IgE Mediated Shellfish Allergy in Children—A Review
Abstract
:1. Introduction
2. Taxonomy
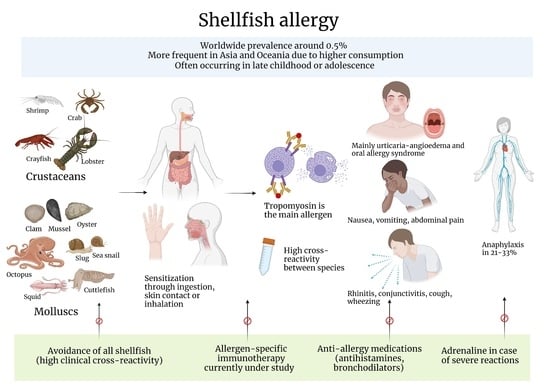
3. Epidemiology
| Country | Year | Age (Years) | Diagnosis | Prevalence | Relative Frequency among Children with Food Allergy |
|---|---|---|---|---|---|
| Thailand [28] | 2012 | 3–7 | SR + PC | 25.4% (15/59) shrimp 6.8% (4/59) crab 1.7% (1/59) squid 1.7% (1/59) mollusk | |
| Lithuania [27] | 2012 | 5–12 | SR | 2.4% (1/41) crustacean | |
| Hong Kong [14] | 2012 | 11–14 | SR | 37.8% (133/352) crustacean | |
| Taiwan [29] | 2012 | 0–18 | SR | 51.6% (1076/2086) shrimp 34% (710/2086) crab 18.4% (384/2086) mollusk | |
| South Korea [32] | 2012 | 0–6 | SR | 13.8% (86/621) crustacean | |
| China [33] | 2015 | 1–7 | SR | 4.4% (112/2540) shrimp 3.2% (81/2540) crab | |
| Mexico [34] | 2016 | 5–13 | SR | 1.3% (12/1049) shrimp 1.3% (12/1049) other shellfish | |
| South Korea [32] | 2017 | 6–16 | SR | 0.84% (250/29,842) crustacean | |
| Australia [35] | 2018 | 10–14 | SR + PC | 0.3% (15/5016) shellfish | |
| Vietnam [36] | 2019 | 2–6 | SR + PC | 3.83% (330/8620) crustacean 1.03% (88/8620) mollusk | |
| United States [12] | 2018 | 0–17 | SR | 1.3% (499/38,408) shellfish | |
| Kuwait [37] | 2019 | 11–14 | SR | 1.3% (48/3738) shellfish | |
| Europe [38] | 2020 | 7–10 | SR + PC | 0.38–3.75% (64–635/16,935) shrimp | |
| Europe [16] | 2020 | 6–10 | SR + PC | 0.2% (15/6069) crustacean | |
| China, Russia, India [39] | 2020 | 6–11 | SR + PC | 0–1.05% (shrimps) 0.07–0.43% (crabs) | |
| China [40] | 2020 | 3–5 | SR + PC | 0.12% (5/4151) shrimp 0.09% (4/4151) crab | |
| USA [24] | 2021 | 0–18 | SR + PC | 0.8% (307/38,408) | |
| Canada [9] | 2021 | 0–19 | SR + PC | 0.2% (576/288,490) | |
| Brazil [15] | 2022 | 2–5 | SR | 31.9% (15/47) shrimp 31.9% (15/47) mollusk |
4. Clinical Features
5. Allergens
6. Tropomyosin
7. Arginine Kinase
8. Myosin Light Chain
9. Sarcoplasmic Calcium-Binding Protein
10. Other Minor Allergens
11. Cross-Reactivity
12. Diagnosis
13. Differential Diagnosis
14. Management
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sicherer, S.H.; Sampson, H.A. Food allergy. J. Allergy Clin. Immunol. 2010, 125, S116–S125. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.J.; Wainstein, B.K.; Barnes, E.H.; Liew, W.K.; Campbell, D.E. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin. Exp. Allergy 2016, 46, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Davis, G.E. An Updated Classification of the Recent Crustacea; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 2001. [Google Scholar]
- Ponder, W. (Ed.) Phylogeny and Evolution of the Mollusca; University of California Press: Oakland, CA, USA, 2008. [Google Scholar] [CrossRef]
- Rona, R.J.; Keil, T.; Summers, C.; Gislason, D.; Zuidmeer, L.; Sodergren, E.; Sigurdardottir, S.T.; Lindner, T.; Goldhahn, K.; Dahlstrom, J.; et al. The prevalence of food allergy: A meta-analysis. J. Allergy Clin. Immunol. 2007, 120, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Sommergruber, K.; Hilger, C.; Santos, A.; De Las Vecillas, L.; Dramburg, S. Molecular Allergology User’s Guide 2.0. Cow’s Milk Allergy 2022, 34, 285–294. [Google Scholar] [CrossRef]
- Moonesinghe, H.; Mackenzie, H.; Venter, C.; Kilburn, S.; Turner, P.; Weir, K.; Dean, T. Prevalence of fish and shellfish allergy: A systematic review. Ann. Allergy Asthma Immunol. 2016, 117, 264–272.e4. [Google Scholar] [CrossRef]
- Ben-Shoshan, M.; Harrington, D.W.; Soller, L.; Fragapane, J.; Joseph, L.; Pierre, Y.S.; Godefroy, S.B.; Elliot, S.J.; Clarke, A.E. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J. Allergy Clin. Immunol. 2010, 125, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.G.; Kosowan, L.; Soller, L.; Chan, E.S.; Nankissoor, N.N.; Phung, R.R.; Abrams, E.M. Prevalence of Physician-Reported Food Allergy in Canadian Children. J. Allergy Clin. Immunol. Pract. 2020, 9, 193–199. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Muñoz-Furlong, A.; Sampson, H.A. Prevalence of seafood allergy in the United States determined by a random telephone survey. J. Allergy Clin. Immunol. 2004, 114, 159–165. [Google Scholar] [CrossRef]
- Kamdar, T.A.; Peterson, S.; Lau, C.H.; Saltoun, C.A.; Gupta, R.S.; Bryce, P.J. Prevalence and characteristics of adult-onset food allergy. J. Allergy Clin. Immunol. Pract. 2014, 3, 114–115.e1. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Blumenstock, J.A.; Jiang, J.; Davis, M.M.; Nadeau, K.C. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 2018, 142, e20181235. [Google Scholar] [CrossRef] [Green Version]
- Wong, L.H.; Tham, E.H.; Lee, B.W. An update on shellfish allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.K.; Lee, S.L.; Wong, W.H.S.; Ip, P.; Lau, Y.L. Prevalence of self-reported food allergy in Hong Kong children and teens--a population survey. Asian Pac. J. Allergy Immunol. 2012, 30, 275–284. [Google Scholar] [PubMed]
- Correia, J.A.D.S.; Antunes, A.A.; Taborda-Barata, L.; Boechat, J.L.; Sarinho, E.S.C. Prevalence of reported food allergies in Brazilian preschoolers living in a small Brazilian city. Allergy Asthma Clin. Immunol. 2022, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Grabenhenrich, L.; Trendelenburg, V.; Bellach, J.; Yürek, S.; Reich, A.; Fiandor, A.; Rivero, D.; Sigurdardottir, S.; Clausen, M.; Papadopoulos, N.G.; et al. Frequency of food allergy in school-aged children in eight European countries—The EuroPrevall-iFAAM birth cohort. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 2294–2308. [Google Scholar] [CrossRef]
