 3204. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.K.; Cepinskas, G.; Fraser, D.D. Endothelial Glycocalyx Degradation in Critical Illness and Injury. Front. Med. 2022, 9, 898592. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, U.; Eichenberg, T.; Parthaune, T.; Haiduk, C.; Saalbach, A.; Milkova, L.; Ludwig, A.; Grosche, J.; Averbeck, M.; Gebhardt, C.; et al. ADAM10 is the constitutive functional sheddase of CD44 in human melanoma cells. J. Investig. Dermatol. 2009, 129, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Kawano, Y.; Tsuiki, H.; Sasaki, J.I.; Nakao, M.; Matsumoto, M.; Suga, M.; Ando, M.; Nakajima, M.; Saya, H. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene 1999, 18, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Pardue, E.L.; Ibrahim, S.; Ramamurthi, A. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 2008, 4, 203–214. [Google Scholar] [CrossRef]
- Ding, H.Y.; Xie, Y.N.; Dong, Q.; Kimata, K.; Nishida, Y.; Ishiguro, N.; Zhuo, L.S. Roles of hyaluronan in cardiovascular and nervous system disorders. J. Zhejiang Univ. Sci. B 2019, 20, 428–436. [Google Scholar] [CrossRef]
- West, D.C.; Kumar, S. Hyaluronan and angiogenesis. Ciba Found. Symp. 1989, 143, 187–201, discussion 81–85. [Google Scholar]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and Reactive Oxygen Species Signaling-Novel Cues from the Matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef]
- Day, A.J.; de la Motte, C.A. Hyaluronan cross-linking: A protective mechanism in inflammation? Trends Immunol. 2005, 26, 637–643. [Google Scholar] [CrossRef]
- Band, P.A.; Heeter, J.; Wisniewski, H.G.; Liublinska, V.; Pattanayak, C.W.; Karia, R.J.; Stabler, T.; Balazs, E.A.; Kraus, V.B. Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthr. Cartil. 2015, 23, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Heldin, P.; Kolliopoulos, C.; Lin, C.Y.; Heldin, C.H. Involvement of hyaluronan and CD44 in cancer and viral infections. Cell. Signal. 2020, 65, 109427. [Google Scholar] [CrossRef] [PubMed]
- Karousou, E.; Misra, S.; Ghatak, S.; Dobra, K.; Götte, M.; Vigetti, D.; Passi, A.; Karamanos, N.K.; Skandalis, S.S. Roles and targeting of the HAS/hyaluronan/CD44 molecular system in cancer. Matrix Biol. 2017, 59, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Kalay, N.; Elcik, D.; Savaş, G.; Altın, P.; Şakalar, Ç.; Kaya, Ö.; Aytekin, M. Elevated hyaluronan levels in patients with rheumatic mitral stenosis and pulmonary arterial thromboembolism. Heart Lung Circ. 2014, 23, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Unterleuthner, D.; Neuhold, P.; Schwarz, K.; Janker, L.; Neuditschko, B.; Nivarthi, H.; Crncec, I.; Kramer, N.; Unger, C.; Hengstschläger, M.; et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 2020, 23, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Perrotta, P.; Emini Veseli, B.; Van der Veken, B.; Roth, L.; Martinet, W.; De Meyer, G.R.Y. Pharmacological strategies to inhibit intra-plaque angiogenesis in atherosclerosis. Vasc. Pharmacol. 2019, 112, 72–78. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 8), C7–C12. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Block, L.H.; Mirtsou-Fidani, V.; Argiriadis, P.; Karakiulakis, G. The differential distribution of hyaluronic acid in the layers of human atheromatic aortas is associated with vascular smooth muscle cell proliferation and migration. Atherosclerosis 1998, 138, 79–89. [Google Scholar] [CrossRef]
- Krolikoski, M.; Monslow, J.; Puré, E. The CD44-HA axis and inflammation in atherosclerosis: A temporal perspective. Matrix Biol. 2019, 78–79, 201–218. [Google Scholar] [CrossRef]
