Central Regulation of Eating Behaviors in Humans: Evidence from Functional Neuroimaging Studies
Abstract
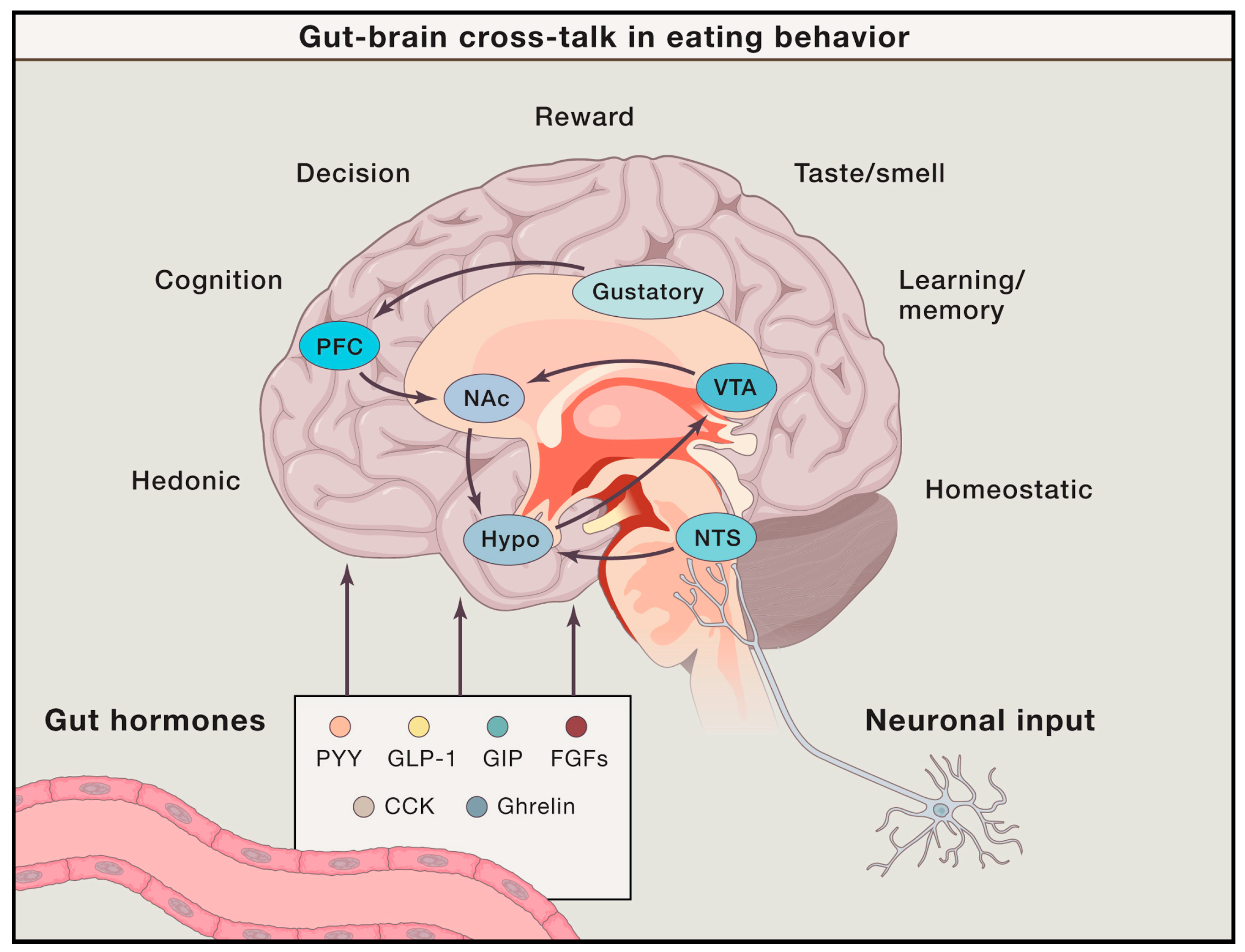
:1. Introduction
2. Eating Behavior and Brain Functions
2.1. Energy Homeostasis, Reward, and Food Craving
2.2. Appetite Control and Cognitive Inhibition of Eating Behavior
2.3. Functional Brain Activities Elicited by Real Food Consumption
2.4. Functional Brain Activities Elicited by Food Images and Food Imagery
2.5. Neuroimaging Studies in Eating Disorders, Obesity, and Disorders of Gut–Brain Interaction
2.5.1. Anorexia Nervosa (AN)
2.5.2. Bulimia Nervosa (BN)
2.5.3. Binge Eating Disorder (BED)
2.5.4. Obesity
2.5.5. Disorders of Gut–Brain Interaction
3. Future Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemmensen, C.; Müller, T.D.; Woods, S.C.; Berthoud, H.-R.; Seeley, R.J.; Tschöp, M.H. Gut-Brain Cross-Talk in Metabolic Control. Cell 2017, 168, 758–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthoud, H.-R. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol. Behav. 2007, 91, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wu, Y.; Liu, F.; Zhu, Y.; Jin, H.; Zhang, H.; Wan, Y.; Li, C.; Yu, D. An update on the prevalence of eating disorders in the general population: A systematic review and meta-analysis. Eat. Weight. Disord.-Stud. Anorex. Bulim. Obes. 2021, 27, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Brownell, K.D.; Walsh, B.T. Eating Disorders and Obesity: A Comprehensive Handbook; Guilford Publications: New York, NY, USA, 2017. [Google Scholar]
- Smeets, P.A.M.; Dagher, A.; Hare, T.A.; Kullmann, S.; van der Laan, L.N.; Poldrack, R.A.; Preissl, H.; Small, D.; Stice, E.; Veldhuizen, M.G. Good practice in food-related neuroimaging. Am. J. Clin. Nutr. 2019, 109, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.E.; Alonso-Alonso, M.; Audette, M.; Malbert, C.H.; Stice, E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin. 2015, 8, 1–31. [Google Scholar] [CrossRef]
- Ekstrand, C. Neuroimaging; Oxford Research Encyclopedia of Psychology (Oxford University Press): Oxford, UK, 2022. [Google Scholar]
- Chen, E.Y.; Eickhoff, S.B.; Giovannetti, T.; Smith, D.V. Obesity is associated with reduced orbitofrontal cortex volume: A coordinate-based meta-analysis. NeuroImage Clin. 2020, 28, 102420. [Google Scholar] [CrossRef]
- Titova, O.E.; Hjorth, O.C.; Schiöth, H.B.; Brooks, S.J. Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: A meta-analysis of VBM studies. BMC Psychiatry 2013, 13, 110. [Google Scholar] [CrossRef] [Green Version]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Anand, B.K.; Brobeck, J.R. Localization of a “Feeding Center” in the Hypothalamus of the Rat. Proc. Soc. Exp. Biol. Med. 1951, 77, 323–325. [Google Scholar] [CrossRef]
- Hamilton, C.L.; Ciaccia, P.J.; Lewis, D.O. Feeding behavior in monkeys with and without lesions of the hypothalamus. Am. J. Physiol. Content 1976, 230, 818–830. [Google Scholar] [CrossRef]
- Anand, B.K.; Chhina, G.S.; Singh, B. Effect of Glucose on the Activity of Hypothalamic “Feeding Centers”. Science 1962, 138, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.K.; Chhina, G.S.; Sharma, K.N.; Dua, S.; Singh, B. Activity of single neurons in the hypothalamic feeding centers: Effect of glucose. Am. J. Physiol. Content 1964, 207, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Ritter, R.C.; Slusser, P.G.; Stone, S. Glucoreceptors Controlling Feeding and Blood Glucose: Location in the Hindbrain. Science 1981, 213, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Dallaporta, M.; Himmi, T.; Perrin, J.; Orsini, J.-C. Solitary tract nucleus sensitivity to moderate changes in glucose level. Neuroreport 1999, 10, 2657–2660. [Google Scholar] [CrossRef]
- Koekkoek, L.L.; Mul, J.D.; la Fleur, S.E. Glucose-Sensing in the Reward System. Front. Neurosci. 2017, 11, 716. [Google Scholar] [CrossRef] [Green Version]
- Zigman, J.M.; Jones, J.E.; Lee, C.E.; Saper, C.B.; Elmquist, J.K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006, 494, 528–548. [Google Scholar] [CrossRef] [Green Version]