- Osterballe, M.; Hansen, T.K.; Mortz, C.G.; Host, A.; Bindslev-Jensen, C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr. Allergy Immunol. 2005, 16, 567–573. [Google Scholar] [CrossRef]
- Thong, B.Y.H.; Cheng, Y.K.; Leong, K.P.; Tang, C.Y.; Chng, H.H. Immediate food hypersensitivity among adults at-tending a clinical immunology/allergy centre in Singapore. Singap. Med. J. 2007, 48, 236. [Google Scholar]
- Liew, W.K.; Chiang, W.C.; Goh, A.E.; Lim, H.H.; Chay, O.M.; Chang, S.; Tan, J.H.; Shih, E.; Kidon, M. Paediatric anaphylaxis in a Singaporean children cohort: Changing food allergy triggers over time. Asia Pac. Allergy 2013, 3, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Lertnawapan, R.; Maek-A-Nantawat, W. Anaphylaxis and Biphasic Phase in Thailand: 4-year Observation. Allergol. Int. 2011, 60, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Smit, D.V.; Cameron, P.A.; Rainer, T.H. Anaphylaxis presentations to an emergency department in Hong Kong: Incidence and predictors of biphasic reactions. J. Emerg. Med. 2005, 28, 381–388. [Google Scholar] [CrossRef]
- Leung, T.; Sy, H.; Tsen, C.; Tang, M.; Wong, G. Is food allergy Increasing in Hong Kong Chinese children? In Allergy; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Ross, M.P.; Ferguson, M.; Street, D.; Klontz, K.; Schroeder, T.; Luccioli, S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J. Allergy Clin. Immunol. 2008, 121, 166–171. [Google Scholar] [CrossRef]
- Wang, H.T.; Warren, C.M.; Gupta, R.S.; Davis, C.M. Prevalence and Characteristics of Shellfish Allergy in the Pediatric Population of the United States. J. Allergy Clin. Immunol. Pract. 2020, 8, 1359–1370.e2. [Google Scholar] [CrossRef] [PubMed]
- Conrado, A.B.; Patel, N.; Turner, P.J. Global patterns in anaphylaxis due to specific foods: A systematic review. J. Allergy Clin. Immunol. 2021, 148, 1515–1525.e3. [Google Scholar] [CrossRef] [PubMed]
- Shek, L.P.-C.; Cabrera-Morales, E.A.; Soh, S.E.; Gerez, I.; Ng, P.Z.; Yi, F.C.; Ma, S.; Lee, B.W. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J. Allergy Clin. Immunol. 2010, 126, 324–331.e7. [Google Scholar] [CrossRef]
- Kavaliūnas, A.; Šurkienė, G.; Dubakienė, R.; Stukas, R.; Žagminas, K.; Šaulytė, J.; Burney, P.G.J.; Kummeling, I.; Mills, C. EuroPrevall Survey on Prevalence and Pattern of Self-Reported Adverse Reactions to Food and Food Allergies among Primary Schoolchildren in Vilnius, Lithuania. Medicina 2012, 48, 38–71. [Google Scholar] [CrossRef] [Green Version]
- Lao-Araya, M.; Trakultivakorn, M. Prevalence of Food Allergy Among Preschool Children in Northern Thailand. J. Allergy Clin. Immunol. 2011, 127, AB186. [Google Scholar] [CrossRef]
- Wu, T.-C.; Tsai, T.-C.; Huang, C.-F.; Chang, F.-Y.; Lin, C.-C.; Huang, I.-F.; Chu, C.-H.; Lau, B.-H.; Wu, L.; Peng, H.-J.; et al. Prevalence of food allergy in Taiwan: A questionnaire-based survey. Intern. Med. J. 2012, 42, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.M.; Aktas, O.N.; Gupta, R.S.; Davis, C.M. Prevalence and characteristics of adult shellfish allergy in the United States. J. Allergy Clin. Immunol. 2019, 144, 1435–1438.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffarelli, C.; Di Mauro, D.; Mastrorilli, C.; Bottau, P.; Cipriani, F.; Ricci, G. Solid Food Introduction and the Development of Food Allergies. Nutrients 2018, 10, 1790. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Kim, D.; Ahn, K.; Kim, J.; Han, Y. Prevalence of Immediate-Type Food Allergy in Early Childhood in Seoul. Allergy Asthma Immunol. Res. 2014, 6, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.-Q.; Luo, J.-Y.; Huang, H.-M.; Zheng, P.-Y.; Luo, W.-T.; Wei, N.-L.; Sun, B.-Q. Food allergy and related risk factors in 2540 preschool children: An epidemiological survey in Guangdong Province, southern China. World J. Pediatr. 2015, 11, 219–225. [Google Scholar] [CrossRef]
- Ontiveros, N.; Valdez-Meza, E.; Vergara-Jiménez, M.; Canizalez-Román, A.; Borzutzky, A.; Cabrera-Chávez, F. Parent-reported prevalence of food allergy in Mexican schoolchildren: A population-based study. Allergol. Immunopathol. 2016, 44, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Koplin, J.J.; Dharmage, S.C.; Field, M.J.; Sawyer, S.M.; McWilliam, V.; Peters, R.L.; Gurrin, L.C.; Vuillermin, P.J.; Douglass, J.; et al. Prevalence of clinic-defined food allergy in early adolescence: The SchoolNuts study. J. Allergy Clin. Immunol. 2017, 141, 391–398.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.T.K.; Nguyen, D.H.; Vu, A.T.L.; Ruethers, T.; Taki, A.C.; Lopata, A.L. A cross-sectional, population-based study on the prevalence of food allergies among children in two different socio-economic regions of Vietnam. Pediatr. Allergy Immunol. 2019, 30, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ziyab, A.H. Prevalence of food allergy among schoolchildren in Kuwait and its association with the coexistence and severity of asthma, rhinitis, and eczema: A cross-sectional study. World Allergy Organ. J. 2019, 12, 100024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, S.A.; Clausen, M.; Knulst, A.C.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Bieli, C.; Dubakiene, R.; Fernandez-Perez, C.; Jedrzejczak-Czechowicz, M.; et al. Prevalence of Food Sensitization and Food Allergy in Children across Europe. J. Allergy Clin. Immunol. Pract. 2020, 8, 2736–2746.e9. [Google Scholar] [CrossRef]
