Nutritional Strategies for the Prevention and Management of Cow’s Milk Allergy in the Pediatric Age
Abstract
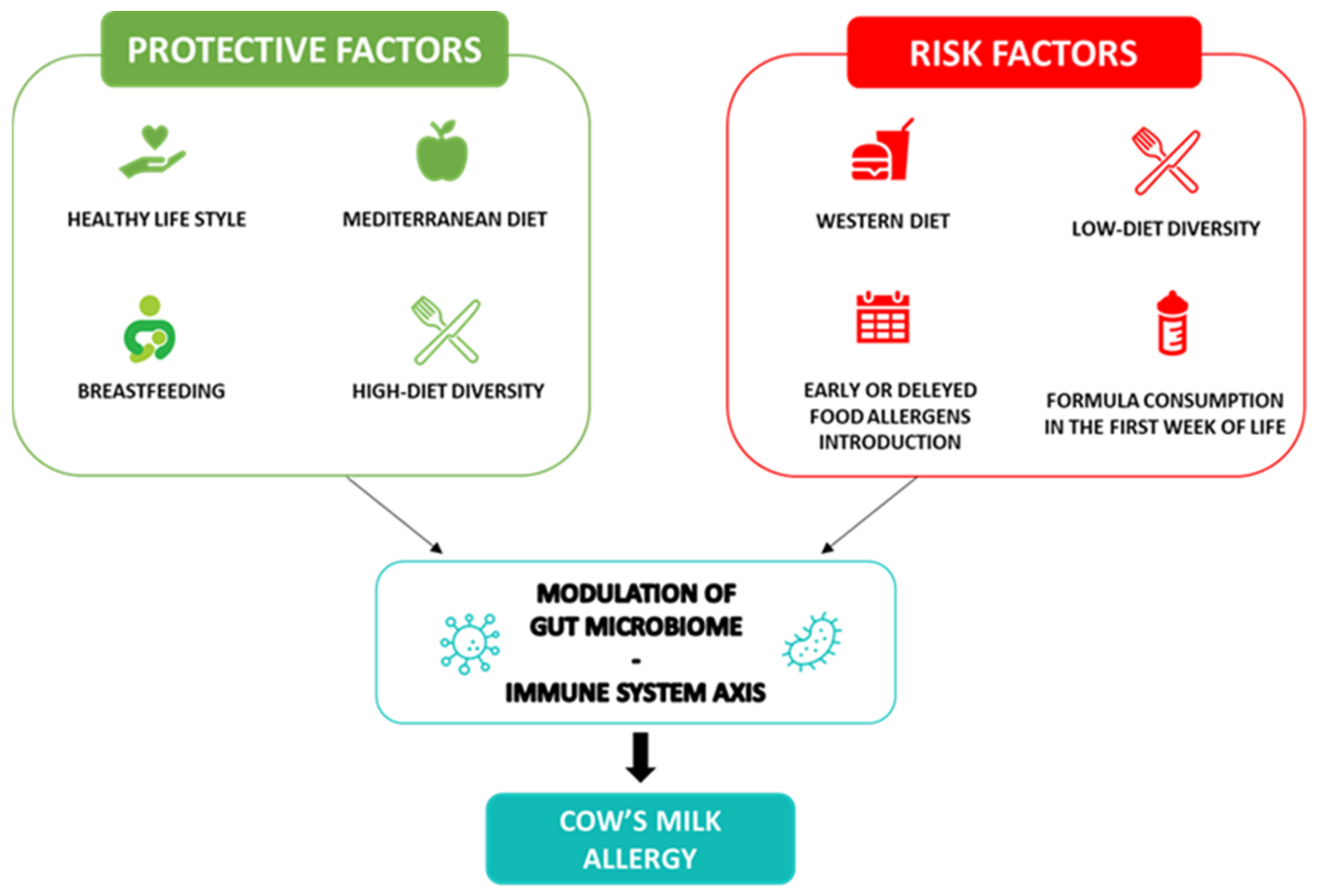
:1. Introduction
2. Cow’s Milk Allergy Preventive Nutritional Strategies
3. Nutritional Strategies for the Management of Cow’s Milk Allergy
3.1. Accidental Exposures Prevention
- (1)
- Contamination: They should be careful about the contact or contamination of foods with cow’s milk protein, especially in places such as bakeries and restaurants, laying out side-by-side of these foods or using the same knife while cutting or using the same fork while serving increases the risk of contamination;
- (2)
- Food labels: All ingredient labels on food packages should be read carefully. Foods containing casein, whey, lactalbumin, albumin, etc. should be avoided [40]. There is no consensus yet on the restriction of foods containing advisory labeling such as “may contain milk” because the allergic risks of these products are not yet fully known and the amount of cow’s milk protein contamination of them are variable [41];
- (3)
- Cross reactions: Due to the high cross-reactivity with cow’s milk protein of sheep, goats, buffalo, ibex, deer, donkey, camel and horse milk, parents should be aware of cross-reactions that may occur and should strictly avoid the consumption of these alternative milks;
- (4)
- Non-food products: Drugs, cosmetics and supplements may contain cow’s milk. The labels of these products should also be read carefully.