- Chai, S.; Chai, Q.; Danielsen, C.C.; Hjorth, P.; Nyengaard, J.R.; Ledet, T.; Yamaguchi, Y.; Rasmussen, L.M.; Wogensen, L. Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ. Res. 2005, 96, 583–591. [Google Scholar] [CrossRef]
- Homann, S.; Grandoch, M.; Kiene, L.S.; Podsvyadek, Y.; Feldmann, K.; Rabausch, B.; Nagy, N.; Lehr, S.; Kretschmer, I.; Oberhuber, A.; et al. Hyaluronan synthase 3 promotes plaque inflammation and atheroprogression. Matrix Biol. 2018, 66, 67–80. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; Parker, R.I. Platelet Activation and Endothelial Cell Dysfunction. Crit. Care Clin. 2020, 36, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Le Master, E.; Granados, S.T.; Levitan, I. Impairment of endothelial glycocalyx in atherosclerosis and obesity. Curr. Top. Membr. 2023, 91, 1–19. [Google Scholar] [PubMed]
- Weinbaum, S.; Cancel, L.M.; Fu, B.M.; Tarbell, J.M. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc. Eng. Technol. 2021, 12, 37–71. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, G. Serum Sodium. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Oxford, UK, 1990. [Google Scholar]
- Hyndman, K.A.; Mironova, E.V.; Giani, J.F.; Dugas, C.; Collins, J.; McDonough, A.A.; Stockand, J.D.; Pollock, J.S. Collecting Duct Nitric Oxide Synthase 1ß Activation Maintains Sodium Homeostasis During High Sodium Intake Through Suppression of Aldosterone and Renal Angiotensin II Pathways. J. Am. Heart Assoc. 2017, 6, e006896. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H.; Wilhelmi, M. Vascular glycocalyx sodium store—Determinant of salt sensitivity? Blood Purif. 2015, 39, 7–10. [Google Scholar] [CrossRef]
- Korte, S.; Wiesinger, A.; Straeter, A.S.; Peters, W.; Oberleithner, H.; Kusche-Vihrog, K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflug. Arch. 2012, 463, 269–278. [Google Scholar] [CrossRef]
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.H.W.; Mullens, W. The pathophysiological role of interstitial sodium in heart failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef]
- Titze, J.; Machnik, A. Sodium sensing in the interstitium and relationship to hypertension. Curr. Opin. Nephrol. Hypertens. 2010, 19, 385–392. [Google Scholar] [CrossRef]
- Kusche-Vihrog, K.; Oberleithner, H. An emerging concept of vascular salt sensitivity. F1000 Biol. Rep. 2012, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.T.; Edwards, D.G.; Farquhar, W.B. The Influence of Dietary Salt Beyond Blood Pressure. Curr. Hypertens. Rep. 2019, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, W.B.; Edwards, D.G.; Jurkovitz, C.T.; Weintraub, W.S. Dietary sodium and health: More than just blood pressure. J. Am. Coll. Cardiol. 2015, 65, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H.; Peters, W.; Kusche-Vihrog, K.; Korte, S.; Schillers, H.; Kliche, K.; Oberleithner, K. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflug. Arch. 2011, 462, 519–528. [Google Scholar] [CrossRef]
- Sulyok, E.; Farkas, B.; Nagy, B.; Várnagy, Á.; Kovács, K.; Bódis, J. Tissue Sodium Accumulation: Pathophysiology and Clinical Implications. Antioxidants 2022, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Alu, A.; Wei, Y.; Wei, X.; Luo, M. The modulatory effect of high salt on immune cells and related diseases. Cell Prolif. 2022, 55, e13250. [Google Scholar] [CrossRef]
- Kirabo, A. A new paradigm of sodium regulation in inflammation and hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R706–R710. [Google Scholar] [CrossRef]
- Oberleithner, H.; Wälte, M.; Kusche-Vihrog, K. Sodium renders endothelial cells sticky for red blood cells. Front. Physiol. 2015, 6, 188. [Google Scholar] [CrossRef]