- Li, J.; Ogorodova, L.M.; Mahesh, P.A.; Wang, M.H.; Fedorova, O.S.; Leung, T.F.; Fernandez-Rivas, M.; Mills, E.C.; Potts, J.; Kummeling, I.; et al. Comparative Study of Food Allergies in Children from China, India, and Russia: The EuroPrevall-INCO Surveys. J. Allergy Clin. Immunol. Pract. 2019, 8, 1349–1358.e16. [Google Scholar] [CrossRef]
- Dai, H.; Wang, F.; Wang, L.; Wan, J.; Xiang, Q.; Zhang, H.; Zhao, W.; Zhang, W. An epidemiological investigation of food allergy among children aged 3 to 6 in an urban area of Wenzhou, China. BMC Pediatr. 2020, 20, 220. [Google Scholar] [CrossRef]
- Caffarelli, C.; Garrubba, M.; Greco, C.; Mastrorilli, C.; Dascola, C.P. Asthma and Food Allergy in Children: Is There a Connection or Interaction? Front. Pediatr. 2016, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Caffarelli, C.; Cavagni, G.; Menzies, I.S.; Bertolini, P.; Atherton, D.J. Elimination diet and intestinal permeability in atopic eczema: A preliminary study. Clin. Exp. Allergy 1993, 23, 28–31. [Google Scholar] [CrossRef]
- Wu, A.Y.; Williams, G.A. Clinical characteristics and pattern of skin test reactivities in shellfish allergy patients in Hong Kong. Allergy Asthma Proc. 2004, 25, 237–242. [Google Scholar]
- Jirapongsananuruk, O.; Sripramong, C.; Pacharn, P.; Udompunturak, S.; Chinratanapisit, S.; Piboonpocanun, S.; Visitsunthorn, N.; Vichyanond, P. Specific allergy to Penaeus monodon (seawater shrimp) or Macrobrachium rosenbergii (freshwater shrimp) in shrimp-allergic children. Clin. Exp. Allergy 2008, 38, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Thalayasingam, M.; Gerez, I.F.A.; Yap, G.C.; Llanora, G.V.; Chia, I.P.; Chua, L.; Lee, C.J.A.O.; Ta, L.D.H.; Cheng, Y.K.; Thong, B.Y.H.; et al. Clinical and immunochemical profiles of food challenge proven or anaphylactic shrimp allergy in tropical Singapore. Clin. Exp. Allergy 2015, 45, 687–697. [Google Scholar] [CrossRef]
- Thong, B.Y.; Arulanandam, S.; Tan, S.C.; Tan, T.C.; Chan, G.Y.; Tan, J.W.; Yeow, M.C.; Tang, C.Y.; Hou, J.; Leong, K.P. Shellfish/crustacean oral allergy syndrome among national service pre-enlistees in Singapore. Asia Pac. Allergy 2018, 8, e18. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P. The Kiss of Death: A Severe Allergic Reaction to a Shellfish Induced by a Good-Night Kiss. Mayo Clin. Proc. 2003, 78, 221–222. [Google Scholar] [CrossRef]
- Dascola, C.P.; Caffarelli, C. Exercise-induced anaphylaxis: A clinical view. Ital. J. Pediatr. 2012, 38, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffarelli, C.; Perrone, F.; Terzi, V. Exercise-induced anaphylaxis related to cuttlefish intake. Eur. J. Pediatr. 1996, 155, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.V.; Swanson, M.C.; Jones, R.T.; Vives, R.; Rodriguez, J.; Yunginger, J.W.; Crespo, J.F. Detection and quantitation of raw fish aeroallergens from an open-air fish market. J. Allergy Clin. Immunol. 2000, 105, 166–169. [Google Scholar] [CrossRef]
- Jeebhay, M.F.; Robins, T.G.; Lehrer, S.B.; Lopata, A.L. Occupational seafood allergy: A review. Occup. Environ. Med. 2001, 58, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Goetz, D.W.; A Whisman, B. Occupational asthma in a seafood restaurant worker: Cross-reactivity of shrimp and scallops. Ann. Allergy Asthma Immunol. 2000, 85, 461–466. [Google Scholar] [CrossRef]
- Leonardi, S.; Pecoraro, R.; Filippelli, M.; Del Giudice, M.M.; Marseglia, G.L.; Salpietro, C.; Arrigo, T.; Stringari, G.; Ricò, S.; La Rosa, M.; et al. Allergic reactions to foods by inhalation in children. Allergy Asthma Proc. 2014, 35, 288–294. [Google Scholar] [CrossRef]
- Lopata, A.L.; Jeebhay, M.F. Airborne Seafood Allergens as a Cause of Occupational Allergy and Asthma. Curr. Allergy Asthma Rep. 2013, 13, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Algarra, A.C.; Miguel, R.A.; de Frutos, F.O.; Romero, F.T. Hand Lesions after Contact with Shellfish: Beyond Patch Testing. Actas Dermosifiliogr. 2021, 112, 274–275. [Google Scholar] [CrossRef]
- Bonlokke, J.H.; Bang, B.; Aasmoe, L.; Rahman, A.M.A.; Syron, L.N.; Andersson, E.; Dahlman-Höglund, A.; Lopata, A.L.; Jeebhay, M. Exposures and Health Effects of Bioaerosols in Seafood Processing Workers—A Position Statement. J. Agromedicine 2019, 24, 441–448. [Google Scholar] [CrossRef]
- Kamath, S.D.; Thomassen, M.R.; Saptarshi, S.R.; Nguyen, H.M.; Aasmoe, L.; Bang, B.E.; Lopata, A.L. Molecular and immunological approaches in quantifying the air-borne food allergen tropomyosin in crab processing facilities. Int. J. Hyg. Environ. Health 2014, 217, 740–750. [Google Scholar] [CrossRef]
- Jeebhay, M.F.; Moscato, G.; Bang, B.E.; Folletti, I.; Lipińska-Ojrzanowska, A.; Lopata, A.L.; Pala, G.; Quirce, S.; Raulf, M.; Sastre, J.; et al. Food processing and occupational respiratory allergy—An EAACI position paper. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 1852–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baynova, K.; Leguísamo, S.; Bartolomé, B.; Prados, M. Occupational allergic contact urticaria caused by the crustaceans Palaemon serratus and Procambarus clarkii. Contact Dermat. 2015, 73, 53–54. [Google Scholar] [CrossRef]
- Zotova, V.; Clarke, A.E.; Chan, E.S.; Asai, Y.; Chin, R.; Van Lambalgen, C.; Harada, L.; Ben-Shoshan, M. Low resolution rates of seafood allergy. J. Allergy Clin. Immunol. Pract. 2018, 7, 690–692. [Google Scholar] [CrossRef]
- Ittiporn, S.; Pacharn, P.; Visitsunthorn, N.; Vichyanond, P.; Jirapongsananuruk, O. The Natural History of Shrimp Allergy in Thai Patients. J. Allergy Clin. Immunol. 2017, 139, AB131. [Google Scholar] [CrossRef]
- WHO/IUIS; Allergen Nomenclature Sub-Committee. Allergen Nomenclature. Available online: http://allergen.org/ (accessed on 9 July 2023).