3.2. Cow’s Milk Protein Elimination Diet
3.3. Proposal of the Most Complete Compounds Supplement for Nutrients Deficiencies Prevention and to Positively Drive the Disease Course in CMA Pediatric Patients
3.4. Follow-Up of CMA Patients
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, S.A.; Fiocchi, A.; Baars, T.; Jordakieva, G.; Nowak-Wegrzyn, A.; Pali-Schöll, I.; Passanisi, S.; Pranger, C.L.; Roth-Walter, F.; Takkinen, K.; et al. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines up-date—III—Cow’s milk allergens and mechanisms triggering immune activation. World Allergy Organ. J. 2022, 15, 100668. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Masilamani, M.; Li, X.M.; Sampson, H.A. The false alarm hypothesis: Food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, J.A.; Leonard, S.; Groetch, M.; Assa’ad, A.; Cianferoni, A.; Clark, A.; Crain, M.; Fausnight, T.; Fleischer, D.; Green, T.; et al. Conducting an Oral Food Challenge: An Update to the 2009 Adverse Reactions to Foods Committee Work Group Report. J. Allergy Clin. Immunol. Pract. 2020, 8, 75–90.e17. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, R.; Leone, L.; Cosenza, L.; Berni Canani, R. Increasing rate of hospitalizations for food-induced anaphylaxis in Italian children: An analysis of the Italian ministry of health database. J. Allergy Clin. Immunol. 2015, 135, 833–835.e3. [Google Scholar] [CrossRef]
- Flom, J.D.; Sicherer, S.H. Epidemiology of cow’s milk. Allergy Nutr. 2019, 11, 1051. [Google Scholar]
- Neeland, M.R.; Martino, D.J.; Allen, K.J. The role of gene-environment interactions in the development of food allergy. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1371–1378. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef]
- Aitoro, R.; Paparo, L.; Amoroso, A.; Di Costanzo, M.; Cosenza, L.; Granata, V.; Di Scala, C.; Nocerino, R.; Trinchese, G.; Montella, M.; et al. Gut Microbiota as a Target for Preventive and Therapeutic Intervention against Food Allergy. Nutrients 2017, 9, 672. [Google Scholar] [CrossRef] [Green Version]
- Devonshire, A.; Gautam, Y.; Johansson, E.; Mersha, T.B. Multi-omics profiling approach in food allergy. World Allergy Organ. J. 2023, 16, 100777. [Google Scholar] [CrossRef]
- Lee, K.H.; Song, Y.; Wu, W.; Yu, K.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Carucci, L.; Coppola, S.; Luzzetti, A.; Voto, L.; Giglio, V.; Paparo, L.; Nocerino, R.; Berni Canani, R. Immunonutrition for Pediatric Patients with Cow’s Milk Allergy: How Early Interventions Could Impact Long-Term Outcomes. Front. Allergy 2021, 2, 676200. [Google Scholar] [CrossRef]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary factors during pregnancy and atopic outcomes in childhood: A systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020, 31, 889–912. [Google Scholar] [CrossRef]
- Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur. J. Nutr. 2021, 60, 1429–1442. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Selma-Royo, M.; González, S.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Distinct maternal microbiota clusters are associated with diet during pregnancy: Impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020, 11, 962–978. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Venter, C.; Palumbo, M.P.; Glueck, D.H.; Sauder, K.A.; O’Mahony, L.; Fleischer, D.M.; Ben-Abdallah, M.; Ringham, B.M.; Dabelea, D. The maternal diet index in pregnancy is associated with offspring allergic diseases: The Healthy Start study. Allergy 2022, 77, 162–172. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Rifas-Shiman, S.L.; Platts-Mills, T.A.; Workman, L.; Sordillo, J.E.; Camargo, C.A., Jr.; Gillman, M.W.; Gold, D.R.; Litonjua, A.A. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. J. Allergy Clin. Immunol. 2014, 133, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Uthoff, H.; Spenner, A.; Reckelkamm, W.; Ahrens, B.; Wölk, G.; Hackler, R.; Hardung, F.; Schaefer, J.; Scheffold, A.; Renz, H.; et al. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J. Immunol. 2003, 171, 3485–3492. [Google Scholar] [CrossRef] [Green Version]
