Abstract
Emerging evidence demonstrates that alterations to the gut microbiota can affect mood, suggesting that the microbiota–gut–brain (MGB) axis contributes to the pathogenesis of depression. Many of these pathways overlap with the way in which the gut microbiota are thought to contribute to metabolic disease progression and obesity. In rodents, prebiotics and probiotics have been shown to modulate the composition and function of the gut microbiota. Together with germ-free rodent models, probiotics have provided compelling evidence for a causal relationship between microbes, microbial metabolites, and altered neurochemical signalling and inflammatory pathways in the brain. In humans, probiotic supplementation has demonstrated modest antidepressant effects in individuals with depressive symptoms, though more studies in clinically relevant populations are needed. This review critically discusses the role of the MGB axis in depression pathophysiology, integrating preclinical and clinical evidence, as well as the putative routes of communication between the microbiota–gut interface and the brain. A critical overview of the current approaches to investigating microbiome changes in depression is provided. To effectively translate preclinical breakthroughs in MGB axis research into novel therapies, rigorous placebo-controlled trials alongside a mechanistic and biochemical understanding of prebiotic and probiotic action are required from future research.
1. Introduction
Research on the microbiota–gut–brain (MGB) axis has increased exponentially over the past decade, exposing the far-reaching influence of the gut microbiome in health and disease [1]. The gut microbiome has become a target for novel therapeutics that aim to modify gut microbial metabolism for the benefit of host immunity, energy balance, and mental health. Prebiotics and probiotics have become a mainstay of microbiota–gut–brain axis research, both as a tool to manipulate the host microbiota and as a potential therapeutic for mood disorders including major depressive disorder (MDD) [2]. Probiotics are defined as bacteria that elicit beneficial effects on host physiology when ingested [2]. Prebiotics are substrates (typically fibre, carbohydrates, or saccharides indigestible by the host) that also confer a positive health effect when metabolised by commensal microbes, but do not include the microbes themselves [3].
Depression is common, debilitating, and lacks effective treatment options for the majority of those affected [4]. Conventional antidepressants, while very effective in some people, have not reduced the prevalence of mood disorders in the last few decades, and this is despite a massive increase in the availability and prescription of these medicines at the level of primary care, particularly in the UK [5]. NHS data have shown that the prescription of antidepressants is doubling every 10 years. In 1998 there were about 18 million prescriptions, 36 million in 2008 [6], and the latest data from 2018 showed 71 million prescriptions being given out [7]. This increase can be partly attributed due to longer treatment regimens and an improvement in awareness, identification, and treatment of mental illness in the community over the last few decades. Over a similar period (1993–present), however, the prevalence of common mental disorders has remained comparatively stable [8], with between 15 and 20% of adults (and more women than men) experiencing a common mental disorder, for the most part anxiety or depression, within the last week at the time of polling. Because current antidepressant and anxiolytic agents are not necessarily reducing the prevalence of mental illness in society, adjunct therapies should continue to be investigated. Herein, we review and discuss the efficacy, safety, and potential mechanisms by which prebiotics and probiotics may exert beneficial effects on brain function to mitigate MDD risk.
2. The Gut Microbiome
Comprehending the significance of very large (or very small) numbers is difficult, despite being inherent to descriptions of the biological and physical world. The human microbiome tends to be introduced with massive orders of magnitude, for example the trillions of microbes known to inhabit the digestive tract. These quantifications gloss over the fact that our interpretation of the microbiome remains limited by our own understanding and means of characterising it. The human microbiome is mostly bacterial, but also incorporates commensal residing viruses, archaea, and fungi, the functional significance of which is much less understood. On average, there are 10 commensal bacteria for every nucleated cell in the human body [9], the vast majority of which are concentrated in the colon (large intestine). The modulation and impact of colonic ‘gut’ microbes on brain health is therefore the focus of this review.
Commensal gut microbes are essential to human health. Most prominently, they have broad metabolic functions, are involved in the education and development of host immunity, and protect against the seeding of pathogenic enteric microbes [10]. Through these intimate relationships with host physiology, research on the gut microbiome has permeated all aspects of biomedical science. Initial characterisations of the human microbiome found it to be highly variable between individuals [11]. Moreover, microbiota composition has not been found to reliably predict healthy or disease-associated phenotypes [11,12], despite a shift in microbiome composition compared to healthy controls being associated with obesity and other metabolic disorders, autoimmune disorders, and psychiatric diseases. This is because associations do not distinguish meaningful differences from meaningless ones, and there is no distinction between cause and effect. This uncertainty remains a challenge of microbiome research in health and disease. This somewhat humbling revelation has led to large-scale, ‘multi-omic’ longitudinal studies to robustly characterise strain-specific microbiota profiles in healthy and disease states, with a focus on functional immune and metabolic outcomes [13,14,15,16,17]. However, these studies are still in their infancy. Further investigations that aim to characterise microbial function and specific contributions to host physiology are critical to convert basic research into clinical applications. Effort is also required to achieve improved socioeconomic and ethnic diversity of humans participating in microbiome research, so that new findings can be applied to underrepresented populations with distinct microbiome signatures [18,19].