- Reese, G.; Ayuso, R.; Lehrer, S.B. Tropomyosin: An Invertebrate Pan–Allergen. Int. Arch. Allergy Immunol. 1999, 119, 247–258. [Google Scholar] [CrossRef]
- Rolland, J.M.; Varese, N.P.; Abramovitch, J.B.; Anania, J.; Nugraha, R.; Kamath, S.; Hazard, A.; Lopata, A.L.; O’Hehir, R.E. Effect of Heat Processing on IgE Reactivity and Cross-Reactivity of Tropomyosin and Other Allergens of Asia-Pacific Mollusc Species: Identification of Novel Sydney Rock Oyster Tropomyosin Sac g 1. Mol. Nutr. Food Res. 2018, 62, e1800148. [Google Scholar] [CrossRef] [Green Version]
- Kamath, S.D.; Rahman, A.M.A.; Komoda, T.; Lopata, A.L. Impact of heat processing on the detection of the major shellfish allergen tropomyosin in crustaceans and molluscs using specific monoclonal antibodies. Food Chem. 2013, 141, 4031–4039. [Google Scholar] [CrossRef] [PubMed]
- Gámez, C.; Zafra, M.P.; Sanz, V.; Mazzeo, C.; Ibáñez, M.D.; Sastre, J.; del Pozo, V. Simulated gastrointestinal digestion reduces the allergic reactivity of shrimp extract proteins and tropomyosin. Food Chem. 2015, 173, 475–481. [Google Scholar] [CrossRef]
- Liu, G.-M.; Huang, Y.-Y.; Cai, Q.-F.; Weng, W.-Y.; Su, W.-J.; Cao, M.-J. Comparative study of in vitro digestibility of major allergen, tropomyosin and other proteins between Grass prawn (Penaeus monodon) and Pacific white shrimp (Litopenaeus vannamei). J. Sci. Food Agric. 2010, 91, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, K.; Wangorsch, A.; Garoffo, L.P.; Reuter, A.; Conti, A.; Taylor, S.L.; Lidholm, J.; DeWitt, Å.M.; Enrique, E.; Vieths, S.; et al. Generation of a comprehensive panel of crustacean allergens from the North Sea Shrimp Crangon crangon. Mol. Immunol. 2011, 48, 1983–1992. [Google Scholar] [CrossRef]
- Gámez, C.; Sánchez-García, S.; Ibáñez, M.D.; López, R.; Aguado, E.; López, E.; Sastre, B.; Sastre, J.; del Pozo, V. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy 2011, 66, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, R.; Sánchez-Garcia, S.; Lin, J.; Fu, Z.; Ibáñez, M.D.; Carrillo, T.; Blanco, C.; Goldis, M.; Bardina, L.; Sastre, J.; et al. Greater epitope recognition of shrimp allergens by children than by adults suggests that shrimp sensitization decreases with age. J. Allergy Clin. Immunol. 2010, 125, 1286–1293.e3. [Google Scholar] [CrossRef] [PubMed]
- Ruethers, T.; Taki, A.C.; Johnston, E.; Nugraha, R.; Le, T.T.K.; Kalic, T.; McLean, T.; Kamath, S.D.; Lopata, A.L. Seafood allergy: A comprehensive review of fish and shellfish allergens. Mol. Immunol. 2018, 100, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Huang, C.H.; Lee, B.W. Shellfish and House Dust Mite Allergies: Is the Link Tropomyosin? Allergy Asthma Immunol. Res. 2016, 8, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, I.; del Barrio, M.; Vidarte, L.; del Pozo, V.; Pastor, C.; Gonzalez-Cabrero, J.; Cárdaba, B.; Rojo, M.; Mínguez, A.; Cortegano, I.; et al. Molecular cloning and characterization of an IgE-reactive protein from Anisakis simplex: Ani s 1. Mol. Biochem. Parasitol. 2000, 107, 263–268. [Google Scholar] [CrossRef]
- Leung, P.S.; Chen, Y.C.; Mykles, D.L.; Chow, W.K.; Li, C.P.; Chu, K.H. Molecular identification of the lobster muscle protein tropomyosin as a seafood allergen. Mol. Mar. Biol. Biotechnol. 1998, 7, 12–20. [Google Scholar]
- Khor, S.-S.; Morino, R.; Nakazono, K.; Kamitsuji, S.; Akita, M.; Kawajiri, M.; Yamasaki, T.; Kami, A.; Hoshi, Y.; Tada, A.; et al. Genome-wide association study of self-reported food reactions in Japanese identifies shrimp and peach specific loci in the HLA-DR/DQ gene region. Sci. Rep. 2018, 8, 1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayuso, R.; Reese, G.; Leong-Kee, S.; Plante, M.; Lehrer, S.B. Molecular Basis of Arthropod Cross-Reactivity: IgE-Binding Cross-Reactive Epitopes of Shrimp, House Dust Mite and Cockroach Tropomyosins. Int. Arch. Allergy Immunol. 2002, 129, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.G.; Villalta, D.; Mistrello, G.; Amato, S.; Asero, R. Shrimp allergy beyond tropomyosin in Italy: Clinical rele-vance of arginine kinase, sarcoplasmic calcium binding protein and hemocyanin. Eur. Ann. Allergy Clin. Immunol. 2014, 46, 172–177. [Google Scholar] [PubMed]
- Chen, H.-L.; Mao, H.-Y.; Cao, M.-J.; Cai, Q.-F.; Su, W.-J.; Zhang, Y.-X.; Liu, G.-M. Purification, physicochemical and immunological characterization of arginine kinase, an allergen of crayfish (Procambarus clarkii). Food Chem. Toxicol. 2013, 62, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.D.; Rahman, A.M.A.; Voskamp, A.; Komoda, T.; Rolland, J.M.; O’Hehir, R.E.; Lopata, A.L. Effect of heat processing on antibody reactivity to allergen variants and fragments of black tiger prawn: A comprehensive allergenomic approach. Mol. Nutr. Food Res. 2014, 58, 1144–1155. [Google Scholar] [CrossRef]
- Gautrin, D.; Cartier, A.; Howse, D.; Horth-Susin, L.; Jong, M.; Swanson, M.; Lehrer, S.; Fox, G.; Neis, B. Occupational asthma and allergy in snow crab processing in Newfoundland and Labrador. Occup. Environ. Med. 2009, 67, 17–23. [Google Scholar] [CrossRef]
- Rahman, A.M.A.; Kamath, S.D.; Gagné, S.; Lopata, A.L.; Helleur, R. Comprehensive Proteomics Approach in Characterizing and Quantifying Allergenic Proteins from Northern Shrimp: Toward Better Occupational Asthma Prevention. J. Proteome Res. 2013, 12, 647–656. [Google Scholar] [CrossRef]
- Bose, U.; Broadbent, J.A.; Juhász, A.; Karnaneedi, S.; Johnston, E.B.; Stockwell, S.; Byrne, K.; Limviphuvadh, V.; Maurer-Stroh, S.; Lopata, A.L.; et al. Protein extraction protocols for optimal proteome measurement and arginine kinase quantitation from cricket Acheta domesticus for food safety assessment. Food Chem. 2021, 348, 129110. [Google Scholar] [CrossRef]
- Ayuso, R.; Grishina, G.; Bardina, L.; Carrillo, T.; Blanco, C.; Ibáñez, M.D.; Sampson, H.A.; Beyer, K. Myosin light chain is a novel shrimp allergen, Lit v 3. J. Allergy Clin. Immunol. 2008, 122, 795–802. [Google Scholar] [CrossRef]