- Järvinen, K.M.; Martin, H.; Oyoshi, M.K. Immunomodulatory effects of breast milk on food allergy. Ann. Allergy Asthma Immunol. 2019, 123, 133–143. [Google Scholar] [CrossRef]
- Yeruva, L.; Munblit, D.; Collado, M.C. Editorial: Impact of Early Life Nutrition on Immune System Development and Related Health Outcomes in Later Life. Front. Immunol. 2021, 25, 668569. [Google Scholar] [CrossRef] [PubMed]
- Adel-Patient, K.; Bernard, H.; Fenaille, F.; Hazebrouck, S.; Junot, C.; Verhasselt, V. Prevention of Allergy to a Major Cow’s Milk Allergen by Breastfeeding in Mice Depends on Maternal Immune Status and Oral Exposure During Lactation. Front. Immunol. 2020, 21, 1545. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, A.; Venturelli, N.; Buccigrosso, T.M.; Osganian, S.K.; Lee, J.; Blumberg, R.S.; Oyoshi, M.K. Maternal IgG immune complexes induce food allergen-specific tolerance in offspring. J. Exp. Med. 2018, 215, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutrients 2018, 10, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonk, M.M.; Blokhuis, B.R.J.; Diks, M.A.P.; Wagenaar, L.; Smit, J.J.; Pieters, R.H.H.; Garssen, J.; Knippels, L.M.J.; van Esch, B.C.A.M. Butyrate Enhances Desensitization Induced by Oral Immunotherapy in Cow’s Milk Allergic Mice. Mediat. Inflamm. 2019, 16, 9062537. [Google Scholar] [CrossRef] [Green Version]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; van den Berg, M.P.M.; de Bruijn, M.J.W.; van IJcken, W.F.J.; Junt, T.; Tam, S.Y.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy 2020, 75, 1966–1978. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as a bioactive human milk protective component against food allergy. Allergy 2021, 76, 1398–1415. [Google Scholar] [CrossRef]
- WHO. Maternal, Infant and Young Child Nutrition. Available online: https://apps.who.int/iris/handle/10665/250636 (accessed on 26 June 2023).
- Muraro, A.; Halken, S.; Arshad, S.H.; Beyer, K.; Dubois, A.E.; Du Toit, G.; Eigenmann, P.A.; Grimshaw, K.E.; Hoest, A.; Lack, G.; et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 2014, 69, 590–601. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Plaut, M.; Bahnson, H.T.; Mitchell, H.; Radulovic, S.; Chan, S.; Fox, A.; Turcanu, V.; et al. Identifying infants at high risk of peanut allergy: The Learning Early About Peanut Allergy (LEAP) screening study. J. Allergy Clin. Immunol. 2013, 131, 135–143.e12. [Google Scholar] [CrossRef]
- Lack, G. Update on risk factors for food allergy. J. Allergy Clin. Immunol. 2012, 129, 1187–1197. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
- Muraro, A.; de Silva, D.; Halken, S.; Worm, M.; Khaleva, E.; Arasi, S.; Dunn-Galvin, A.; Nwaru, B.I.; De Jong, N.W.; Rodríguez Del Río, P.; et al. Managing food allergy: GA2LEN guideline 2022. World Allergy Organ. J. 2022, 15, 100687. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T.; Standards of Care Committee (SOCC) of the British Society for Allergy and Clinical Immunology (BSACI). BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Wright, K.; Feeney, M.; Yerlett, N.; Meyer, R. Nutritional Management of Children with Food Allergies. Curr. Treat. Options Allergy 2022, 9, 375–393. [Google Scholar] [CrossRef]
- Stróżyk, A.; Ruszczyński, M.; Horvath, A.; Dahdah, L.; Fiocchi, A.; Nowak-Węgrzyn, A.; Shamir, R.; Spergel, J.; Vandenplas, Y.; Venter, C.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update—IV—A quality appraisal with the AGREE II instrument. World Allergy Organ. J. 2022, 15, 100613. [Google Scholar] [CrossRef]