3. The Microbiota–Gut–Brain Axis: Putative Mechanisms of Bug-to-Brain Communications
One of the most exciting and rapidly evolving fields of neuroscience and biological psychiatry is the MGB axis, which explores the mutual influences and communications between the gut, its commensal microbes, and brain function. Humans have never existed without a symbiotic relationship with microbes. By extension, the brain has never been without signals from the gut and its resident microbes. While much remains to be understood about how microbiota influence the brain and systemic physiology, significant progress in the last decade has provided some insight into the functional role that microbiota play in brain health and disease [1].
The MGB axis has been implicated in the pathogenesis of a wide range of neurological and psychiatric disorders. Dysbiosis of the gut microbiota, which refers to an imbalance in the composition or function of the microbiota, has been associated with various neurological and psychiatric disorders, including autism spectrum disorder, depression, and anxiety [1].
In addition, important work in germ-free mice established the important role that the gut microbiota play to support the development of normal brain function and behaviour [20,21,22,23,24,25]. Through this research, several bidirectional routes of communication have been identified (Figure 1). Principally, these include interaction with the nervous system, in particular the vagus nerve afferents, modulation of the host immune system, and the metabolism and production of small molecules including SCFAs and neuroactive compounds. The following subsections provide a detailed overview of the MGB axis with respect to each specific pathway of communication. Most likely, these pathways operate in parallel.
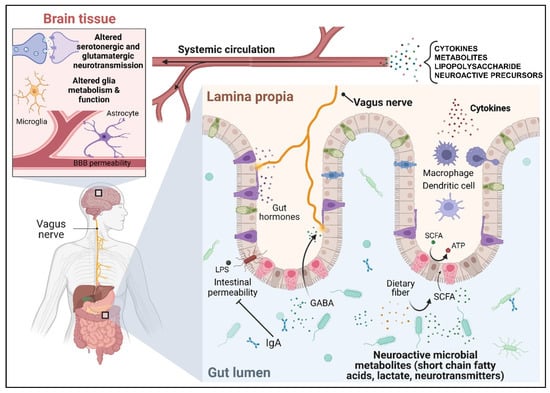
Figure 1.
Summary of the putative mechanisms of communication between the gut microbiota and the brain.
3.1. The Microbiota–Gut–Brain Axis Communicates through the Vagus Nerve
The vagus nerve, the tenth cranial nerve, is the chief neural component of the brain–gut axis, and the primary parasympathetic nerve linking the brain with the viscera. Composed of 80% afferent (and 20% efferent) fibres, vagal afferent endings can respond to both distension of the gastrointestinal (GI) tract via mechanosensors as well as gut peptide hormones, neurotransmitters, and microbial metabolites through a diverse array of chemosensitive and other receptors [26].
Research utilising vagotomies has contributed most to our understanding of how the vagus nerve contributes to brain–gut communication, including signals from gut bacteria. In humans, vagotomy was a consequence of gastrectomy which was, historically, a treatment for duodenal ulcers. In a historical study of 118 patients following partial gastrectomy, a high incidence (43%) of psychiatric symptoms was observed [27]. Complete vagotomy has also been used for the treatment of morbid obesity [28], whereby the main cognitive symptom reported post-operatively was unexplained fatigue alongside altered hedonic perceptions of taste [29]. Reciprocally, vagus nerve stimulation has been used as an adjunct treatment for severe depression [30].
In rodents, intraperitoneal injection of lipopolysaccharide (LPS) is used to model sickness behaviour associated with peripheral and central inflammatory responses. LPS is an integral structural component of the outer membrane of Gram-negative bacteria and a potent activator of the human immune system. Several studies noted that vagotomy reduced the effect of LPS on facets of sickness behaviour, including general activity two hours post-injection [31], social behaviour [32], and food-motivated behaviour [33]. Levels of peripheral IL-1B, increased after LPS treatment, were unaffected by vagotomy [31,32]. Cumulatively, these studies point to the contribution of the vagus nerve to changes in behaviour arising from peritoneal inflammation. Conversely, vagotomy does not prevent an LPS-induced increase in brain IL-1B in rats [34]. While sickness behaviour was not measured, the results indicate that the vagus nerve is not solely responsible for communicating gastrointestinal perturbations to the brain.