- Pascal, M.; Grishina, G.; Yang, A.C.; Sánchez-García, S.; Lin, J.; Towle, D.; Ibañez, M.D.; Sastre, J.; Sampson, H.A.; Ayuso, R. Molecular Diagnosis of Shrimp Allergy: Efficiency of Several Allergens to Predict Clinical Reactivity. J. Allergy Clin. Immunol. Pract. 2015, 3, 521–529.e10. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Chen, H.-L.; Maleki, S.J.; Cao, M.-J.; Zhang, L.-J.; Su, W.-J.; Liu, G.-M. Purification, Characterization, and Analysis of the Allergenic Properties of Myosin Light Chain in Procambarus clarkii. J. Agric. Food Chem. 2015, 63, 6271–6282. [Google Scholar] [CrossRef] [PubMed]
- Gelis, S.; Rueda, M.; Valero, A.; Fernández, E.; Moran, M.; Fernández-Caldas, E. Shellfish Allergy: Unmet Needs in Diagnosis and Treatment. J. Investig. Allergol. Clin. Immunol. 2020, 30, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Cao, M.-J.; Cai, Q.-F.; Su, W.-J.; Mao, H.-Y.; Liu, G.-M. Purification and characterisation of sarcoplasmic calcium-binding protein, a novel allergen of red swamp crayfish (Procambarus clarkii). Food Chem. 2013, 139, 213–223. [Google Scholar] [CrossRef]
- Shiomi, K.; Sato, Y.; Hamamoto, S.; Mita, H.; Shimakura, K. Sarcoplasmic Calcium-Binding Protein: Identification as a New Allergen of the Black Tiger Shrimp Penaeus monodon. Int. Arch. Allergy Immunol. 2008, 146, 91–98. [Google Scholar] [CrossRef]
- Ayuso, R.; Grishina, G.; Ibáñez, M.D.; Blanco, C.; Carrillo, T.; Bencharitiwong, R.; Sánchez, S.; Nowak-Wegrzyn, A.; Sampson, H.A. Sarcoplasmic calcium-binding protein is an EF-hand–type protein identified as a new shrimp allergen. J. Allergy Clin. Immunol. 2009, 124, 114–120. [Google Scholar] [CrossRef]
- Chao, E.; Kim, H.-W.; Mykles, D.L. Cloning and tissue expression of eleven troponin-C isoforms in the American lobster, Homarus americanus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 157, 88–101. [Google Scholar] [CrossRef]
- Hindley, J.; Wunschmann, S.; Satinover, S.; Woodfolk, J.; Chew, F.; Chapman, M.; Pomes, A. Bla g 6: A troponin C allergen from Blattella germanica with IgE binding calcium dependence. J. Allergy Clin. Immunol. 2006, 117, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y.; Kim, C.-R.; Un, S.; Yi, M.-H.; Lee, I.-Y.; Park, J.W.; Hong, C.-S.; Yong, T.-S. Allergenicity of Recombinant Troponin C from Tyrophagus putrescentiae. Int. Arch. Allergy Immunol. 2009, 151, 207–213. [Google Scholar] [CrossRef]
- Chuang, J.-G.; Su, S.-N.; Chiang, B.-L.; Lee, H.-J.; Chow, L.-P. Proteome mining for novel IgE-binding proteins from the German cockroach (Blattella germanica) and allergen profiling of patients. Proteomics 2010, 10, 3854–3867. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.-W.; Hurlburt, B.K.; Li, G.-L.; Zhang, Y.-X.; Fei, D.-X.; Shen, H.-W.; Cao, M.-J.; Liu, G.-M. Identification of triosephosphate isomerase as a novel allergen in Octopus fangsiao. Mol. Immunol. 2017, 85, 35–46. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.; Fernández-Caldas, E.; Marañón, F.; Sastre, J.; Bernal, M.L.; Rodríguez, J.; Bedate, C.A. Molecular Cloning of Paramyosin, a New Allergen of Anisakis simplex. Int. Arch. Allergy Immunol. 2000, 123, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.M.A.; Kamath, S.D.; Lopata, A.L.; Robinson, J.J.; Helleur, R.J. Biomolecular characterization of allergenic proteins in snow crab (Chionoecetes opilio) and de novo sequencing of the second allergen arginine kinase using tandem mass spectrometry. J. Proteom. 2011, 74, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gámez, C.; Zafra, M.P.; Boquete, M.; Sanz, V.; Mazzeo, C.; Ibáñez, M.D.; Sánchez-García, S.; Sastre, J.; del Pozo, V. New shrimp IgE-binding proteins involved in mite-seafood cross-reactivity. Mol. Nutr. Food Res. 2014, 58, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Khurana, T.; Collison, M.; Chew, F.T.; Slater, J.E. Blag 3: A novel allergen of German cockroach identified using cockroach-specific avian single-chain variable fragment antibody. Ann. Allergy Asthma Immunol. 2014, 112, 140–145.e1. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, L.; Li, S.; Zhang, J.; She, T.; Yan, J.; Bian, Y.; Li, H. Identification of the major allergenic epitopes of Eriocheir sinensis roe hemocyanin: A novel tool for food allergy diagnoses. Mol. Immunol. 2016, 74, 125–132. [Google Scholar] [CrossRef]
- Khanaruksombat, S.; Srisomsap, C.; Chokchaichamnankit, D.; Punyarit, P.; Phiriyangkul, P. Identification of a novel allergen from muscle and various organs in banana shrimp (Fenneropenaeus merguiensis). Ann. Allergy Asthma Immunol. 2014, 113, 301–306. [Google Scholar] [CrossRef]
- Piboonpocanun, S.; Jirapongsananuruk, O.; Tipayanon, T.; Boonchoo, S.; Goodman, R.E. Identification of hemocyanin as a novel non-cross-reactive allergen from the giant freshwater shrimp Macrobrachium rosenbergii. Mol. Nutr. Food Res. 2011, 55, 1492–1498. [Google Scholar] [CrossRef]
- Bessot, J.C.; Metz-Favre, C.; Rame, J.M.; De Blay, F.; Pauli, G. Tropomyosin or not tropomyosin, what is the relevant allergen in house dust mite and snail cross allergies? Eur. Ann. Allergy Clin. Immunol. 2010, 42, 3–10. [Google Scholar]
- Zhang, Y.; Matsuo, H.; Morita, E. Cross-reactivity among shrimp, crab and scallops in a patient with a seafood allergy. J. Dermatol. 2006, 33, 174–177. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Hong, C.-S.; Yong, T.-S. Allergenic tropomyosins and their cross-reactivities. Protein Pept. Lett. 2006, 13, 835–845. [Google Scholar] [CrossRef]
- Crespo, J.F.; Pascual, C.; Helm, R.; Sanchez-Pastor, S.; Ojeda, I.; Romualdo, L.; Martin-Esteban, M.; Ojeda, J.A. Cross-reactivity of IgE-binding components between boiled Atlantic shrimp and German cockroach. Allergy 1995, 50, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.S.; Chow, W.K.; Duffey, S.; Kwan, H.S.; Gershwin, M.; Chu, K.H. IgE reactivity against a cross-reactive allergen in crustacea and mollusca: Evidence for tropomyosin as the common allergen. J. Allergy Clin. Immunol. 1996, 98, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; Eigenmann, P.A.; Sicherer, S.H. Clinical Relevance of Cross-Reactivity in Food Allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Azofra, J.; Echechipía, S.; Irazábal, B.; Muñoz, D.; Bernedo, N.; García, B.; Gastaminza, G.; Goikoetxea, M.; Joral, A.; Lasa, E.; et al. Heterogeneity in Allergy to Mollusks: A Clinical-Immunological Study in a Population From the North of Spain. J. Investig. Allergol. Clin. Immunol. 2017, 27, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thalayasingam, M.; Loo, E.; Tan, M.; Bever, H.; Shek, L. A review of oral food challenges in children presenting to a single tertiary centre with perceived or true food allergies. Singap. Med. J. 2015, 56, 622–625. [Google Scholar] [CrossRef] [Green Version]