- Fiocchi, A.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R.; Martelli, A.; Terracciano, L.; Bahna, S.L.; Rancé, F.; et al. Diagnosis and Rationale for Action Against Cow’s Milk Allergy (DRACMA): A summary report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128.e12. [Google Scholar] [CrossRef]
- Dupont, C.; Chouraqui, J.P.; de Boissieu, D.; Bocquet, A.; Bresson, J.L.; Briend, A.; Darmaun, D.; Frelut, M.L.; Ghisolfi, J.; Girardet, J.P.; et al. Dietary treatment of cows’ milk protein allergy in childhood: A commentary by the Committee on Nutrition of the French Society of Paediatrics. Br. J. Nutr. 2012, 107, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Crotty, M.P.; Taylor, S.L. Risks associated with foods having advisory milk labeling. J. Allergy Clin. Immunol. 2010, 125, 935–937. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. Pediatr. Allergy Immunol. 2010, 21, 1–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kwon, J.; Noh, G.; Lee, S.S. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr. Res. Pract. 2013, 7, 488–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, H.; Groetch, M.; Wang, J. Growth and nutritional concerns in children with food allergy. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, M.; D’Auria, E.; Caffarelli, C.; Verduci, E.; Barberi, S.; Indinnimeo, L.; Iacono, I.D.; Martelli, A.; Riva, E.; Bernardini, R. Nutritional management and follow up of infants and children with food allergy: Italian Society of Pediatric Nutrition/Italian Society of Pediatric Allergy and Immunology Task Force Position Statement. Ital. J. Pediatr. 2014, 40, 1. [Google Scholar] [CrossRef] [Green Version]
- Hebeisen, D.F.; Hoeflin, F.; Reusch, H.P.; Junker, E.; Lauterburg, B.H. Increased concentrations of omega-3 fatty acids in milk and platelet rich plasma of grass-fed cows. Int. J. Vitam. Nutr. Res. 1993, 63, 229–233. [Google Scholar]
- Aldámiz-Echevarría, L.; Bilbao, A.; Andrade, F.; Elorz, J.; Prieto, J.A.; Rodríguez-Soriano, J. Fatty acid deficiency profile in children with food allergy managed with elimination diets. Acta Paediatr. 2008, 97, 1572–1576. [Google Scholar] [CrossRef]
- Isolauri, E.; Sutas, Y.; Salo, M.K.; Isosomppi, R.; Kaila, M. Elimination diet in cow’s milk Allergy: Risk of impaired growth in young children. J. Pediatr. 1998, 132, 1004–1009. [Google Scholar] [CrossRef]
- Christie, L.; Hine, R.J.; Parker, J.G.; Burks, W. Food allergies in children affect nutrient intake and growth. J. Am. Diet. Assoc. 2002, 102, 1648–1651. [Google Scholar] [CrossRef]
- Tanner, J.M. A concise history of growth studies from Buffon to Boas. In Human Growth: A Comprehensive Treatise, In Neurobiology and Nutrition; Faulkner, F., Tanner, J.M., Eds.; Plenum: New York, NY, USA, 1979; Volume 3, pp. 515–593. [Google Scholar]
- Millward, D.J.; Rivers, J.P. The need for indispensable amino acids: The concept of the anabolic drive. Diabetes Metab. Rev. 1989, 5, 191–211. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Heine, R.G.; Høst, A.; Järvi, A.; Kuitunen, M.; Mukherjee, R.; Ribes-Koninckx, C.; Szajewska, H.; et al. Growth in Infants with Cow’s Milk Protein Allergy Fed an Amino Acid-Based Formula. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 392–402. [Google Scholar] [CrossRef]
- Doulgeraki, A.E.; Manousakis, E.M.; Papadopoulos, N.G. Bone health assessment of food allergic children on restrictive diets: A practical guide. J. Pediatr. Endocrinol. Metab. 2017, 30, 133–139. [Google Scholar] [CrossRef]
- Jensen, V.B.; Jorgensen, I.M.; Rasmussen, K.B.; Molgaard, C.; Prahl, P. Bone mineral status in children with cow’s milk allergy. Pediatr. Allergy Immunol. 2004, 15, 562–565. [Google Scholar] [CrossRef]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [Green Version]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef]
- Mailhot, G.; Perrone, V.; Alos, N.; Dubois, J.; Delvin, E.; Paradis, L.; Des Roches, A. Cow’s Milk Allergy and Bone Mineral Density in Prepubertal Children. Pediatrics 2016, 137, e20151742. [Google Scholar] [CrossRef] [Green Version]
