Association between Consumption of Ultra-Processed Food and Body Composition of Adults in a Capital City of a Brazilian Region
Abstract
1. Introduction
2. Materials and Methods
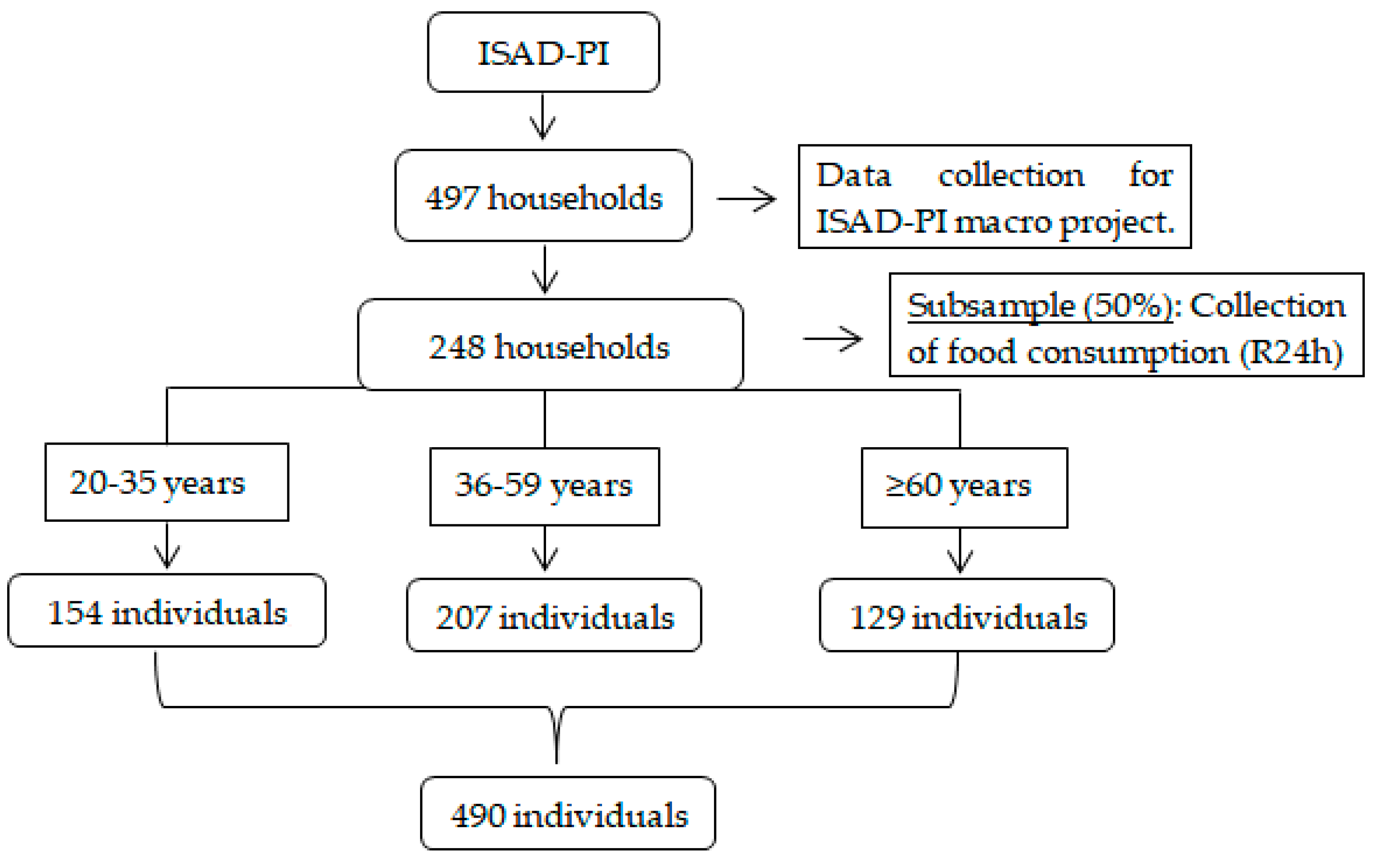
2.1. Study Design and Population
2.2. Sample Size
2.3. Data Collection and Anthropometric Measurements
2.4. Dietary Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azevedo, E.C.; Diniz Ada, S.; Monteiro, J.S.; Cabral, P.C. Dietary risk patterns for non-communicable chronic diseases and their association with body fat—A systematic review. Cien Saude Colet. 2014, 19, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Konigorski, S.; Pischon, T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism 2019, 92, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, R.A.; Meliscki, G.C.; Silva, B.R.; Navarro, A.M.; Bollela, V.R.; Schmidt, A.; Foss-Freitas, M.C. Comparing the Ability of Anthropometric Indicators in Identifying Metabolic Syndrome in HIV Patients. PLoS ONE 2016, 11, e0149905. [Google Scholar] [CrossRef]
- Piqueras, P.; Ballester, A.; Durá-Gil, J.V.; Martinez-Hervas, S.; Redón, J.; Real, J.T. Anthropometric Indicators as a Tool for Diagnosis of Obesity and Other Health Risk Factors: A Literature Review. Front. Psychol. 2021, 12, 631179. [Google Scholar] [CrossRef]
- Hirani, V. Generalised and abdominal adiposity are important risk factors for chronic disease in older people: Results from a nationally representative survey. J. Nutr. Health Aging 2011, 15, 469–478. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef]
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfør, T.M.; Alexander, J. Diets and drugs for weight loss and health in obesity—An update. Biomed. Pharmacother. 2021, 140, 111789. [Google Scholar] [CrossRef]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-processed Food Intake and Obesity: What Really Matters for Health—Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef]
- Moubarac, J.C.; Parra, D.; Cannon, G.; Monteiro, C.A. Food classification systems based on food processing: Significance and implications for policies and actions. A systematic literature review and assessment. Curr. Obes. Rep. 2014, 3, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Lakhssassi, S.; Briffaz, A. Beyond nutrient-based food indices: A data mining approach to search for a quantitative holistic index reflecting the degree of food processing and including physicochemical properties. Food Funct. 