- Mitra, R.; O’Neil, G.L.; Harding, I.C.; Cheng, M.J.; Mensah, S.A.; Ebong, E.E. Glycocalyx in Atherosclerosis-Relevant Endothelium Function and as a Therapeutic Target. Curr. Atheroscler. Rep. 2017, 19, 63. [Google Scholar] [CrossRef]
- Mutchler, S.M.; Kleyman, T.R. New insights regarding epithelial Na+ channel regulation and its role in the kidney, immune system and vasculature. Curr. Opin. Nephrol. Hypertens. 2019, 28, 113–119. [Google Scholar] [CrossRef]
- Ertuglu, L.A.; Kirabo, A. Dendritic Cell Epithelial Sodium Channel in Inflammation, Salt-Sensitive Hypertension, and Kidney Damage. Kidney360 2022, 3, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, N.R.; Foss, J.D.; Kryshtal, D.O.; Tsyba, N.; Kumaresan, S.; Xiao, L.; Mernaugh, R.L.; Itani, H.A.; Loperena, R.; Chen, W.; et al. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017, 21, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Pitzer, A.; Elijovich, F.; Laffer, C.L.; Ertuglu, L.A.; Sahinoz, M.; Saleem, M.; Krishnan, J.; Dola, T.; Aden, L.A.; Sheng, Q.; et al. DC ENaC-Dependent Inflammasome Activation Contributes to Salt-Sensitive Hypertension. Circ. Res. 2022, 131, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Datla, S.R.; Griendling, K.K. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 2010, 56, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Patik, J.C.; Lennon, S.L.; Farquhar, W.B.; Edwards, D.G. Mechanisms of Dietary Sodium-Induced Impairments in Endothelial Function and Potential Countermeasures. Nutrients 2021, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri Barbaro, N.; Van Beusecum, J.; Xiao, L.; do Carmo, L.; Pitzer, A.; Loperena, R.; Foss, J.D.; Elijovich, F.; Laffer, C.L.; Montaniel, K.R.; et al. Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc. Res. 2021, 117, 1358–1371. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Ertuglu, L.A.; Mutchler, A.P.; Yu, J.; Kirabo, A. Inflammation and oxidative stress in salt sensitive hypertension; The role of the NLRP3 inflammasome. Front. Physiol. 2022, 13, 1096296. [Google Scholar] [CrossRef]
- Okamoto, T.; Suzuki, K. The Role of Gap Junction-Mediated Endothelial Cell-Cell Interaction in the Crosstalk between Inflammation and Blood Coagulation. Int. J. Mol. Sci. 2017, 18, 2254. [Google Scholar] [CrossRef]
- Chadjichristos, C.E.; Scheckenbach, K.E.L.; Van Veen, T.; Richani Sarieddine, M.Z.; De Wit, C.; Yang, Z.; Roth, I.; Bacchetta, M.; Viswambharan, H.; Foglia, B.; et al. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation 2010, 121, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 80, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Patrick, D.M.; Aden, L.A.; Kirabo, A. Mechanisms of isolevuglandin-protein adduct formation in inflammation and hypertension. Prostaglandins Other Lipid Mediat. 2018, 139, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.B.; Davies, S.S.; Kirabo, A. Dendritic cells and isolevuglandins in immunity, inflammation, and hypertension. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H368–H374. [Google Scholar] [CrossRef]
- Paudel, P.; van Hout, I.; Bunton, R.W.; Parry, D.J.; Coffey, S.; McDonald, F.J.; Fronius, M. Epithelial Sodium Channel δ Subunit Is Expressed in Human Arteries and Has Potential Association with Hypertension. Hypertension 2022, 79, 1385–1394. [Google Scholar] [CrossRef]
- Yen, W.; Cai, B.; Yang, J.; Zhang, L.; Zeng, M.; Tarbell, J.M.; Fu, B.M. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS ONE 2015, 10, e0117133. [Google Scholar] [CrossRef]
- Florian, J.A.; Kosky, J.R.; Ainslie, K.; Pang, Z.; Dull, R.O.; Tarbell, J.M. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 2003, 93, e136–e142. [Google Scholar] [CrossRef]