- Howick, K.; Griffin, B.T.; Cryan, J.F.; Schellekens, H. From Belly to Brain: Targeting the Ghrelin Receptor in Appetite and Food Intake Regulation. Int. J. Mol. Sci. 2017, 18, 273. [Google Scholar] [CrossRef] [Green Version]
- Burger, K.S.; Stice, E. Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am. J. Clin. Nutr. 2013, 97, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.D.; McCutcheon, J.E.; Cone, J.J.; Ragozzino, M.E.; Roitman, M.F. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur. J. Neurosci. 2011, 34, 1997–2006. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, L.; Hoebel, B.G. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988, 42, 1705–1712. [Google Scholar] [CrossRef]
- Martel, P.; Fantino, M. Mesolimbic dopaminergic system activity as a function of food reward: A microdialysis study. Pharmacol. Biochem. Behav. 1996, 53, 221–226. [Google Scholar] [CrossRef]
- Andrews, Z.B.; Horvath, T.L. Tasteless Food Reward. Neuron 2008, 57, 806–808. [Google Scholar] [CrossRef] [Green Version]
- de Araujo, I.E.; Oliveira-Maia, A.J.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G.; Nicolelis, M.A.; Simon, S.A. Food Reward in the Absence of Taste Receptor Signaling. Neuron 2008, 57, 930–941. [Google Scholar] [CrossRef] [Green Version]
- Palmiter, R.D. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007, 30, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.P.; Karim, H.T.; Aizenstein, H.J.; Helbling, N.L.; Toledo, F.G.S. Insulin sensitivity predicts brain network connectivity following a meal. Neuroimage 2018, 171, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Al Massadi, O.; Nogueiras, R.; Dieguez, C.; Girault, J.-A. Ghrelin and food reward. Neuropharmacology 2019, 148, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Omrani, A.; de Vrind, V.A.J.; Lodder, B.; Stoltenborg, I.; Kooij, K.; Wolterink-Donselaar, I.G.; Luijendijk-Berg, M.C.M.; Garner, K.M.; Van’t Sant, L.J.; Rozeboom, A.; et al. Identification of Novel Neurocircuitry Through Which Leptin Targets Multiple Inputs to the Dopamine System to Reduce Food Reward Seeking. Biol. Psychiatry 2021, 90, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M.; Mingote, S.M.; Weber, S.M. Beyond the reward hypothesis: Alternative functions of nucleus accumbens dopamine. Curr. Opin. Pharmacol. 2005, 5, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, S.; Miras, A.D.; Chhina, N.; Prechtl, C.G.; Sleeth, M.L.; Daud, N.M.; Ismail, N.A.; Durighel, G.; Ahmed, A.R.; Olbers, T.; et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014, 63, 891–902. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.-W.; Gao, X.-B. A neural circuit for gut-induced reward. Cell 2018, 175, 665–678.e623. [Google Scholar] [CrossRef] [Green Version]
- De Araujo, I.E.; Schatzker, M.; Small, D.M. Rethinking food reward. Annu. Rev. Psychol. 2020, 71, 139–164. [Google Scholar] [CrossRef] [PubMed]
- DiFeliceantonio, A.G.; Coppin, G.; Rigoux, L.; Thanarajah, S.E.; Dagher, A.; Tittgemeyer, M.; Small, D.M. Supra-additive effects of combining fat and carbohydrate on food reward. Cell Metab. 2018, 28, 33–44.e33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.W.; Fellows, L.K.; Dagher, A. Behavioral and Neural Valuation of Foods Is Driven by Implicit Knowledge of Caloric Content. Psychol. Sci. 2014, 25, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Simpson, K.A.; Minnion, J.S.; Shillito, J.C.; Bloom, S.R. The role of gut hormones and the hypothalamus in appetite regulation. Endocr. J. 2010, 57, 359–372. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.J.; Moran, T.H. Sub-diaphragmatic vagal afferent integration of meal-related gastrointestinal signals. Neurosci. Biobehav. Rev. 1996, 20, 47–56. [Google Scholar] [CrossRef]
- Goldstein, N.; McKnight, A.D.; Carty, J.R.E.; Arnold, M.; Betley, J.N.; Alhadeff, A.L. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 2021, 33, 676–687.e5. [Google Scholar] [CrossRef]
- Pelchat, M.L.; Johnson, A.; Chan, R.; Valdez, J.; Ragland, J.D. Images of desire: Food-craving activation during fMRI. Neuroimage 2004, 23, 1486–1493. [Google Scholar] [CrossRef]