2018, 9, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; Castro, I.R.; Cannon, G. A new classification of foods based on the extent and purpose of their processing. Cad. Saude Publica 2010, 26, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D.; Ricardo, C.; et al. NOVA. The star shines bright [Food classification. Public health]. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Batal, M.; Johnson-Down, L.; Moubarac, J.C.; Ing, A.; Fediuk, K.; Sadik, K.; Tikhonov, C.; Chan, L.; Willows, N. Quantifying associations of the dietary share of ultra-processed foods with overall diet quality in First Nations peoples in the Canadian provinces of British Columbia, Alberta, Manitoba and Ontario. Public Health Nutr. 2018, 21, 103–113. [Google Scholar] [CrossRef]
- Marrón-Ponce, J.A.; Sánchez-Pimienta, T.G.; Louzada, M.L.D.C.; Batis, C. Energy contribution of NOVA food groups and sociodemographic determinants of ultra-processed food consumption in the Mexican population. Public Health Nutr. 2018, 21, 87–93. [Google Scholar] [CrossRef]
- Phillips, N.E.; Mareschal, J.; Schwab, N.; Manoogian, E.N.C.; Borloz, S.; Ostinelli, G.; Gauthier-Jaques, A.; Umwali, S.; Gonzalez Rodriguez, E.; Aeberli, D.; et al. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients 2021, 13, 1042. [Google Scholar] [CrossRef]
- Gibney, M.J.; Forde, C.G.; Mullally, D.; Gibney, E.R. Ultra-processed foods in human health: A critical appraisal. Am. J. Clin. Nutr. 2017, 106, 717–724, Erratum in: Am. J. Clin. Nutr. 2018, 107, 482–483. [Google Scholar] [CrossRef]
- Sadler, C.R.; Grassby, T.; Hart, K.; Raats, M.; Sokolovi´c, M.; Timotijevic, L. Processed food classification: Conceptualisation and challenges. Trends Food Sci. Technol. 2021, 112, 149–162. [Google Scholar] [CrossRef]
- Braesco, V.; Souchon, I.; Sauvant, P.; Haurogné, T.; Maillot, M.; Féart, C.; Darmon, N. Ultra-processed foods: How functional is the NOVA system? Eur. J. Clin. Nutr. 2022, 76, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, R.; Mambrini, S.P.; Pellizzari, M.; Foppiani, A.; Bertoli, S.; Battezzati, A.; Leone, A. Ultra-processed foods and obesity and adiposity parameters among children and adolescents: A systematic review. Eur. J. Nutr. 2022, 61, 2297–2311. [Google Scholar] [CrossRef]
- Harsha, P.S.C.; Lavelli, V. Use of Grape Pomace Phenolics to Counteract Endogenous and Exogenous Formation of Advanced Glycation End-Products. Nutrients 2019, 11, 1917. [Google Scholar] [CrossRef]
- Velayoudom-Cephise, F.L.; Cano-Sanchez, M.; Bercion, S.; Tessier, F.; Yu, Y.; Boulanger, E.; Neviere, R. Receptor for advanced glycation end products modulates oxidative stress and mitochondrial function in the soleus muscle of mice fed a high-fat diet. Appl. Physiol. Nutr. Metab. 2020, 45, 1107–1117. [Google Scholar] [CrossRef]