- Bartosch, A.M.W.; Mathews, R.; Tarbell, J.M. Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling. Biophys. J. 2017, 113, 101–108. [Google Scholar] [CrossRef]
- Schierke, F.; Wyrwoll, M.J.; Wisdorf, M.; Niedzielski, L.; Maase, M.; Ruck, T.; Meuth, S.G.; Kusche-Vihrog, K. Nanomechanics of the endothelial glycocalyx contribute to Na(+)-induced vascular inflammation. Sci. Rep. 2017, 7, 46476. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Garcia-Valencia, O.; Milic, N.M.; Codsi, E.; Cubro, H.; Nath, M.C.; White, W.M.; Nath, K.A.; Garovic, V.D. Early Onset Preeclampsia Is Associated with Glycocalyx Degradation and Reduced Microvascular Perfusion. J. Am. Heart Assoc. 2019, 8, e010647. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Nuckols, V.R.; Pierce, G.L. Maternal microvascular dysfunction during preeclamptic pregnancy. Clin. Sci. 2021, 135, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, M.; Pickard, J.; Unnikrishnan, S.; Acton, S.T.; Ley, K. Analysis of leukocyte rolling in vivo and in vitro. Methods Enzymol. 2006, 416, 346–371. [Google Scholar]
- Machin, D.R.; Bloom, S.I.; Campbell, R.A.; Phuong, T.T.T.; Gates, P.E.; Lesniewski, L.A.; Rondina, M.T.; Donato, A.J. Advanced age results in a diminished endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H531–H539. [Google Scholar] [CrossRef]
- Bhat, S.; Marklund, M.; Henry, M.E.; Appel, L.J.; Croft, K.D.; Neal, B.; Wu, J.H.Y. A Systematic Review of the Sources of Dietary Salt Around the World. Adv. Nutr. 2020, 11, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Liang, L.; Pu, D.; Zhang, Y. Analysis of Sodium Content in 4082 Kinds of Commercial Foods in China. Nutrients 2022, 14, 2908. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.E.; Jones, A.; Okoro, C.E.; Alfa, V.O.; Okoli, R.; Shedul, G.L.; Orji, I.A.; Osagie, S.; Chopra, A.; Van Horn, L.V.; et al. Sodium Content and Labelling of Packaged Foods and Beverages in Nigeria: A Cross-Sectional Study. Nutrients 2022, 15, 27. [Google Scholar] [CrossRef]
- Hunter, S.D.; Kavouras, S.A.; Rahimi, M. Exploring heated exercise as a means of preventing the deleterious effects of high-sodium intake in Black women. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H833–H839. [Google Scholar] [CrossRef]
- Wenner, M.M.; Welti, L.M.; Dow, C.A.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Aerobic exercise training reduces ET-1-mediated vasoconstriction and improves endothelium-dependent vasodilation in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H732–H738. [Google Scholar] [CrossRef]
- Dow, C.A.; Stauffer, B.L.; Brunjes, D.L.; Greiner, J.J.; DeSouza, C.A. Regular aerobic exercise reduces endothelin-1-mediated vasoconstrictor tone in overweight and obese adults. Exp. Physiol. 2017, 102, 1133–1142. [Google Scholar] [CrossRef]
- Walker, J.C.; Dando, R. Sodium Replacement with KCl and MSG: Attitudes, Perception and Acceptance in Reduced Salt Soups. Foods 2023, 12, 2063. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Lee, S.M.; Kim, K.O. Use of Consumer Acceptability as a Tool to Determine the Level of Sodium Reduction: A Case Study on Beef Soup Substituted with Potassium Chloride and Soy-Sauce Odor. J. Food Sci. 2015, 80, S2570–S2577. [Google Scholar] [CrossRef] [PubMed]
- Levings, J.L.; Gunn, J.P. The imbalance of sodium and potassium intake: Implications for dietetic practice. J. Acad. Nutr. Diet. 2014, 114, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Moran, A.E.; Whelton, P.K. Whelton, Treatment of Hypertension: A Review. JAMA 2022, 328, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Song, H.; Zou, T.; Raza, A.; Li, P.; Li, K.; Xiong, J. Reduction of sodium chloride: A review. J. Sci. Food Agric. 2022, 102, 3931–3939. [Google Scholar] [CrossRef]