- Koban, L.; Wager, T.D.; Kober, H. A neuromarker for drug and food craving distinguishes drug users from non-users. Nat. Neurosci. 2023, 26, 316–325. [Google Scholar] [CrossRef]
- Rolls, E.T.; McCabe, C. Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur. J. Neurosci. 2007, 26, 1067–1076. [Google Scholar] [CrossRef]
- Lee, I.-S.; Kullmann, S.; Scheffler, K.; Preissl, H.; Enck, P. Fat label compared with fat content: Gastrointestinal symptoms and brain activity in functional dyspepsia patients and healthy controls. Am. J. Clin. Nutr. 2018, 108, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-S.; Preissl, H.; Giel, K.; Schag, K.; Enck, P. Attentional and physiological processing of food images in functional dyspepsia patients: A pilot study. Sci. Rep. 2018, 8, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, T.A.; Camerer, C.F.; Rangel, A. Self-Control in Decision-Making Involves Modulation of the vmPFC Valuation System. Science 2009, 324, 646–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.M.; Higgs, S.; Dourish, C.T.; Hansen, P.C.; Harmer, C.J.; McCabe, C. Satiation attenuates BOLD activity in brain regions involved in reward and increases activity in dorsolateral prefrontal cortex: An fMRI study in healthy volunteers. Am. J. Clin. Nutr. 2015, 101, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.J.; O’Daly, O.; Uher, R.; Friederich, H.-C.; Giampietro, V.; Brammer, M.; Williams, S.C.R.; Schiöth, H.B.; Treasure, J.; Campbell, I.C. Thinking about Eating Food Activates Visual Cortex with Reduced Bilateral Cerebellar Activation in Females with Anorexia Nervosa: An fMRI Study. PLoS ONE 2012, 7, e34000. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, S.; Geisler, D.; Ritschel, F.; King, J.A.; Seidel, M.; Boehm, I.; Breier, M.; Clas, S.; Weiss, J.; Marxen, M.; et al. Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. J. Psychiatry Neurosci. 2015, 40, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Amlung, M.; Petker, T.; Jackson, J.; Balodis, I.; MacKillop, J. Steep discounting of delayed monetary and food rewards in obesity: A meta-analysis. Psychol. Med. 2016, 46, 2423–2434. [Google Scholar] [CrossRef] [Green Version]
- Stojek, M.K.M.; MacKillop, J. Relative reinforcing value of food and delayed reward discounting in obesity and disordered eating: A systematic review. Clin. Psychol. Rev. 2017, 55, 1–11. [Google Scholar] [CrossRef]
- Rösch, S.A.; Schmidt, R.; Lührs, M.; Ehlis, A.-C.; Hesse, S.; Hilbert, A. Evidence of fNIRS-Based Prefrontal Cortex Hypoactivity in Obesity and Binge-Eating Disorder. Brain Sci. 2020, 11, 19. [Google Scholar] [CrossRef]
- García-García, I.; Horstmann, A.; Jurado, M.A.; Garolera, M.; Chaudhry, S.J.; Margulies, D.S.; Villringer, A.; Neumann, J. Reward processing in obesity, substance addiction and non-substance addiction. Obes. Rev. 2014, 15, 853–869. [Google Scholar] [CrossRef] [Green Version]
- Grant, S.; London, E.D.; Newlin, D.B.; Villemagne, V.L.; Liu, X.; Contoreggi, C.; Phillips, R.L.; Kimes, A.S.; Margolin, A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. USA 1996, 93, 12040–12045. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Volkow, N.D.; Fowler, J.S.; Cervany, P.; Hitzemann, R.J.; Pappas, N.R.; Wong, C.T.; Felder, C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999, 64, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Skunde, M.; Walther, S.; Bendszus, M.; Herzog, W.; Friederich, H.-C. Neural signature of food reward processing in bulimic-type eating disorders. Soc. Cogn. Affect. Neurosci. 2016, 11, 1393–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, C.J.; Reichelt, A.C.; Hall, P.A. The Prefrontal Cortex and Obesity: A Health Neuroscience Perspective. Trends Cogn. Sci. 2019, 23, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Rolls, E.T.; Feng, R.; Cheng, W.; Feng, J. Orbitofrontal cortex connectivity is associated with food reward and body weight in humans. Soc. Cogn. Affect. Neurosci. 2023, 18, nsab083. [Google Scholar] [CrossRef]