Studies conducted more recently have suggested that the vagus nerve may also mediate the effects of gut bacteria on brain function and behaviour. Oral gavage treatment with Lactobacillus rhamnosus (JB-1) reduced anxiety and depressive-like behaviour in the elevated plus maze (EPM) and forced swim test (FST) in healthy mice, respectively [35]. These changes were associated with altered messenger (m)RNA levels of GABAA and GABAB receptors in the brain. Vagotomy prevented the behavioural effects of L. rhamnosus (JB-1) administration as well as the associated changes in GABAA expression in the amygdala and hippocampus. This implicates the vagus nerve in some of the observed molecular and behavioural effects of the probiotic strain. Similarly, it was shown that anxiety-like behaviour associated with dextran sodium sulphate-induced colitis was mitigated by Bifidobacterium longum (NCC3001), but only in animals with an intact vagus nerve [36]. Lastly, the bacterial species Lactobacillus reuteri was shown to act specifically via the vagus nerve in its ability to restore normal social behaviour in mouse models of autism spectrum disorder (ASD) when administered in drinking water [37]. This was thought to be independent of other commensal microbes, as L. reuteri was also able to rescue aberrant social behaviour in germ-free (GF) mice, and supplementing ASD-like mice with L. reuteri did not change the overall gut microbiota composition of the animals. Despite the rigorous nature of this study in elucidating the basic mechanism of action of L. reuteri, it remains unclear from all these studies how microbes interact with the vagus nerve. This is an important issue to resolve, as the latter study described here suggests that L. reuteri has the capacity to rescue social behaviour and restore oxytocin levels in the paraventricular nucleus of the hypothalamus as one strain among hundreds, at least. Understanding and harnessing this mechanism will be important in translating and applying microbiome-based therapeutics in the future via modulation of the vagus nerve.
3.2. The Microbiota–Gut–Brain Axis Communicates through the Immune System
Inflammation is associated with depression in a bidirectional manner [38]. Chronic gut, liver, and brain inflammatory diseases such as inflammatory bowel disease (IBD), non-alcoholic fatty liver disease (NAFLD), and multiple sclerosis (MS) are associated with increased rates of depression [39,40,41]. The peripheral immune system, therefore, is a high plausible indirect route of communication between the collective gut microbiota, brain function, and behaviour.
Studies in GF mice established the essential role for commensal microbes in the maturation of the host innate and adaptive immune systems [42] mediated by bacterial polysaccharide A [43]. GF mice have an underdeveloped immune system, including reduced number of adaptive immune cells and reduced development of secondary lymphoid structures [44]. In the GI tract itself, converging mechanisms compartmentalise commensal microbes and prevent intrusion into sterile tissue and aberrant immune responses. A dense and insoluble luminal mucous barrier, largely formed of the mucin MUC2, is maintained by goblet cells in the epithelial lining of the large intestine. Within the mucous layer, dendritic cells sample microbial epitopes in a perpetual state of immunotolerance induced by MUC2-associated glycans [42,45]. In parallel, microbial production of butyrate induces the formation of regulatory T cells [46,47] which, together with the tolerogenic dendritic cells, promote B cell secretory IgA production to further prevent pro-inflammatory innate immune activation in the gut [48,49].
Collectively, it has become apparent that the qualitative composition of the gut is crucial to maintaining systemic immune homeostasis. It is not just the presence or absence of microbes that affect host immunity, but rather the balance of an unfathomably large ecosystem. Dysbiosis of the microbiome by diet or other means therefore has the potential to disrupt immune homeostasis. Given that up to 80% of human immune cells reside in the GI tract [50], it is conceivable that the downstream implications of disrupted immune homeostasis in the gut, such as increased circulating pro-inflammatory cytokines, induce or exacerbate chronic inflammatory diseases and psychiatric disorders [44].
In a maternal immune activation (MIA) model of ASD in mice, offspring showed increased intestinal permeability, gut microbiota dysbiosis, and increased colonic inflammation by increased IL-6 expression alongside reduced social behaviour [51]. Treatment of the offspring with Bacteroides fragilis normalised levels of GI tract inflammation and reversed aberrant social behaviour, supporting an immune-mediated link between the microbiota, gut barrier integrity, and brain and behaviour [51]. Distal to the gut, a complex microbiota has also been shown to be crucial for microglia maturation and ongoing function [52]. Researchers found that microglia from GF mice, under homeostatic “steady-state” conditions, were immature and had a diminished immune response [52]. Accordingly, microglia from GF mice failed to mount an appropriate immune response to intracranial injections of LPS or lymphocytic choriomeningitis virus, characterised by reduced mRNA expression levels of TNF and IL-1B. The precise coordinates of the intracranial injection was not reported, which limits the interpretability and reproducibility of this finding. Antibiotic depletion of the microbiota produced a similar immature microglial phenotype in adult mice, suggesting an ongoing and highly plastic relationship between the gut microbiota and brain microglia [52].