- Chokshi, N.Y.; Maskatia, Z.K.; Miller, S.; Guffey, D.; Minard, C.G.; Davis, C.M. Risk factors in pediatric shrimp allergy. Allergy Asthma Proc. 2015, 36, 65–71. [Google Scholar] [CrossRef]
- Pedrosa, M.; Boyano-Martínez, T.; García-Ara, C.; Quirce, S. Shellfish Allergy: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2014, 49, 203–216. [Google Scholar] [CrossRef]
- Boquete, M.; Iraola, V.; Morales, M.; Pinto, H.; Francisco, C.; Carballás, C.; Carnés, J. Seafood hypersensitivity in mite sensitized individuals: Is tropomyosin the only responsible allergen? Ann. Allergy Asthma Immunol. 2011, 106, 223–229. [Google Scholar] [CrossRef]
- Wang, J.; Calatroni, A.; Visness, C.M.; Sampson, H.A. Correlation of specific IgE to shrimp with cockroach and dust mite exposure and sensitization in an inner-city population. J. Allergy Clin. Immunol. 2011, 128, 834–837. [Google Scholar] [CrossRef]
- Chiang, W.C.; Kidon, M.I.; Liew, W.K.; Goh, A.; Tang, J.P.L.; Chay, O.M. The changing face of food hypersensitivity in an Asian community. Clin. Exp. Allergy 2007, 37, 1055–1061. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, J.; Wei, N.; Feng, M.; Xian, M.; Shi, X.; Zheng, Z.; Su, Q.; Wong, G.W.K.; Li, J. Cockroach is a major cross-reactive allergen source in shrimp-sensitized rural children in southern China. Allergy 2017, 73, 585–592. [Google Scholar] [CrossRef]
- Chomchai, S.; Laoraksa, P.; Virojvatanakul, P.; Boonratana, P.; Chomchai, C. Prevalence and cluster effect of self-reported allergic reactions among insect consumers. Asian Pac. J. Allergy Immunol. 2020, 38, 40–46. [Google Scholar] [CrossRef]
- Beaumont, P.; Courtois, J.; Van der Brempt, X.; Tollenaere, S. Food-induced anaphylaxis to Tenebrio molitor and allergens implicated. Rev. Française D’allergologie 2019, 59, 389–393. [Google Scholar] [CrossRef]
- Broekman, H.; Verhoeckx, K.C.; Jager, C.F.D.H.; Kruizinga, A.G.; Pronk-Kleinjan, M.; Remington, B.; Bruijnzeel-Koomen, C.A.; Houben, G.F.; Knulst, A.C. Majority of shrimp-allergic patients are allergic to mealworm. J. Allergy Clin. Immunol. 2016, 137, 1261–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peixoto, S.; Monteiro, T.; Carvalho, M.; Santos, M.; Matos, C.; Bartolomé, B.; Labrador-Horrillo, M.; Quaresma, M. Vertebrate Tropomyosin as an Allergen. J. Investig. Allergol. Clin. Immunol. 2018, 28, 51–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Fernández, J.; Alguacil-Guillén, M.; Cuéllar, C.; Daschner, A. Possible Allergenic Role of Tropomyosin in Patients with Adverse Reactions after Fish Intake. Immunol. Investig. 2018, 47, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Dondi, A.; Dascola, C.P.; Ricci, G. Skin prick test to foods in childhood atopic eczema: Pros and cons. Ital. J. Pediatr. 2013, 39, 48. [Google Scholar] [CrossRef] [Green Version]
- Asero, R.; Scala, E.; Villalta, D.; Pravettoni, V.; Arena, A.; Billeri, L.; Colombo, G.; Cortellini, G.; Cucinelli, F.; De Cristofaro, M.L.; et al. Shrimp Allergy: Analysis of Commercially Available Extracts for In Vivo Diagnosis. J. Investig. Allergol. Clin. Immunol. 2017, 27, 175–182. [Google Scholar] [CrossRef]
- Yang, A.C.; Arruda, L.K.; Santos, A.B.R.; Barbosa, M.C.; Chapman, M.D.; Galvão, C.E.; Kalil, J.; Morato-Castro, F.F. Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion. J. Allergy Clin. Immunol. 2010, 125, 872–878. [Google Scholar] [CrossRef]
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat Allergy in Children: A Comprehensive Update. Medicina 2019, 55, 400. [Google Scholar] [CrossRef] [Green Version]
- Wai, C.Y.; Leung, N.Y.; Leung, A.S.; Shum, Y.; Leung, P.S.; Chu, K.H.; Kwan, Y.W.; Lee, Q.U.; Wong, J.S.; Lam, I.C.; et al. Cell-Based Functional IgE Assays Are Superior to Conventional Allergy Tests for Shrimp Allergy Diagnosis. J. Allergy Clin. Immunol. Pract. 2020, 9, 236–244.e9. [Google Scholar] [CrossRef] [PubMed]
- Tsedendorj, O.; Chinuki, Y.; Ueda, K.; Kohno, K.; Adachi, A.; Morita, E. Tropomyosin is a minor but distinct allergen in patients with shrimp allergies in Japan. J. Cutan. Immunol. Allergy 2018, 1, 100–108. [Google Scholar] [CrossRef]
- Asero, R.; Mistrello, G.; Amato, S.; Ariano, R.; Colombo, G.; Conte, M.; Crivellaro, M.; De Carli, M.; Della Torre, F.; Emiliani, F.; et al. Shrimp Allergy in Italian Adults: A Multicenter Study Showing a High Prevalence of Sensitivity to Novel High Molecular Weight Allergens. Int. Arch. Allergy Immunol. 2011, 157, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.Y.; Leung, N.Y.H.; Leung, A.S.Y.; Ngai, S.M.; Pacharn, P.; Yau, Y.S.; Duque, J.S.D.R.; Kwan, M.Y.W.; Jirapongsananuruk, O.; Chan, W.H.; et al. Comprehending the allergen repertoire of shrimp for precision molecular diagnosis of shrimp allergy. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 3041–3051. [Google Scholar] [CrossRef]
- Jarupalee, T.; Chatchatee, P.; Komolpis, K.; Suratannon, N.; Roytrakul, S.; Yingchutrakul, Y.; Yimchuen, W.; Butta, P.; Jacquet, A.; Palaga, T. Detecting Allergens From Black Tiger Shrimp Penaeus monodon That Can Bind and Cross-link IgE by ELISA, Western Blot, and a Humanized Rat Basophilic Leukemia Reporter Cell Line RS-ATL8. Allergy, Asthma Immunol. Res. 2018, 10, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Wai, C.Y.; Leung, P.S. Emerging approaches in the diagnosis and therapy in shellfish allergy. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 202–212. [Google Scholar] [CrossRef]
- Bird, J.A.; Leonard, S.; Groetch, M.; Assa’Ad, A.; Cianferoni, A.; Clark, A.; Crain, M.; Fausnight, T.; Fleischer, D.; Green, T.; et al. Conducting an Oral Food Challenge: An Update to the 2009 Adverse Reactions to Foods Committee Work Group Report. J. Allergy Clin. Immunol. Pract. 2020, 8, 75–90.e17. [Google Scholar] [CrossRef]