- Çelik, M.N.; Köksal, E. Nutritional Targets in Cow’s Milk Protein Allergy: A Comprehensive Review. Curr. Nutr. Rep. 2022, 11, 329–336. [Google Scholar] [CrossRef]
- Pereira, A.P.D.S.; Mendonça, R.B.; Fonseca, F.L.A.; Mallozi, M.C.; Sarni, R.O.S. Vitamin D deficiency in children and adolescents with food allergy: Association with number of allergens, sun exposure and nutritional status. Allergol. Immunopathol. 2022, 50, 10–16. [Google Scholar] [CrossRef]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Bergwitz, C.; Jüppner, H. Regulation of Phosphate Homeostasis by PTH, Vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Sahay, M.; Sahay, R. Rickets-vitamin D deficiency and dependency. Indian J. Endocrinol. Metab. 2012, 16, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian pediatric society and the Italian society of preventive and social pediatrics, jointly with the Italian federation of pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Identification of the vitamin D receptor in osteoblasts and chondrocytes but not osteoclasts in mouse bone. J. Bone Miner. Res. 2014, 29, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Nilsson, O.; Baron, J. Recent insights into the regulation of the growth plate. J. Mol. Endocrinol. 2014, 53, T1–T9. [Google Scholar] [CrossRef] [Green Version]
- Cousins, R.J.; Liuzzi, J.P.; Lichten, L.A. Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 2006, 281, 24085–24089. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.H.; Peerson, J.M.; Baker, S.K.; Hess, S.Y. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr. Bull. 2009, 30, S12–S40. [Google Scholar] [CrossRef]
- Brown, K.H.; Peerson, J.M.; Rivera, J.; Allen, L.H. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2002, 75, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Imdad, A.; Bhutta, Z.A. Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: A meta-analysis of studies for input to the lives saved tool. BMC Public Health 2011, 11, S22. [Google Scholar] [CrossRef] [Green Version]
- Mayo-Wilson, E.; Junior, J.A.; Imdad, A.; Dean, S.; Chan, X.H.; Chan, E.S.; Jaswal, A.; Bhutta, Z.A. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst. Rev. 2014, 15, CD009384. [Google Scholar] [CrossRef]
- Dean, S.; Bhutta, Z.A. Preventive zinc supplementation for children, and the effect of additional iron: A systematic review and meta-analysis. BMJ Open 2014, 4, e004647. [Google Scholar]
- Ramakrishnan, U.; Nguyen, P.; Martorell, R. Effects of micronutrients on growth of children under 5 y of age: Meta-analyses of single and multiple nutrient interventions. Am. J. Clin. Nutr. 2009, 89, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Stammers, A.L.; Lowe, N.M.; Medina, M.W.; Patel, S.; Dykes, F.; Perez-Rodrigo, C.; Serra-Majam, L.; Nissensohn, M.; Moran, V.H. The relationship between zinc intake and growth in children aged 1–8 years: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 147–153. [Google Scholar] [CrossRef]
- Flammarion, S.; Santos, C.; Guimber, D.; Jouannic, L.; Thumerelle, C.; Gottrand, F.; Deschildre, A. Diet and nutritional status of children with food allergies. Pediatr. Allergy Immunol. 2011, 22, 161–165. [Google Scholar] [CrossRef]
- Millward, D.J. Nutrition, infection and stunting: The roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res. Rev. 2017, 30, 50–72. [Google Scholar] [CrossRef]
- Nakajima, S.; Naruto, T.; Miyamae, T.; Imagawa, T.; Mori, M.; Nishimaki, S.; Yokota, S. Interleukin-6 inhibits early differentiation of ATDC5 chondrogenic progenitor cells. Cytokine 2009, 47, 91–97. [Google Scholar] [CrossRef]
- Sederquist, B.; Fernandez-Vojvodich, P.; Zaman, F.; Sävendahl, L. Recent research on the growth plate: Impact of inflammatory cytokines on longitudinal bone growth. J. Mol. Endocrinol. 2014, 53, T35–T44. [Google Scholar] [CrossRef]