- Wagas, K.; Chen, J.; Lu, T.; van der Eerden, B.C.J.; Rivadeneira, F.; Uitterlinden, A.G.; Voortman, T.; Zillikens, M.C. Dietary advanced glycation end-products (dAGEs) intake and its relation to sarcopenia and frailty—The Rotterdam Study. Bone 2022, 165, 116564. [Google Scholar] [CrossRef] [PubMed]
- Rudakoff, L.C.S.; Magalhães, E.I.S.; Viola, P.C.A.F.; Oliveira, B.R.; Coelho, C.C.N.S.; Bragança, M.L.B.M.; Arruda, S.P.M.; Cardoso, V.C.; Bettiol, H.; Barbieri, M.A. Ultra-processed food consumption is associated with increase in fat mass and decrease in lean mass in Brazilian women: A cohort study. Front. Nutr. 2022, 9, 1006018. [Google Scholar] [CrossRef] [PubMed]
- Canhada, S.L.; Luft, V.C.; Giatti, L.; Duncan, B.B.; Chor, D.; Fonseca, M.; Matos, S.M.A.; Molina, M.; Barreto, S.M.; Levy, R.B.; et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public. Health Nutr. 2020, 23, 1076–1086. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-processed diets cause excess calorie intake and weight gain: An inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019, 30, 67–77.e63. [Google Scholar] [CrossRef]
- Nascimento, L.M.; Monteiro, N.V.N.; Vilar, T.M.; Ibiapina, C.R.L.S.; Frota, K.M.G. The influence of ultra-processed food consumption in anthropometric and atherogenic indices of adolescents. Rev. Nutr. 2021, 34, e200036. [Google Scholar] [CrossRef]
- Konieczna, J.; Morey, M.; Abete, I.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Vioque, J.; Gonzalez-Palacios, S.; Daimiel, L.; Salas-Salvado, J.; Fiol, M.; et al. Contribution of ultra-processed foods in visceral fat deposition and other adiposity indicators: Prospective analysis nested in the PREDIMED-Plus trial. Clin. Nutr. 2021, 40, 4290–4300. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gomez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef] [PubMed]
- Alves Junior, C.A.S.; Mocellin, M.C.; Gonçalves, E.C.A.; Silva, D.A.S.; Trindade, E.B.S.M. Anthropometric Indicators as Body Fat Discriminators in Children and Adolescents: A Systematic Review and Meta-Analysis. Adv. Nutr. 2017, 8, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, Z.; Dychała, E.; Pisula-Lewandowska, A.; Danel, D.P. Comparison of Skinfold Thickness Measured by Caliper and Ultrasound Scanner in Normative Weight Women. Int. J. Environ. Res. Public Health 2022, 19, 16230. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Censo Demográfico 2010: Características Gerais da População e dos Domicílios, Resultados do Universo. Rio de Janeiro: Instituto. 2010. Available online: https://censo2010.ibge.gov.br/resultados.html (accessed on 12 August 2021).