- Wierenga, C.E.; Bischoff-Grethe, A.; Melrose, A.J.; Irvine, Z.; Torres, L.; Bailer, U.F.; Simmons, A.; Fudge, J.L.; McClure, S.M.; Ely, A.; et al. Hunger Does Not Motivate Reward in Women Remitted from Anorexia Nervosa. Biol. Psychiatry 2015, 77, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Yeung, A.W.K.; Goto, T.K.; Leung, W.K. Affective value, intensity and quality of liquid tastants/food discernment in the human brain: An activation likelihood estimation meta-analysis. Neuroimage 2018, 169, 189–199. [Google Scholar] [CrossRef]
- Small, D.M.; Jones-Gotman, M.; Zatorre, R.J.; Petrides, M.; Evans, A.C. Flavor processing: More than the sum of its parts. Neuroreport 1997, 8, 3913–3917. [Google Scholar] [CrossRef]
- Cerf, B.; Lebihan, D.; Van de Moortele, P.; Mac Leod, P.; Faurion, A. Functional Lateralization of Human Gustatory Cortex Related to Handedness Disclosed by fMRI Study a. Ann. N. Y. Acad. Sci. 1998, 855, 575–578. [Google Scholar] [CrossRef]
- Grabenhorst, F.; D’Souza, A.A.; Parris, B.A.; Rolls, E.T.; Passingham, R.E.J.N. A common neural scale for the subjective pleasantness of different primary rewards. Neuroimage 2010, 51, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Canna, A.; Prinster, A.; Cantone, E.; Ponticorvo, S.; Russo, A.G.; Di Salle, F.; Esposito, F. Intensity-related distribution of sweet and bitter taste fMRI responses in the insular cortex. Hum. Brain Mapp. 2019, 40, 3631–3646. [Google Scholar] [CrossRef] [Green Version]
- Avery, J.A.; Liu, A.G.; Ingeholm, J.E.; Riddell, C.D.; Gotts, S.J.; Martin, A. Taste Quality Representation in the Human Brain. J. Neurosci. 2020, 40, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.A.; de Graaf, C.; Stafleu, A.; van Osch, M.J.; Nievelstein, R.A.; van der Grond, J. Effect of satiety on brain activation during chocolate tasting in men and women. Am. J. Clin. Nutr. 2006, 83, 1297–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uher, R.; Treasure, J.; Heining, M.; Brammer, M.J.; Campbell, I.C. Cerebral processing of food-related stimuli: Effects of fasting and gender. Behav. Brain Res. 2006, 169, 111–119. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, I.E.; Rolls, E.T. Representation in the Human Brain of Food Texture and Oral Fat. J. Neurosci. 2004, 24, 3086–3093. [Google Scholar] [CrossRef] [Green Version]
- Felsted, J.A.; Ren, X.; Chouinard-Decorte, F.; Small, D.M. Genetically Determined Differences in Brain Response to a Primary Food Reward: Figure 1. J. Neurosci. 2010, 30, 2428–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stice, E.; Yokum, S.; Burger, K.; Epstein, L.; Smolen, A. Multilocus Genetic Composite Reflecting Dopamine Signaling Capacity Predicts Reward Circuitry Responsivity. J. Neurosci. 2012, 32, 10093–10100. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.; Stice, E.; Yokum, S.; Bohon, C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite 2011, 57, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Small, D.M.; Jones-Gotman, M.; Daghera, A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 2003, 19, 1709–1715. [Google Scholar] [CrossRef]
- Frank-Podlech, S.; Heinze, J.M.; Machann, J.; Scheffler, K.; Camps, G.; Fritsche, A.; Rosenberger, M.; Hinrichs, J.; Veit, R.; Preissl, H. Functional Connectivity Within the Gustatory Network Is Altered by Fat Content and Oral Fat Sensitivity—A Pilot Study. Front. Neurosci. 2019, 13, 725. [Google Scholar] [CrossRef]
- Frank, S.; Linder, K.; Kullmann, S.; Heni, M.; Ketterer, C.; Çavuşoğlu, M.; Krzeminski, A.; Fritsche, A.; Häring, H.-U.; Preissl, H.; et al. Fat intake modulates cerebral blood flow in homeostatic and gustatory brain areas in humans. Am. J. Clin. Nutr. 2012, 95, 1342–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, A.W.K.; Wong, N.S.M.; Lau, H.; Eickhoff, S.B. Human brain responses to gustatory and food stimuli: A meta-evaluation of neuroimaging meta-analyses. Neuroimage 2019, 202, 116111. [Google Scholar] [CrossRef]