- Sampson, H.A.; van Wijk, R.G.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.J.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology–European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar] [CrossRef]
- Caffarelli, C.; Franceschini, F.; Caimmi, D.; Mori, F.; Diaferio, L.; Di Mauro, D.; Mastrorilli, C.; Arasi, S.; Barni, S.; Bottau, P.; et al. SIAIP position paper: Provocation challenge to antibiotics and non-steroidal anti-inflammatory drugs in children. Ital. J. Pediatr. 2018, 44, 147. [Google Scholar] [CrossRef] [Green Version]
- Agyemang, A.; Nowak-Wegrzyn, A. Food Protein-Induced Enterocolitis Syndrome: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2019, 57, 261–271. [Google Scholar] [CrossRef]
- Rahmati, A.R.; Kiani, B.; Afshari, A.; Moghaddas, E.; Williams, M.; Shamsi, S. World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: A systematic review. Parasitol. Res. 2020, 119, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Guardone, L.; Armani, A.; Nucera, D.; Costanzo, F.; Mattiucci, S.; Bruschi, F. Human anisakiasis in Italy: A retrospective epidemiological study over two decades. Parasite 2018, 25, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-J.; Lee, J.-C.; Kim, M.-J.; Hur, G.-Y.; Shin, S.-Y.; Park, H.-S. The Clinical Characteristics of Anisakis Allergy in Korea. Korean J. Intern. Med. 2009, 24, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Baird, F.J.; Gasser, R.B.; Jabbar, A.; Lopata, A.L. Foodborne anisakiasis and allergy. Mol. Cell. Probes 2014, 28, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Audicana, M.T. Anisakis, Something Is Moving inside the Fish. Pathogens 2022, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Morishima, R.; Motojima, S.; Tsuneishi, D.; Kimura, T.; Nakashita, T.; Fudouji, J.; Ichikawa, S.; Ito, H.; Nishino, H. Anisakis is a major cause of anaphylaxis in seaside areas: An epidemiological study in Japan. Allergy Eur. J. Allergy Clin. Immunol. 2019, 75, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Moneo, I.; Carballeda-Sangiao, N.; González-Muñoz, M. New Perspectives on the Diagnosis of Allergy to Anisakis spp. Curr. Allergy Asthma Rep. 2017, 17, 27. [Google Scholar] [CrossRef]
- Mines, D.; Stahmer, S.; Shepherd, S.M. POISONINGS: Food, fish, shellfish. Emerg. Med. Clin. N. Am. 1997, 15, 157–177. [Google Scholar] [CrossRef]
- Balaban, N.; Rasooly, A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000, 61, 1–10. [Google Scholar] [CrossRef]
- Marcus, E.N.; Burns, M.M. Overview of Shellfish, Pufferfish, and Other Marine toxin Poisoning; UpToDate: Waltham, MA, USA, 2022. [Google Scholar]
- Refaat, M.M.; Attia, M.Y.; Saber, H.M. Desensitization Efficacy by Sublingual Immunotherapy of Shrimps Extract in Asthmatic, Rhinitis and Urticaria Allergic Patients. Food Nutr. Sci. 2014, 05, 1704–1710. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.I.; Sindher, S.B.; Chinthrajah, R.S.; Nadeau, K.; Davis, C.M. Shrimp-allergic patients in a multi-food oral immunotherapy trial. Pediatr. Allergy Immunol. 2021, 33, e13679. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.Y.H.; Wai, C.Y.Y.; Shu, S.A.; Chu, K.H.; Chang, C.C.; Leung, P.S. Low-Dose Allergen-Specific Immunotherapy Induces Tolerance in a Murine Model of Shrimp Allergy. Int. Arch. Allergy Immunol. 2017, 174, 86–96. [Google Scholar] [CrossRef]
- Wai, C.Y.Y.; Leung, N.Y.H.; Ho, M.H.K.; Gershwin, L.J.; Shu, S.A.; Leung, P.S.C.; Chu, K.H. Immunization with Hypoallergens of Shrimp Allergen Tropomyosin Inhibits Shrimp Tropomyosin Specific IgE Reactivity. PLoS ONE 2014, 9, e111649. [Google Scholar] [CrossRef] [PubMed]
- Reese, G.; Viebranz, J.; Leong-Kee, S.M.; Plante, M.; Lauer, I.; Randow, S.; Moncin, M.S.-M.; Ayuso, R.; Lehrer, S.B.; Vieths, S. Reduced Allergenic Potency of VR9-1, a Mutant of the Major Shrimp Allergen Pen a 1 (Tropomyosin). J. Immunol. 2005, 175, 8354–8364. [Google Scholar] [CrossRef] [Green Version]
- Lopata, A.L.; O’Hehir, R.E.; Lehrer, S.B. Shellfish allergy. Clin. Exp. Allergy 2010, 40, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Ravkov, E.V.; Pavlov, I.Y.; Martins, T.B.; Gleich, G.J.; Wagner, L.A.; Hill, H.R.; Delgado, J.C. Identification and validation of shrimp-tropomyosin specific CD4 T cell epitopes. Hum. Immunol. 2013, 74, 1542–1549. [Google Scholar] [CrossRef] [Green Version]
- Wai, C.Y.Y.; Leung, N.Y.H.; Leung, P.S.C.; Chu, K.H. T cell epitope immunotherapy ameliorates allergic responses in a murine model of shrimp allergy. Clin. Exp. Allergy 2016, 46, 491–503. [Google Scholar] [CrossRef]
- Renand, A.; Newbrough, S.; Wambre, E.; DeLong, J.H.; Robinson, D.; Kwok, W.W. Arginine kinase Pen m 2 as an important shrimp allergen recognized by T H 2 cells. J. Allergy Clin. Immunol. 2014, 134, 1456–1459. [Google Scholar] [CrossRef]
- Gao, Q.; Hong, J.; Xiao, X.; Cao, H.; Yuan, R.; Liu, Z.; Chen, T. T cell epitope of arginine kinase with CpG co-encapsulated nanoparticles attenuates a shrimp allergen-induced Th2-bias food allergy. Biosci. Biotechnol. Biochem. 2019, 84, 804–814. [Google Scholar] [CrossRef]
- Pevec, B.; Pevec, M.R.; Markovic, A.S.; Batista, I. House dust mite subcutaneous immunotherapy does not induce new sensitization to tropomyosin: Does it do the opposite? J. Investig. Allergol. Clin. Immunol. 2014, 24, 29–34. [Google Scholar]
- Cortellini, G.; Spadolini, I.; Santucci, A.; Cova, V.; Conti, C.; Corvetta, A.; Passalacqua, G. Improvement of shrimp allergy after sublingual immunotherapy for house dust mites: A case report. Eur. Ann. Allergy Clin. Immunol. 2011, 43, 162–164. [Google Scholar] [PubMed]
- Peroni, D.; Piacentini, G.L.; Bodini, A.; Boner, A.L. Snail anaphylaxis during house dust mite immunotherapy. Pediatr. Allergy Immunol. 2000, 11, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Ree, R.; Antonicelli, L.; Akkerdaas, J.H.; Garritani, M.S.; Aalberse, R.C.; Bonifazi, F. Possible induction of food allergy during mite immunotherapy. Allergy 1996, 51, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Van Ree, R.; Antonicelli, L.; Akkerdaas, J.H.; Pajno, G.B.; Barberio, G.; Corbetta, L.; Ferro, G.; Zambito, M.; Garritani, M.S.; Aalberse, R.C.; et al. Asthma after consumption of snails in house-dust-mite-allergic patients: A case of IgE cross-reactivity. Allergy Eur. J. Allergy Clin. Immunol. 1996, 51, 387–393. [Google Scholar] [CrossRef]