- Liao, C.R.; Wang, S.N.; Zhu, S.Y.; Wang, Y.Q.; Li, Z.Z.; Liu, Z.Y.; Jiang, W.S.; Chen, J.T.; Wu, Q. Advanced oxidation protein products increase TNF-α and IL-1β expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 2020, 28, 101306. [Google Scholar] [CrossRef]
- Seifarth, C.; Csaki, C.; Shakibaei, M. Anabolic actions of IGF-I and TGF-beta1 on Interleukin-1beta-treated human articular chondrocytes: Evaluation in two and three dimensional cultures. Histol. Histopathol. 2009, 24, 1245–1262. [Google Scholar]
- Mårtensson, K.; Chrysis, D.; Sävendahl, L. Interleukin-1beta and TNF-alpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J. Bone Miner. Res. 2004, 19, 1805–1812. [Google Scholar] [CrossRef]
- Cole, C.R. Preventing hidden hunger in children using micronutrient supplementation. J. Pediatr. 2012, 161, 777–778. [Google Scholar] [CrossRef]
- Berry, M.J.; Adams, J.; Voutilainen, H.; Feustel, P.J.; Celestin, J.; Järvinen, K.M. Impact of elimination diets on growth and nutritional status in children with multiple food allergies. Pediatr. Allergy Immunol. 2015, 26, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.; McCartney, A.L.; Gibson, G.R. Probiotics and prebiotics in infant nutrition. Proc. Nutr. Soc. 2007, 66, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Bird, J.A.; Fiocchi, A.; Knol, J.; Meyer, R.; Salminen, S.; Sitang, G.; Szajewska, H.; Papadopoulos, N. The potential for pre-, pro- and synbiotics in the management of infants at risk of cow’s milk allergy or with cow’s milk allergy: An exploration of the rationale, available evidence and remaining questions. World Allergy Organ. J. 2019, 12, 100034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Li, X.; Yang, Y.; Shoaie, S.; Zhang, C.; Ji, B.; Wei, Y. Advances in the Relationships Between Cow’s Milk Protein Allergy and Gut Microbiota in Infants. Front. Microbiol. 2021, 16, 716667. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4. [Google Scholar] [CrossRef] [Green Version]
- Nocerino, R.; Di Costanzo, M.; Bedogni, G.; Cosenza, L.; Maddalena, Y.; Di Scala, C.; Della Gatta, G.; Carucci, L.; Voto, L.; Coppola, S.; et al. Dietary Treatment with Extensively Hydrolyzed Casein Formula Containing the Probiotic Lactobacillus rhamnosus GG Prevents the Occurrence of Functional Gastrointestinal Disorders in Children with Cow’s Milk Allergy. J. Pediatr. 2019, 213, 137–142.e2. [Google Scholar] [CrossRef] [Green Version]
- Bode, L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J. Nutr. 2006, 136, 2127–2130. [Google Scholar] [CrossRef] [Green Version]
- Kulinich, A.; Liu, L. Human milk oligosaccharides: The role in the fine-tuning of innate immune responses. Carbohydr. Res. 2016, 432, 62–70. [Google Scholar] [CrossRef]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 29, nzy005. [Google Scholar] [CrossRef] [Green Version]
- Maryniak, N.Z.; Sancho, A.I.; Hansen, E.B.; Bøgh, K.L. Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy. Foods 2022, 11, 926. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Karaman, M.; Firinci, F.; Cilaker, S.; Uysal, P.; Tugyan, K.; Yilmaz, O.; Uzuner, N.; Karaman, O. Anti-inflammatory effects of curcumin in a murine model of chronic asthma. Allergol. Immunopathol. 2012, 40, 210–214. [Google Scholar] [CrossRef]
- Yang, N.; Wang, J.; Liu, C.; Song, Y.; Zhang, S.; Zi, J.; Zhan, J.; Masilamani, M.; Cox, A.; Nowak-Wegrzyn, A.; et al. Berberine and limonin suppress IgE production by human B cells and peripheral blood mononuclear cells from food-allergic patients. Ann. Allergy Asthma Immunol. 2014, 113, 556–564.e4. [Google Scholar] [CrossRef]
- Integratori Alimentari e Linee Guida Ministeriali (LGM). Available online: https://www.salute.gov.it/portale/temi/p2_6.jsp?area=Alimenti%20particolari%20e%20integratori&id=1267&menu=integratori (accessed on 26 June 2023).