- Plassmann, H.; O’Doherty, J.P.; Rangel, A. Appetitive and Aversive Goal Values Are Encoded in the Medial Orbitofrontal Cortex at the Time of Decision Making. J. Neurosci. 2010, 30, 10799–10808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, W.K.; Martin, A.; Barsalou, L.W. Pictures of Appetizing Foods Activate Gustatory Cortices for Taste and Reward. Cereb. Cortex 2005, 15, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Laharnar, N.; Kullmann, S.; Veit, R.; Canova, C.; Hegner, Y.L.; Fritsche, A.; Preissl, H. Processing of food pictures: Influence of hunger, gender and calorie content. Brain Res. 2010, 1350, 159–166. [Google Scholar] [CrossRef]
- van der Laan, L.N.; de Ridder, D.T.; Viergever, M.A.; Smeets, P.A. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. Neuroimage 2011, 55, 296–303. [Google Scholar] [CrossRef]
- Litt, A.; Plassmann, H.; Shiv, B.; Rangel, A. Dissociating Valuation and Saliency Signals during Decision-Making. Cereb. Cortex 2011, 21, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Miao, M.; Gan, Y. A systematic and meta-analytic review on the neural correlates of viewing high- and low-calorie foods among normal-weight adults. Neurosci. Biobehav. Rev. 2022, 138, 104721. [Google Scholar] [CrossRef]
- Brooks, S.J.; Cedernaes, J.; Schiöth, H.B. Increased Prefrontal and Parahippocampal Activation with Reduced Dorsolateral Prefrontal and Insular Cortex Activation to Food Images in Obesity: A Meta-Analysis of fMRI Studies. PLoS ONE 2013, 8, e60393. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hu, X.; Wang, J.; Chen, J.; Guo, Q.; Li, C.; Enck, P. Processing of Food, Body and Emotional Stimuli in Anorexia Nervosa: A Systematic Review and Meta-analysis of Functional Magnetic Resonance Imaging Studies. Eur. Eat. Disord. Rev. 2012, 20, 439–450. [Google Scholar] [CrossRef]
- Avery, J.A.; Liu, A.G.; Ingeholm, J.E.; Gotts, S.J.; Martin, A. Viewing images of foods evokes taste quality-specific activity in gustatory insular cortex. Proc. Natl. Acad. Sci. USA 2021, 118, e2010932118. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takeda, M.; Hattori, N.; Fukunaga, M.; Sasabe, T.; Inoue, N.; Nagai, Y.; Sawada, T.; Sadato, N.; Watanabe, Y. Functional imaging of gustatory perception and imagery: “top-down” processing of gustatory signals. Neuroimage 2004, 23, 1271–1282. [Google Scholar] [CrossRef]
- Holsen, L.M.; Lawson, E.A.; Blum, J.; Ko, E.; Makris, N.; Fazeli, P.K.; Klibanski, A.; Goldstein, J.M. Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. J. Psychiatry Neurosci. 2012, 37, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, E.A.; Holsen, L.M.; DeSanti, R.; Santin, M.; Meenaghan, E.; Herzog, D.B.; Goldstein, J.M.; Klibanski, A. Increased hypothalamic–pituitary–adrenal drive is associated with decreased appetite and hypoactivation of food-motivation neurocircuitry in anorexia nervosa. Eur. J. Endocrinol. 2013, 169, 639–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, G.K.W.; Shott, M.E.; Riederer, J.; Pryor, T.L. Altered structural and effective connectivity in anorexia and bulimia nervosa in circuits that regulate energy and reward homeostasis. Transl. Psychiatry 2016, 6, e932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, N.; Smeets, P.A.M.; van Elburg, A.A.; Danner, U.N.; van Meer, F.; Hoek, H.W.; Adan, R.A.H. Altered Food-Cue Processing in Chronically Ill and Recovered Women with Anorexia Nervosa. Front. Behav. Neurosci. 2015, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Scaife, J.C.; Godier, L.R.; Reinecke, A.; Harmer, C.J.; Park, R.J. Differential activation of the frontal pole to high vs. low calorie foods: The neural basis of food preference in Anorexia Nervosa? Psychiatry Res. Neuroimaging 2016, 258, 44–53. [Google Scholar] [CrossRef]
- Oudijn, M.S.; Storosum, J.G.; Nelis, E.; Denys, D. Is deep brain stimulation a treatment option for anorexia nervosa? BMC Psychiatry 2013, 13, 277. [Google Scholar] [CrossRef] [Green Version]
- Foerde, K.; Steinglass, J.E.; Shohamy, D.; Walsh, B.T. Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nat. Neurosci. 2015, 18, 1571–1573. [Google Scholar] [CrossRef]