| Common Name | Scientific Name | Allergen | Protein Type | Molecular Weight (kDa) |
|---|---|---|---|---|
| a. Crustacea allergens | ||||
| SHRIMP | ||||
| North Sea shrimp | Crangon crangon | Cra c 1 | Tropomyosin | 38 |
| Cra c 2 | Arginine kinase | 45 | ||
| Cra c 4 | Sarcoplasmic calcium-binding protein | 25 | ||
| Cra c 5 | Myosin light chain 1 | 17.5 | ||
| Cra c 6 | Troponin C | 21 | ||
| Cra c 8 | Triosephosphate isomerase | 28 | ||
| White shrimp | Litopenaeus vannamei | Lit v 1 | Tropomyosin | 36 |
| Lit v 2 | Arginine kinase | 40 | ||
| Lit v 3 | Myosin light chain 2 | 20 | ||
| Lit v 4 | Sarcoplasmic calcium-binding protein | 20 | ||
| Lit v 13 | Fatty acid-binding protein | 15 | ||
| Black tiger shrimp | Penaeus monodon | Pen m 1 | Tropomyosin | 38 |
| Pen m 2 | Arginine kinase | 34 | ||
| Pen m 3 | Myosin light chain 2 | 20 | ||
| Pen m 4 | Sarcoplasmic calcium-binding protein | 20 | ||
| Pen m 6 | Troponin C | 16.8 | ||
| Pen m 7 | Hemocyanin | 76 | ||
| Pen m 8 | Triosephosphate isomerase | 27 | ||
| Pen m 13 | Cytoplasmic fatty acid-binding protein | 20 | ||
| Pen m 14 | Glycogen phosphorylase-like protein | 95 | ||
| Brown shrimp | Penaeus aztecus | Pen a 1 | Tropomyosin | 36 |
| Shrimp | Penaeus indicus | Pen i 1 | Tropomyosin | 34 |
| White-legged freshwater shrimp | Exopalaemon modestus | Exo m 1 | Tropomyosin | 38 |
| Shrimp | Metapenaeus ensis | Met e 1 | Tropomyosin | 34 |
| Northern shrimp | Pandalus borealis | Pan b 1 | Tropomyosin | 37 |
| CRAB | ||||
| Mud crab | Scylla paramamosain | Scy p 1 | Tropomyosin | 38 |
| Scy p 2 | Arginine kinase | 40 | ||
| Scy p 3 | Myosin light chain | 18 | ||
| Scy p 4 | Sarcoplasmic Ca+ binding protein | 20 | ||
| Scy p 8 | Triosephosphate isomerase | 28 | ||
| Scy p 9 | Filamin C | 90 | ||
| Warrior swimming brown crab | Callinectes bellicosus | Cal b 2 | Arginine kinase | 40 |
| Crab | Charybdis feriatus | Cha f 1 | Tropomyosin | 34 |
| Chinese mitten crab | Eriocheir sinensis | Eri s 2 | Ovary development-related protein | 28.2 |
| Blue swimmer crab | Portunus pelagicus | Por p 1 | Tropomyosin | 39 |
| LOBSTER | ||||
| American lobster | Homarus americanus | Hom a 1 | Tropomyosin | 34 |
| Hom a 3 | Myosin light chain 2 | 23 | ||
| Hom a 6 | Troponin C | 20 | ||
| Spiny lobster | Panulirus stimpsoni | Pan s 1 | Tropomyosin | 34 |
| b. Mollusca allergens | ||||
| BIVALVIA | ||||
| Pacific oyster | Crassostrea gigas | Cra g 1 | Tropomyosin | 38 |
| Pacific oyster | Crassostrea angulata | Cra a 2 | Arginine kinase | 38 |
| Cockle | Fulvia mutica | Cra a 4 | Sarcoplasmic calcium-binding protein | 20–25 |
| Sydney rock oyster | Saccostrea glomerata | Sac g 1 | Tropomyosin | 38 |
| GASTEROPODA | ||||
| Brown garden snail | Helix aspersa | Hel as 1 | Paramyosin | 99 |
| Veined rapa whelk | Rapana venosa | Rap v 2 | Paramyosin | |
| CEFALOPODA | ||||
| Perlemoen abalone | Haliotes midae | Hal m 1 | Tropomyosin | 49 |
| Jade tiger abaolone | Haliotis laevigata, Haliotis rubra | Hal l 1 | Tropomyosin | 33.4 |
| Japanese flying squid | Todarodes pacificus | Tod p 1 | Tropomyosin | 38 |
| Name | Affected Shellfish | Cause | Onset (h after Ingestion) | Clinical Findings |
|---|---|---|---|---|
| IMMUNOLOGICAL REACTIONS | ||||
| IgE-mediated shellfish allergy | Crustaceans and mollusks | IgE-mediated adverse reaction to shellfish | Minutes–4 h | Oral allergy syndrome, urticaria, rhinitis, nausea, vomiting, anaphylaxis |
| Food Protein-Induced Enterocolitis Syndrome (FPIES) | Crustaceans and mollusks | T-cell-mediated intestinal inflammation (not clearly understood pathogenesis) | 1–4 h | Profuse vomiting, diarrhea, sepsis-like picture |
| Anisakis allergy | Crustaceans and mollusks | IgE-mediated adverse reaction to Anisakis infesting seafood | 2–24 h | Urticaria, angioedema, abdominal pain, anaphylaxis |
| SHELLFISH CONTAMINATION | ||||
| Staphylococcus aureus food poisoning | Crustaceans and mollusks | Ingestion of fish contaminated by hands at room temperature | 1–6 h | Nausea, vomiting, abdominal pain, fever |
| Bacterial or viral contamination (e.g., Vibrio Cholerae, Hepatitis A) | Crustaceans and mollusks | Ingestion of raw fish harvested in contaminated waters | 2–24 h | Nausea, vomiting, diarrhea, abdominal pain, fever |
| Anisakiasis | Crustaceans and mollusks | Ingestion of raw, undercooked or pickled fish with alive parasites (Anisakis) | 2–24 h | Nausea, vomiting, abdominal pain |
| SHELLFISH POISONING | ||||
| Paralytic shellfish poisoning | Bivalve mollusks | Saxitoxin formed by algae | 1–2 h | Paresthesias, dizziness, ataxia |
| Neurotoxic shellfish poisoning | Bivalve mollusks | Brevetoxin formed by algae | 3–4 h | Nausea, vomiting, diarrhea, abdominal pain Paresthesias, dizziness, ataxia rhinorrhea, bronchoconstriction |
| Diarrhetic shellfish poisoning | Bivalve mollusks | Okadaic acid formed by algae | 1–15 h | Nausea, vomiting, diarrhea, abdominal pain |
| Amnesic shellfish poisoning | Bivalve mollusks | Domoic acid formed by algae | 24–48 h | Disorientation, amnesia, headache, diarrhea, abdominal pain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetti, A.; Pession, A.; Bettini, I.; Ricci, G.; Giannì, G.; Caffarelli, C. IgE Mediated Shellfish Allergy in Children—A Review. Nutrients 2023, 15, 3112. https://doi.org/10.3390/nu15143112
Giannetti A, Pession A, Bettini I, Ricci G, Giannì G, Caffarelli C. IgE Mediated Shellfish Allergy in Children—A Review. Nutrients. 2023; 15(14):3112. https://doi.org/10.3390/nu15143112
Chicago/Turabian StyleGiannetti, Arianna, Andrea Pession, Irene Bettini, Giampaolo Ricci, Giuliana Giannì, and Carlo Caffarelli. 2023. "IgE Mediated Shellfish Allergy in Children—A Review" Nutrients 15, no. 14: 3112. https://doi.org/10.3390/nu15143112
APA StyleGiannetti, A., Pession, A., Bettini, I., Ricci, G., Giannì, G., & Caffarelli, C. (2023). IgE Mediated Shellfish Allergy in Children—A Review. Nutrients, 15(14), 3112. https://doi.org/10.3390/nu15143112