- Drummond, H.A.; Gebremedhin, D.; Harder, D.R. Degenerin/epithelial Na+ channel proteins: Components of a vascular mechanosensor. Hypertension 2004, 44, 643–648. [Google Scholar] [CrossRef]
- Pérez, F.R.; Venegas, F.; González, M.; Andrés, S.; Vallejos, C.; Riquelme, G.; Sierralta, J.; Michea, L. Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3-kinase/Akt in small-diameter mesenteric arteries. Hypertension 2009, 53, 1000–1007. [Google Scholar] [CrossRef]
- Guan, Z.; Pollock, J.S.; Cook, A.K.; Hobbs, J.L.; Inscho, E.W. Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension 2009, 54, 1062–1069. [Google Scholar] [CrossRef]
- Pitzer, A.L.; Van Beusecum, J.P.; Kleyman, T.R.; Kirabo, A. ENaC in Salt-Sensitive Hypertension: Kidney and Beyond. Curr. Hypertens. Rep. 2020, 22, 69. [Google Scholar] [CrossRef]
- Lemmens-Gruber, R.; Tzotzos, S. The Epithelial Sodium Channel—An Underestimated Drug Target. Int. J. Mol. Sci. 2023, 24, 7775. [Google Scholar] [CrossRef]
- Fedorov, D.A.; Sidorenko, S.V.; Yusipovich, A.I.; Bukach, O.V.; Gorbunov, A.M.; Lopina, O.D.; Klimanova, E.A. Increased Extracellular Sodium Concentration as a Factor Regulating Gene Expression in Endothelium. Biochemistry 2022, 87, 489–499. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Ma, X.; Zhang, D.; Li, D.; Feng, J.; Pan, X.; Lü, J.; Wang, X.; Liu, X. Berberine alleviates endothelial glycocalyx degradation and promotes glycocalyx restoration in LPS-induced ARDS. Int. Immunopharmacol. 2018, 65, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Mulivor, A.W.; Lipowsky, H.H. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation 2009, 16, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Adamson, R.H.; Curry, F.R.; Tarbell, J.M. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H363–H372. [Google Scholar] [CrossRef]
- Ramnath, R.D.; Butler, M.J.; Newman, G.; Desideri, S.; Russell, A.; Lay, A.C.; Neal, C.R.; Qiu, Y.; Fawaz, S.; Onions, K.L.; et al. Blocking matrix metalloproteinase-mediated syndecan-4 shedding restores the endothelial glycocalyx and glomerular filtration barrier function in early diabetic kidney disease. Kidney Int. 2020, 97, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liu, X.H.; Tarbell, J.; Fu, B. Sphingosine 1-phosphate induced synthesis of glycocalyx on endothelial cells. Exp. Cell Res. 2015, 339, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Lepedda, A.J.; Nieddu, G.; Piperigkou, Z.; Kyriakopoulou, K.; Karamanos, N.; Formato, M. Circulating Heparan Sulfate Proteoglycans as Biomarkers in Health and Disease. Semin. Thromb. Hemost. 2021, 47, 295–307. [Google Scholar] [CrossRef]
- Huang, X.; Hu, H.; Sun, T.; Zhu, W.; Tian, H.; Hao, D.; Wang, T.; Wang, X. Plasma Endothelial Glycocalyx Components as a Potential Biomarker for Predicting the Development of Disseminated Intravascular Coagulation in Patients with Sepsis. J. Intensive Care Med. 2021, 36, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef]
- Mony, V.K.; Benjamin, S.; O’Rourke, E.J. A lysosome-centered view of nutrient homeostasis. Autophagy 2016, 12, 619–631. [Google Scholar] [CrossRef]
- Baby, S.; Reljic, T.; Villalba, N.; Kumar, A.; Yuan, S.Y. Endothelial glycocalyx-associated molecules as potential serological markers for sepsis-associated encephalopathy: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0281941. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.G.; Patel, V.; Dull, R.O. Human glycocalyx shedding: Systematic review and critical appraisal. Acta Anaesthesiol. Scand. 2021, 65, 590–606. [Google Scholar] [CrossRef]