- McClelland, J.; Kekic, M.; Bozhilova, N.; Nestler, S.; Dew, T.; Van den Eynde, F.; David, A.S.; Rubia, K.; Campbell, I.C.; Schmidt, U. A Randomised Controlled Trial of Neuronavigated Repetitive Transcranial Magnetic Stimulation (rTMS) in Anorexia Nervosa. PLoS ONE 2016, 11, e0148606. [Google Scholar] [CrossRef] [Green Version]
- Dalton, B.; Foerde, K.; Bartholdy, S.; McClelland, J.; Kekic, M.; Grycuk, L.; Campbell, I.C.; Schmidt, U.; Steinglass, J.E. The effect of repetitive transcranial magnetic stimulation on food choice-related self-control in patients with severe, enduring anorexia nervosa. Int. J. Eat. Disord. 2020, 53, 1326–1336. [Google Scholar] [CrossRef] [Green Version]
- Marsh, R.; Steinglass, J.E.; Gerber, A.J.; O’leary, K.G.; Wang, Z.; Murphy, D.; Walsh, B.T.; Peterson, B.S. Deficient Activity in the Neural Systems That Mediate Self-regulatory Control in Bulimia Nervosa. Arch. Gen. Psychiatry 2009, 66, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.; Horga, G.; Wang, Z.; Wang, P.; Klahr, K.W.; Berner, L.A.; Walsh, B.T.; Peterson, B.S. An fMRI Study of Self-Regulatory Control and Conflict Resolution in Adolescents with Bulimia Nervosa. Am. J. Psychiatry 2011, 168, 1210–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berner, L.A.; Marsh, R. Frontostriatal Circuits and the Development of Bulimia Nervosa. Front. Behav. Neurosci. 2014, 8, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, S.J.; O’Daly, O.G.; Uher, R.; Friederich, H.-C.; Giampietro, V.; Brammer, M.; Williams, S.C.R.; Schiöth, H.B.; Treasure, J.; Campbell, I.C. Differential Neural Responses to Food Images in Women with Bulimia versus Anorexia Nervosa. PLoS ONE 2011, 6, e22259. [Google Scholar] [CrossRef]
- Bohon, C.; Stice, E. Reward abnormalities among women with full and subthreshold bulimia nervosa: A functional magnetic resonance imaging study. Int. J. Eat. Disord. 2010, 44, 585–595. [Google Scholar] [CrossRef]
- Frank, G.K.; Reynolds, J.R.; Shott, M.E.; O’Reilly, R.C. Altered Temporal Difference Learning in Bulimia Nervosa. Biol. Psychiatry 2010, 70, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Kekic, M.; McClelland, J.; Bartholdy, S.; Boysen, E.; Musiat, P.; Dalton, B.; Tiza, M.; David, A.S.; Campbell, I.C.; Schmidt, U. Single-Session Transcranial Direct Current Stimulation Temporarily Improves Symptoms, Mood, and Self-Regulatory Control in Bulimia Nervosa: A Randomised Controlled Trial. PLoS ONE 2017, 12, e0167606. [Google Scholar] [CrossRef] [Green Version]
- Gay, A.; Jaussent, I.; Sigaud, T.; Billard, S.; Attal, J.; Seneque, M.; Galusca, B.; Van Den Eynde, F.; Massoubre, C.; Courtet, P.; et al. A Lack of Clinical Effect of High-frequency rTMS to Dorsolateral Prefrontal Cortex on Bulimic Symptoms: A Randomised, Double-blind Trial. Eur. Eat. Disord. Rev. 2016, 24, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Weygandt, M.; Schaefer, A.; Schienle, A.; Haynes, J.D. Diagnosing different binge-eating disorders based on reward-related brain activation patterns. Hum. Brain Mapp. 2012, 33, 2135–2146. [Google Scholar] [CrossRef]
- Hege, M.A.; Stingl, K.T.; Kullmann, S.; Schag, K.; Giel, K.E.; Zipfel, S.; Preissl, H. Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. Int. J. Obes. 2015, 39, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Veit, R.; Schag, K.; Schopf, E.; Borutta, M.; Kreutzer, J.; Ehlis, A.-C.; Zipfel, S.; Giel, K.E.; Preissl, H.; Kullmann, S. Diminished prefrontal cortex activation in patients with binge eating disorder associates with trait impulsivity and improves after impulsivity-focused treatment based on a randomized controlled IMPULS trial. NeuroImage: Clin. 2021, 30, 102679. [Google Scholar] [CrossRef] [PubMed]
- Devoto, F.; Zapparoli, L.; Bonandrini, R.; Berlingeri, M.; Ferrulli, A.; Luzi, L.; Banfi, G.; Paulesu, E.J.N.; Reviews, B. Hungry brains: A meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci. Biobehav. Rev. 2018, 94, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The orbitofrontal cortex, food reward, body weight and obesity. Soc. Cogn. Affect. Neurosci. 2023, 18, nsab044. [Google Scholar] [CrossRef]