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; WHO Press: Geneva, Switzerland, 2006. [Google Scholar]
- Fiocchi, A.; Terracciano, L.; Bouygue, G.R.; Veglia, F.; Sarratud, T.; Martelli, A.; Restani, P. Incremental prognostic factors associated with cow’s milk allergy outcomes in infant and child referrals: The Milan Cow’s Milk Allergy Cohort study. Ann. Allergy Asthma Immunol. 2008, 101, 166–173. [Google Scholar] [CrossRef]
- Cherkaoui, S.; Bégin, P.; Paradis, L.; Paradis, J.; Des Roches, A. Powder milk: A user-friendly and safe product for heated-milk food challenge? Allergy Asthma Clin. Immunol. 2015, 23, 39. [Google Scholar] [CrossRef] [Green Version]
- Nowak-Węgrzyn, A.; Lawson, K.; Masilamani, M.; Kattan, J.; Bahnson, H.T.; Sampson, H.A. Increased Tolerance to Less Extensively Heat-Denatured (Baked) Milk Products in Milk-Allergic Children. J. Allergy Clin. Immunol. Pract. 2018, 6, 486–495.e5. [Google Scholar] [CrossRef]
- Dunlop, J.H.; Keet, C.A.; Mudd, K.; Wood, R.A. Long-Term Follow-Up After Baked Milk Introduction. J. Allergy Clin. Immunol. Pract. 2018, 6, 1699. [Google Scholar] [CrossRef]
- Tosca, M.A.; Olcese, R.; Marinelli, G.; Schiavetti, I.; Ciprandi, G. Oral Immunotherapy for Children with Cow’s Milk Allergy: A Practical Approach. Children 2022, 30, 1872. [Google Scholar] [CrossRef]
| Preventing allergic reaction |
|
| Avoiding nutritional deficit |
|
| Ensuring optimal body growth |
|
| Minerals | DRVs (7–11 Months) | DRVs (1–3 Years) | DRVs (4–6 Years) | DRVs (7–10 Years) | DRVs (11–14 Years) | DRVs (15–17 Years) |
|---|---|---|---|---|---|---|
| Sodium | NA | NA | NA | NA | NA | NA |
| Potassium | 750 mg/day | 800 mg/day | 1100 mg/day | 1800 mg/day | 2700 mg/day | 3500 mg/day |
| Chlorine | NA | 1.7 g/day | 2 g/day | 2.6 g/day | 3.1 g/day | 3.1 g/day |
| Magnesium | 80 mg/day | 170 mg/day | 230 mg/day | 230 mg/day | 250–300 mg/day | 250–300 mg/day |
| Trace Elements | ||||||
| Iron | 11 mg/day | 7 mg/day | 7 mg/day | 11 mg/day | 11 mg/day | 13 mg/day |
| Zinc | 2.9 mg/day | 4.3 mg/day | 5.5 mg/day | 7.4 μg/day | 10.7 μg/day | 11.9–14.2 μg/day |
| Copper | 0.4 mg/day | 0.7 mg/day | 1 mg/day | 1 mg/day | 1.1–1.3 mg/day | 1.1–1.3 mg/day |
| Manganese | 0.02–0.5 mg/day | 0.5 mg/day | 1 mg/day | 1.5 mg/day | 2 mg/day | 3 mg/day |
| Molybdenum | 10 μg/day | 15 μg/day | 20 μg/day | 30 μg/day | 45 μg/day | 65 μg/day |
| Selenium | 15 μg/day | 15 μg/day | 20 μg/day | 35 μg/day | 55 μg/day | 70 μg/day |
| Chromium | NA | NA | NA | NA | NA | NA |
| Iodine | 70 μg/day | 90 μg/day | 90 μg/day | 90 μg/day | 120 μg/day | 130 μg/day |
| Vitamins | ||||||
| Vit. A | 250 μg RE/day | 250 μg RE/day | 300 μg RE/day | 400 μg RE/day | 600 μg RE/day | 650–750 μg RE/day |
| Vit. D3 | 10 μg/day | 15 μg/day | 15 μg/day | 15 μg/day | 15 μg/day | 15 μg/day |
| Vit. E | 5 mg/day | 6 mg/day | 9 mg/day | 9 mg/day | 11–13 mg/day | 11–13 mg/day |
| Vit. K | 10 μg/day | 12 μg/day | 20 μg/day | 30 μg/day | 45 μg/day | 65 μg/day |
| Thiamine (Vit. B1) | 0.1 mg/MJ | 0.1 mg/MJ | 0.1 mg/MJ | 0.1 mg/MJ | 0.1 mg/MJ | 0.1 mg/MJ |