- Rodrigues, L.A.R.L.; Costa e Silva, D.M.; Oliveira, E.A.R.; Lavôr, L.C.C.; Sousa, R.R.; Carvalho, R.B.N.; Farias Junior, G.; Formiga, L.M.F.; Sousa, A.F.; Cardoso, M.R.A.; et al. Sampling plan and methodological aspects: A household healthcare survey in Piauí. Rev. Saude Publica 2021, 55, 118. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Pesquisa Nacional de Saúde: Percepção do Estado de Saúde, Estilos de Vida e Doenças Crônicas: 2013; IBGE: Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Fisberg, R.M.; Marchioni, D.M.L. Manual de Avaliação do Consumo Alimentar em Estudos Populacionais: A Experiência do Inquérito de Saúde em São Paulo (ISA); Faculdade de Saúde Pública da Universidade de São Paulo: São Paulo, Brazil, 2012; 197p. [Google Scholar]
- Cameron, N. Anthropometric Measurements. In The Measurement of Human Growth; Cameron, N., Ed.; Croom Helm: London, UK, 1984; pp. 56–99. [Google Scholar]
- Jelliffe, D.B.; Jelliffe, E.F.P. Anthropometry: Major measurements. In Community Nutritional Assessment with Special Reference to Less Technically Developed Countries, 2nd ed.; Jelliffe, D.B., Jelliffe, E.F.P., Eds.; Oxford University Press: London, UK, 1989; pp. 68–105. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Technical Report Series 894, 9; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Lipschitz, D.A. Screening for nutritional status in the elderly. Prim. Care 1994, 21, 55–67. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry; WHO Technical Report Series n. 854; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Ashwell, M.; Hsieh, S.D. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [CrossRef]
- Frisancho, A.R. Anthropometric Standards for the Assessment of Growth and Nutritional Status; University of Michigan Press: Ann Arbor, MI, USA, 1991. [Google Scholar]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1991. [Google Scholar]
- Martin, F.G.; Nebuloni, C.C.; Najas, M.S. Correlação entre estado nutricional e força de preensão palmar em idosos. Rev. Bras. Ger. Gerontol. 2012, 15, 493–504. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An overview of USDA’s Dietary Intake Data System. J. Food Compost. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
- Verly-Júnior, E.; Castro, M.A.; Fisberg, R.M.; Marchioni, D.M.L. Precision of Usual Food Intake Estimates According to the Percentage of Individuals with a Second Dietary Measurement. J. Acad. Nutr. Diet. 2012, 112, 1015–1020. [Google Scholar] [CrossRef]
- Pinheiro, A.B.V.; Lacerda, E.M.D.A.; Benzecry, E.H.; Gomes, M.C.D.S.; Costa, V.M.D. Tabela para Avaliação de Consumo Alimentar em Medidas Caseiras, 5th ed.; Atheneu: São Paulo, Brazil, 2005. [Google Scholar]
- TACO. Tabela Brasileira de Composição de Alimentos, 4th ed.; NEPA-UNICAMP: Campinas, Brazil, 2011; 161p. [Google Scholar]
- IBGE. Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares (POF), 2008–2009: Tabela de Composição Nutricional dos Alimentos Consumidos No Brasil; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Philippi, S.T. Tabela de Composição de Alimentos: Suporte para Decisão Nutricional, 6th ed.; Manole: São Paulo, Brazil, 2018; 160p. [Google Scholar]
- Textor, J.; Hardt, J.; Knuppel, S. DAGitty: A graphical tool for analyzing causal digrams. Epidemiology 2011, 22, 745. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.S.; Sattamini, I.F.; Martínez Steele, E.; Louzada, M.L.C.; Claro, R.M.; Monteiro, C.A. Consumption of ultra-processed foods and its association with sociodemographic factors in the adult population of the 27 Brazilian state capitals (2019). Rev. Saude Publica 2021, 55, 47. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, N.; Cediel, G.; Obando, A.; Jaime, P.C.; Parra, D.C. Sociodemographic factors associated with the consumption of ultra-processed foods in Colombia. Rev. Saude Publica 2020, 54, 19. [Google Scholar] [CrossRef]