- DelParigi, A.; Chen, K.; Salbe, A.D.; Hill, J.O.; Wing, R.R.; Reiman, E.M.; Tataranni, P.A. Persistence of abnormal neural responses to a meal in postobese individuals. Int. J. Obes. 2004, 28, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Le, D.S.N.; Pannacciulli, N.; Chen, K.; Del Parigi, A.; Salbe, A.D.; Reiman, E.M.; Krakoff, J. Less activation of the left dorsolateral prefrontal cortex in response to a meal: A feature of obesity. Am. J. Clin. Nutr. 2006, 84, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Pannacciulli, N.; Del Parigi, A.; Chen, K.; Le, D.S.N.; Reiman, E.M.; Tataranni, P.A.J.N. Brain abnormalities in human obesity: A voxel-based morphometric study. Neuroimage 2006, 31, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Weygandt, M.; Mai, K.; Dommes, E.; Leupelt, V.; Hackmack, K.; Kahnt, T.; Rothemund, Y.; Spranger, J.; Haynes, J.-D. The role of neural impulse control mechanisms for dietary success in obesity. NeuroImage 2013, 83, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Spetter, M.S.; Malekshahi, R.; Birbaumer, N.; Lührs, M.; van der Veer, A.H.; Scheffler, K.; Spuckti, S.; Preissl, H.; Veit, R.; Hallschmid, M. Volitional regulation of brain responses to food stimuli in overweight and obese subjects: A real-time fMRI feedback study. Appetite 2017, 112, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Murray, H.B.; Kuo, B.; Eddy, K.T.; Breithaupt, L.; Becker, K.R.; Ba, M.J.D.; Thomas, J.J.; Staller, K. Disorders of gut–brain interaction common among outpatients with eating disorders including avoidant/restrictive food intake disorder. Int. J. Eat. Disord. 2020, 54, 952–958. [Google Scholar] [CrossRef]
- Lee, I.-S.; Wang, H.; Chae, Y.; Preissl, H.; Enck, P. Functional neuroimaging studies in functional dyspepsia patients: A systematic review. Neurogastroenterol. Motil. 2016, 28, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Skrobisz, K.; Piotrowicz, G.; Drozdowska, A.; Markiet, K.; Sabisz, A.; Naumczyk, P.; Rydzewska, G.; Szurowska, E. Use of functional magnetic resonance imaging in patients with irritable bowel syndrome and functional dyspepsia. Gastroenterol. Rev. 2019, 14, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, P.; Sun, R.; Zhang, X.; He, Y.; Li, S.; Yin, T.; Zeng, F. Altered resting-state brain activity in functional dyspepsia patients: A coordinate-based meta-analysis. Front. Neurosci. 2023, 17, 1174287. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.V.; Hyldig, G. Food satisfaction: Integrating feelings before, during and after food intake. Food Qual. Prefer. 2015, 43, 126–134. [Google Scholar] [CrossRef]
- Rolls, E.T. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol. Behav. 2005, 85, 45–56. [Google Scholar] [CrossRef]
- Lee, I.-S.; Preissl, H.; Enck, P. How to Perform and Interpret Functional Magnetic Resonance Imaging Studies in Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2017, 23, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Ledoux, T.; Nguyen, A.S.; Bakos-Block, C.; Bordnick, P. Using virtual reality to study food cravings. Appetite 2013, 71, 396–402. [Google Scholar] [CrossRef]
- Manasse, S.M.; Trainor, C.; Payne-Reichert, A.; Abber, S.R.; Lampe, E.W.; Gillikin, L.M.; Juarascio, A.S.; Forman, E.M. Does virtual reality enhance the effects of inhibitory control training for loss-of-control eating? A pilot factorial experiment. Eat. Behav. 2023, 50, 101749. [Google Scholar] [CrossRef]
- Piper, S.K.; Krueger, A.; Koch, S.P.; Mehnert, J.; Habermehl, C.; Steinbrink, J.; Obrig, H.; Schmitz, C.H. A wearable multi-channel fNIRS system for brain imaging in freely moving subjects. Neuroimage 2014, 85 Pt 1, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Balardin, J.B.; Zimeo Morais, G.A.; Furucho, R.A.; Trambaiolli, L.; Vanzella, P.; Biazoli, C., Jr.; Sato, J.R. Imaging Brain Function with Functional Near-Infrared Spectroscopy in Unconstrained Environments. Front. Hum. Neurosci. 2017, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Yeung, A.W.K. Differences in Brain Responses to Food or Tastants Delivered with and Without Swallowing: A Meta-analysis on Functional Magnetic Resonance Imaging (fMRI) Studies. Chemosens. Percept. 2022, 15, 112–123. [Google Scholar] [CrossRef]