| Riboflavin (Vit. B2) | 0.4 mg/day | 0.6 mg/day | 0.7 mg/day | 1 mg/day | 1.4 mg/day | 1.4 mg/day |
| Niacin (Vit. B3) | 1.6 mg NE/MJ | 1.6 mg NE/MJ | 1.6 mg NE/MJ | 1.6 mg NE/MJ | 1.6 mg NE/MJ | 1.6 mg NE/MJ |
| Pantothenic Acid | 3 mg/day | 4 mg/day | 4 mg/day | 4 mg/day | 5 mg/day | 5 mg/day |
| Vit. B6 | 0.3 mg/day | 0.6 mg/day | 0.6 mg/day | 1 mg/day | 1.4 mg/day | 1.6–1.7 mg/day |
| Folic Acid | 80 μg DFE/day | 120 μg DFE/day | 140 μg DFE/day | 200 μg DFE/day | 270 μg DFE/day | 330 μg DFE/day |
| Vit. B12 | 1.5 μg/day | 1.5 μg/day | 1.5 μg/day | 2.5 μg/day | 3.5 μg/day | 4 μg/day |
| Biotin | 6 μg/day | 20 μg/day | 25 μg/day | 25 μg/day | 35 μg/day | 35 μg/day |
| Vit. C | 20 mg/day | 20 mg/day | 30 mg/day | 45 mg/day | 70 mg/day | 90–100 mg/day |
| Fatty Acids | ||||||
| Alpha-Linolenic acid (ALA) | 0.5 E% | 0.5 E% | 0.5 E% | 0.5 E% | 0.5 E% | 0.5 E% |
| Linoleic acid (LA) | 4 E% | 4 E% | 4 E% | 4 E% | 4 E% | 4 E% |
| Arachidonic acid (ARA) | NA | NA | NA | NA | NA | NA |
| Eicosapentaenoic Acid (EPA) | 100 mg/day | 100–250 mg/day | 250 mg/day | 250 mg/day | 250 mg/day | 250 mg/day |
| Docosanoic Acid (DHA) | 100 mg/day | 100–250 mg/day | 250 mg/day | 250 mg/day | 250 mg/day | 250 mg/day |
| Classification | Description |
|---|---|
| Moderately underweight | Weight-for-age <−2 SD and ≥−3 SD |
| Severely underweight | Weight-for-age <−3 SD |
| Moderate acute malnutrition | Weight-for-length/height or BMI-for-age ≤−2 SD and ≥−3 SD, or mid-upper arm circumference ≥115 mm and <125 mm |
| Severe acute malnutrition | Weight-for-length/height or BMI-for-age <−3 SD, or mid-upper arm circumference <115 mm, or bilateral pitting edema |
| Moderate chronic malnutrition | Length/height-for-age ≤−2 SD and ≥−3 SD |
| Severe chronic malnutrition | Length/height-for-age <−3 SD |
| Moderately wasted | Weight-for-length/height ≤−2 SD and ≥−3 SD |
| Severely wasted | Weight-for-length/height <−3 SD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, S.; Carucci, L.; Oglio, F.; Di Sarra, C.; Ozen, G.; Berni Canani, R. Nutritional Strategies for the Prevention and Management of Cow’s Milk Allergy in the Pediatric Age. Nutrients 2023, 15, 3328. https://doi.org/10.3390/nu15153328
Coppola S, Carucci L, Oglio F, Di Sarra C, Ozen G, Berni Canani R. Nutritional Strategies for the Prevention and Management of Cow’s Milk Allergy in the Pediatric Age. Nutrients. 2023; 15(15):3328. https://doi.org/10.3390/nu15153328
Chicago/Turabian StyleCoppola, Serena, Laura Carucci, Franca Oglio, Claudia Di Sarra, Gulsum Ozen, and Roberto Berni Canani. 2023. "Nutritional Strategies for the Prevention and Management of Cow’s Milk Allergy in the Pediatric Age" Nutrients 15, no. 15: 3328. https://doi.org/10.3390/nu15153328
APA StyleCoppola, S., Carucci, L., Oglio, F., Di Sarra, C., Ozen, G., & Berni Canani, R. (2023). Nutritional Strategies for the Prevention and Management of Cow’s Milk Allergy in the Pediatric Age. Nutrients, 15(15), 3328. https://doi.org/10.3390/nu15153328