- Javed, A.A.; Aljied, R.; Allison, D.J.; Anderson, L.N.; Ma, J.; Raina, P. Body mass index and all-cause mortality in older adults: A scoping review of observational studies. Obes. Rev. 2020, 21, e13035. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, E.L.A.; Oliveira, J.B.; Santana, A.A.; Maia, L.; Ramos, H.E. Prevalência de sobrepeso e obesidade em idosas frequentadoras da Universidade Aberta da Terceira Idade—UATI. Rev. Ciênc Méd Biol. 2020, 19, 543–546. [Google Scholar]
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profle in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef]
- Louzada, M.L.C.; Ricardo, C.Z.; Steele, E.M.; Levy, R.B.; Cannon, G.; Monteiro, C.A. The share of ultra-processed foods determines the overall nutritional quality of diets in Brazil. Public. Health Nutr. 2018, 21, 94–102. [Google Scholar] [CrossRef]
- Louzada, M.L.C.; Baraldi, L.G.; Steele, E.M.; Martins, A.P.; Canella, D.S.; Moubarac, J.C.; Levy, R.B.; Cannon, G.; Afshin, A.; Imamura, F.; et al. Consumption of ultraprocessed foods and obesity in Brazilian adolescents and adults. Prev. Med. 2015, 81, 9–15. [Google Scholar] [CrossRef]
- Juul, F.; Martinez-Steele, E.; Parekh, N.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption and excess weight among US adults. Br. J. Nutr. 2018, 120, 90–100. [Google Scholar] [CrossRef]
- Nardocci, M.; Leclerc, B.S.; Louzada, M.L.; Monteiro, C.A.; Batal, M.; Moubarac, J.C. Consumption of ultra-processed foods and obesity in Canada. Can. J. Public Health 2019, 110, 4–14. [Google Scholar] [CrossRef]
- Liu, J.; Steele, E.M.; Li, Y.; Yi, S.S.; Monteiro, C.A.; Mozaffarian, D. Consumption of Ultraprocessed Foods and Body Fat Distribution Among U.S. Adults. Am. J. Prev. Medic. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Viola, P.C.A.F.; Carvalho, C.A.; Bragança, M.L.B.M.; França, A.K.T.D.C.; Alves, M.T.S.S.B.E.; da Silva, A.A.M. High consumption of ultra-processed foods is associated with lower muscle mass in Brazilian adolescents in the RPS birth cohort. Nutrition. 2020, 79–80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultraprocessed foods and health outcomes: A narrative review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Milagro, F.I.; Aranaz, P.; Martínez, J.A.; Riezu-Boj, J.I. Gut microbiota differences according to ultra-processed food consumption in a spanish population. Nutrients 2021, 13, 2710. [Google Scholar] [CrossRef] [PubMed]
- Brasil (2020). Pesquisa de Orçamentos Familiares 2017–2018: Análise do Consumo Alimentar Pessoal no Brasil/IBGE, Coordenação de Trabalho e Rendimento; IBGE: Rio de Janeiro, Brazil, 2020. [Google Scholar]

| Age (Years) | n (%) |
|---|---|
| 20–35 | 154 (31.4) |
| 36–59 | 207 (42.2) |
| ≥60 | 129 (26.3) |
| Gender | |
| Masculine | 162 (33.1) |
| Feminine | 328 (66.9) |
| Marital status | |
| Single | 286 (58.5) |
| Married | 203 (41.5) |
| Family income (Brazilian minimum wage) | |
| ≤2 | 374 (76.3) |
| >2 | 116 (23.7) |
| Level of education | |
| Not literate | 29 (5.9) |
| Primary education | 149 (30.4) |
| High school | 201 (41) |
| University education | 111 (22.7) |
| Alcohol consumption | |
| No | 294 (60) |
| Yes | 196 (40) |
| Smoking | |
| No | 382 (78) |
| Yes | 108 (22) |
| Physical activity | |
| Insufficiently active | 96 (19.6) |
| Active | 394 (80.4) |
| Mean contribution percentage in the TEV of the food groups NOVA | |
| Food groups NOVA | Mean ± SD |
| Unprocessed or minimally processed | 67.9 ± 18.9 |