- Lin, C.S. Meta-analysis of brain mechanisms of chewing and clenching movements. J. Oral Rehabil. 2018, 45, 627–639. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, M.T.; Smeets, P.A.M.; la Fleur, S.E. Inhibitory control as a potential treatment target for obesity. Nutr. Neurosci. 2022, 26, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Morys, F.; Wu, Q.; Li, J.; Chen, H. Pilot study of food-specific go/no-go training for overweight individuals: Brain imaging data suggest inhibition shapes food evaluation. Soc. Cogn. Affect. Neurosci. 2023, 18, nsab137. [Google Scholar] [CrossRef]
- Jones, A.; Di Lemma, L.C.; Robinson, E.; Christiansen, P.; Nolan, S.; Tudur-Smith, C.; Field, M. Inhibitory control training for appetitive behaviour change: A meta-analytic investigation of mechanisms of action and moderators of effectiveness. Appetite 2016, 97, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Lowe, C.J.; Staines, W.R.; Manocchio, F.; Hall, P.A. The neurocognitive mechanisms underlying food cravings and snack food consumption. A combined continuous theta burst stimulation (cTBS) and EEG study. Neuroimage 2018, 177, 45–58. [Google Scholar] [CrossRef]
- Hall, P.A.; Lowe, C.; Vincent, C. Brain Stimulation Effects on Food Cravings and Consumption: An Update on Lowe et al. (2017) and a Response to Generoso et al. (2017). Psychosom. Med. 2017, 79, 839–842. [Google Scholar] [CrossRef]
- Ljubisavljevic, M.; Maxood, K.; Bjekic, J.; Oommen, J.; Nagelkerke, N. Long-Term Effects of Repeated Prefrontal Cortex Transcranial Direct Current Stimulation (tDCS) on Food Craving in Normal and Overweight Young Adults. Brain Stimul. 2016, 9, 826–833. [Google Scholar] [CrossRef]
- Burgess, E.E.; Sylvester, M.D.; Morse, K.E.; Amthor, F.R.; Mrug, S.; Lokken, K.L.; Osborn, M.K.; Soleymani, T.; Boggiano, M.M. Effects of transcranial direct current stimulation (tDCS) on binge-eating disorder. Int. J. Eat. Disord. 2016, 49, 930–936. [Google Scholar] [CrossRef]
- Gluck, M.E.; Alonso-Alonso, M.; Piaggi, P.; Weise, C.M.; Jumpertz-von Schwartzenberg, R.; Reinhardt, M.; Wassermann, E.M.; Venti, C.A.; Votruba, S.B.; Krakoff, J. Neuromodulation targeted to the prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity 2015, 23, 2149–2156. [Google Scholar] [CrossRef]
- Kohl, S.H.; Veit, R.; Spetter, M.S.; Günther, A.; Rina, A.; Lührs, M.; Birbaumer, N.; Preissl, H.; Hallschmid, M. Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: A randomized controlled trial in overweight and obese subjects. Neuroimage 2019, 191, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.; Campbell, I.C.; Schmidt, U. Neuromodulation and neurofeedback treatments in eating disorders and obesity. Curr. Opin. Psychiatry 2017, 30, 458–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hege, M.A.; Veit, R.; Krumsiek, J.; Kullmann, S.; Heni, M.; Rogers, P.J.; Brunstrom, J.M.; Fritsche, A.; Preissl, H. Eating less or more —Mindset induced changes in neural correlates of pre-meal planning. Appetite 2018, 125, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veit, R.; Horstman, L.I.; Hege, M.A.; Heni, M.; Rogers, P.J.; Brunstrom, J.M.; Fritsche, A.; Preissl, H.; Kullmann, S. Health, pleasure, and fullness: Changing mindset affects brain responses and portion size selection in adults with overweight and obesity. Int. J. Obes. 2020, 44, 428–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, Y.; Lee, I.-S. Central Regulation of Eating Behaviors in Humans: Evidence from Functional Neuroimaging Studies. Nutrients 2023, 15, 3010. https://doi.org/10.3390/nu15133010
Chae Y, Lee I-S. Central Regulation of Eating Behaviors in Humans: Evidence from Functional Neuroimaging Studies. Nutrients. 2023; 15(13):3010. https://doi.org/10.3390/nu15133010
Chicago/Turabian StyleChae, Younbyoung, and In-Seon Lee. 2023. "Central Regulation of Eating Behaviors in Humans: Evidence from Functional Neuroimaging Studies" Nutrients 15, no. 13: 3010. https://doi.org/10.3390/nu15133010