- Rehm, M.; Bruegger, D.; Christ, F.; Conzen, P.; Thiel, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Reichart, B.; et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007, 116, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Torres Filho, I.P.; Torres, L.N.; Salgado, C.; Dubick, M.A. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H1468–H1478. [Google Scholar] [CrossRef] [PubMed]
- Bol, M.E.; Huckriede, J.B.; van de Pas, K.G.H.; Delhaas, T.; Lorusso, R.; Nicolaes, G.A.F.; Sels, J.E.M.; van de Poll, M.C.G. Multimodal measurement of glycocalyx degradation during coronary artery bypass grafting. Front. Med. 2022, 9, 1045728. [Google Scholar] [CrossRef]
- Matyjaszczyk-Gwarda, K.; Kij, A.; Olkowicz, M.; Fels, B.; Kusche-Vihrog, K.; Walczak, M.; Chlopicki, S. Simultaneous quantification of selected glycosaminoglycans by butanolysis-based derivatization and LC-SRM/MS analysis for assessing glycocalyx disruption in vitro and in vivo. Talanta 2022, 238 Pt 1, 123008. [Google Scholar] [CrossRef]
- Yeo, T.W.; Weinberg, J.B.; Lampah, D.A.; Kenangalem, E.; Bush, P.; Chen, Y.; Price, R.N.; Young, S.; Zhang, H.Y.; Millington, D.; et al. Glycocalyx Breakdown Is Associated with Severe Disease and Fatal Outcome in Plasmodium falciparum Malaria. Clin. Infect. Dis. 2019, 69, 1712–1720. [Google Scholar] [CrossRef]
- Oberleithner, H. Quantifying salt sensitivity. Biol. Chem. 2021, 402, 1597–1602. [Google Scholar] [CrossRef]
- Oberleithner, H.; Wilhelmi, M. Salt Sensitivity Determined From Capillary Blood. Kidney Blood Press. Res 2016, 41, 355–364. [Google Scholar] [CrossRef]
- Masenga, S.K.; Pilic, L.; Malumani, M.; Hamooya, B.M. Erythrocyte sodium buffering capacity status correlates with self-reported salt intake in a population from Livingstone, Zambia. PLoS ONE 2022, 17, e0264650. [Google Scholar] [CrossRef]
- Vinaiphat, A.; Pazhanchamy, K.; JebaMercy, G.; Ngan, S.C.; Leow, M.K.S.; Ho, H.H.; Gao, Y.G.; Lim, K.L.; Richards, A.M.; de Kleijn, D.P.; et al. Endothelial Damage Arising From High Salt Hypertension Is Elucidated by Vascular Bed Systematic Profiling. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 427–442. [Google Scholar] [CrossRef] [PubMed]
- McNally, R.J.; Morselli, F.; Farukh, B.; Chowienczyk, P.J.; Faconti, L. A pilot study to evaluate the erythrocyte glycocalyx sensitivity to sodium as a marker for cellular salt sensitivity in hypertension. J. Hum. Hypertens. 2023, 37, 286–291. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sembajwe, L.F.; Ssekandi, A.M.; Namaganda, A.; Muwonge, H.; Kasolo, J.N.; Kalyesubula, R.; Nakimuli, A.; Naome, M.; Patel, K.P.; Masenga, S.K.; et al. Glycocalyx–Sodium Interaction in Vascular Endothelium. Nutrients 2023, 15, 2873. https://doi.org/10.3390/nu15132873
Sembajwe LF, Ssekandi AM, Namaganda A, Muwonge H, Kasolo JN, Kalyesubula R, Nakimuli A, Naome M, Patel KP, Masenga SK, et al. Glycocalyx–Sodium Interaction in Vascular Endothelium. Nutrients. 2023; 15(13):2873. https://doi.org/10.3390/nu15132873
Chicago/Turabian StyleSembajwe, Lawrence Fred, Abdul M. Ssekandi, Agnes Namaganda, Haruna Muwonge, Josephine N. Kasolo, Robert Kalyesubula, Annettee Nakimuli, Mwesigwa Naome, Kaushik P. Patel, Sepiso K. Masenga, and et al. 2023. "Glycocalyx–Sodium Interaction in Vascular Endothelium" Nutrients 15, no. 13: 2873. https://doi.org/10.3390/nu15132873
APA StyleSembajwe, L. F., Ssekandi, A. M., Namaganda, A., Muwonge, H., Kasolo, J. N., Kalyesubula, R., Nakimuli, A., Naome, M., Patel, K. P., Masenga, S. K., & Kirabo, A. (2023). Glycocalyx–Sodium Interaction in Vascular Endothelium. Nutrients, 15(13), 2873. https://doi.org/10.3390/nu15132873