| Ultra-processed food | 19.7 ± 17.9 |
| Mean ± SD | 20–35 Years | 36–59 Years | ≥60 Years | |
|---|---|---|---|---|
| WHtR | 0.56 ± 0.81 | 0.52 ± 0.07 a | 0.57 ± 0.07 b | 0.61 ± 0.07 c |
| TSF | 23.7 ± 10.1 | 23.9 ± 10.6 ab | 25.1 ± 10.4 a | 21.1 ± 8.7 b |
| AC | 31.4 ± 4.6 | 31.2 ± 4.8 a | 32.2 ± 4.7 b | 30.3 ± 4.6 a |
| AMC | 23.9 ± 3.6 | 23.7 ± 3.9 a | 24.3 ± 3.7 a | 23.7 ± 3.0 a |
| CAMA | 39.1 ± 13.7 | 38.3 ± 14.6 a | 40.6 ± 14.5 a | 37.8 ± 10.8 a |
| SSF | 22.7 ± 9.3 | 21.4 ± 9.0 a | 23. 7 ± 9.5 b | - |
| CC | 34.1 ± 3.6 | - | - | - |
| ß (CI95%) | p | ß (CI95%) | p * | |
|---|---|---|---|---|
| 20–35 years (n = 153) | ||||
| WHtR | −0.03 (−0.08/0.02) | 0.27 | −0.01 (−0.02/0.05) | 0.19 |
| TSF | 0.03 (−0.07/0.12) | 0.54 | 0.04 (0.03/0.09) | 0.04 |
| AC | −0.00 (−0.05/0.04) | 0.82 | −0.01 (−0.01/0.02) | 0.38 |
| AMC | −0.01 (−0.04/0.01) | 0.21 | −0.01 (−0.02/0.01) | 0.49 |
| CAMA | −0.05 (−0.13/0.03) | 0.22 | −0.02 (−0.08/0.04) | 0.54 |
| SSF | −0.02 (−0.09/0.06) | 0.65 | −0.003 (−0.05/0.04) | 0.89 |
| 36–59 years (n = 207) | ||||
| WHtR | 0.02 (−0.06/0.06) | 0.92 | −0.03 (−0.02/0.02) | 0.76 |
| TSF | 0.04 (−0.05/0.13) | 0.36 | −0.00 (−0.05/0.04) | 0.92 |
| AC | −0.01 (−0.05/0.03) | 0.63 | −0.02 (−0.03/−0.01) | 0.03 |
| AMC | −0.02 (−0.04/−0.00) | 0.04 | −0.02 (−0.03/−0.00) | 0.01 |
| CAMA | −0.08 (−0.16/−0.006) | 0.04 | −0.07 (−0.12/−0.02) | 0.01 |
| SSF | 0.03 (−0.03/0.10) | 0.32 | 0.01 (−0.04/0.06) | 0.65 |
| ≥60 years (n = 128) | ||||
| WHtR | 0.00 (−0.01/0.01) | 0.89 | −0.03 (−0.04/0.02) | 0.80 |
| TSF | 0.03 (−0.06/0.13) | 0.49 | −0.02 (−0.07/0.04) | 0.59 |
| AC | 0.01 (−0.03/0.06) | 0.52 | 0.01 (−0.004/0.03) | 0.13 |
| AMC | 0.00 (−0.03/0.03) | 0.77 | 0.02 (−0.002/0.04) | 0.08 |
| CAMA | 0.03 (−0.08/0.13) | 0.61 | 0.06 (−0.01/0.13) | 0.10 |
| CC | −0.0005 (−0.04/0.04) | 0.98 | 0.006 (−0.02/0.03) | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteles Nascimento, L.; de Carvalho Lavôr, L.C.; Mendes Rodrigues, B.G.; da Costa Campos, F.; de Almeida Fonseca Viola, P.C.; Lucarini, M.; Durazzo, A.; Arcanjo, D.D.R.; de Carvalho e Martins, M.d.C.; de Macêdo Gonçalves Frota, K. Association between Consumption of Ultra-Processed Food and Body Composition of Adults in a Capital City of a Brazilian Region. Nutrients 2023, 15, 3157. https://doi.org/10.3390/nu15143157
Monteles Nascimento L, de Carvalho Lavôr LC, Mendes Rodrigues BG, da Costa Campos F, de Almeida Fonseca Viola PC, Lucarini M, Durazzo A, Arcanjo DDR, de Carvalho e Martins MdC, de Macêdo Gonçalves Frota K. Association between Consumption of Ultra-Processed Food and Body Composition of Adults in a Capital City of a Brazilian Region. Nutrients. 2023; 15(14):3157. https://doi.org/10.3390/nu15143157
Chicago/Turabian StyleMonteles Nascimento, Larisse, Layanne Cristina de Carvalho Lavôr, Bruna Grazielle Mendes Rodrigues, Felipe da Costa Campos, Poliana Cristina de Almeida Fonseca Viola, Massimo Lucarini, Alessandra Durazzo, Daniel Dias Rufino Arcanjo, Maria do Carmo de Carvalho e Martins, and Karoline de Macêdo Gonçalves Frota. 2023. "Association between Consumption of Ultra-Processed Food and Body Composition of Adults in a Capital City of a Brazilian Region" Nutrients 15, no. 14: 3157. https://doi.org/10.3390/nu15143157
APA StyleMonteles Nascimento, L., de Carvalho Lavôr, L. C., Mendes Rodrigues, B. G., da Costa Campos, F., de Almeida Fonseca Viola, P. C., Lucarini, M., Durazzo, A., Arcanjo, D. D. R., de Carvalho e Martins, M. d. C., & de Macêdo Gonçalves Frota, K. (2023). Association between Consumption of Ultra-Processed Food and Body Composition of Adults in a Capital City of a Brazilian Region. Nutrients, 15(14), 3157. https://doi.org/10.3390/nu15143157