The absence of microbiota has also been associated with an altered microglia phenotype in aged mice [53]. Specific pathogen-free (SPF), but not GF mice, showed an increase in intestinal permeability at ~2 years age compared to ~2 months age. This coincided with a detrimental accumulation of the microbial metabolite N 6-carboxymethyllysine in the aged microglia, which was not found in the brain of GF mice. GF mice showed reduced markers of brain oxidative stress and microglial dysfunction compared to SPF mice, which was attributed to the N 6-carboxymethyllysine. Thus, the commensal microbiota appears to play a role in normal microglia physiology and age-related pathology throughout the lifespan.
3.3. Microbiota–Gut–Brain Axis Communicates through Circulating Gut-Derived Microbial Metabolites and Neurotransmitters
Metabolites produced by microbiota are thought to be the key effector molecules that allow the microbiota to communicate and influence the function of distal organs, including the brain. Using untargeted metabolomics by mass spectrometry (MS) to compare the plasma of GF and SPF mice, Wikoff et al. showed that the gut microbiome affects the blood concentrations of many metabolites [54]. Tryptophan, for example, was present at higher concentrations in GF mice, while serotonin was present at higher concentrations in SPF mice, which may suggest a role for the microbiota in the metabolism of dietary tryptophan to serotonin [54]. Untargeted metabolomics of the brain, blood, and faeces of SPF mice showed that they exhibit elevated levels of neurotransmitters in the gut compared to GF mice, including serotonin, GABA, and glutamate, among others [55]. Other microbial-derived metabolites elevated in the serum of SPF mice compared to GF animals, such as indoxyl sulphate and indole-3-lactate, were also markedly elevated in the brain. In a different study, indoxyl sulphate was one of a panel of microbial metabolites required for normal fear extinction learning in mice, a behaviour that was suggested to rely on microbiota-derived signals throughout the lifespan of the mice [56]. Together, these studies substantiate the link between microbial metabolites, brain function, and altered behaviour.
Several studies have identified one or several metabolites that, when administered to GF/antibiotic-treated mice, partially or fully reproduce the effects of the presence of the gut microbiota on aspects of host immunity, brain function, and behaviour. Microbially derived butyrate and propionate, for example, are potent histone deacetylase (HDAC) inhibitors in vitro and in vivo. In the colonic mucosa, these SCFAs have been shown to act via this mechanism to switch off dendritic cell pro-inflammatory cytokine expression and enhance Foxp3 expression to drive the induction of regulatory T cells and immune tolerance [46]. As previously discussed, ongoing signals from a complex gut microbiota are required for normal microglia function in the brain [52]. By contrast, the microglia of GF mice lack these microbial signals, and display an immature phenotype including increased expression of the survival and growth factors CSF1R and DDIT4, whereby expression is typically attenuated in mature microglia. A 4-week supplementation with a mixture of SCFAs (acetate, propionate, and butyrate) in drinking water was shown to restore normal gene expression profiles in the GF mice, including reduced DDIT4 expression, and reduce the number of CSF1R+ cells to the levels exhibited by SPF mice [52]. In a follow-up study, they identified that the primary metabolite mediating microglial fitness in SPF mice was microbially derived acetate. GF mice showed depleted levels of acetate in the brain and blood compared to SPF mice. Oral gavage of 13C-derived acetate in GF mice was shown to enter brain tissues after six hours, by which point the 13C label had been incorporated into tricarboxylic acid cycle metabolites.
Microbial metabolites can also facilitate the effects of the maternal gut microbiota on offspring brain development [57]. Embryonic brain development markedly differs between GF and SPF mothers who are otherwise healthy. Specifically, axonal projections between the thalamus and cortex fail to connect properly, leading to defects in sensorimotor behaviours. Metabolomic profiling of the maternal sera and offspring brain revealed reduced levels of certain microbial metabolites that, when supplemented to antibiotic-treated dams, restored normal axonogenesis and sensorimotor behaviour in the offspring [57]. In this way, microbial metabolites not only contribute to gut–brain communication across the organism’s lifespan, but also between mother and foetus during the perinatal period.
4. Perturbed Microbiota–Gut–Brain Axis Metabolism in Mood Disorders
4.1. Profiling Gut Microbiota Changes in Mood Disorders: The Way Forward
The gut microbiota can be profiled using several complementary omics approaches, including transcriptomics, metagenomics, metaproteomics, and metabolomics. Transcriptomics, which involves the untargeted analysis of RNA molecules in a sample, provides an insight into the gene expression patterns of the microbiota [58]. Metagenomics, on the other hand, involves the direct sequencing of microbial DNA from environmental samples, providing information on the taxonomic and functional diversity of the microbiota [59]. Metaproteomics involves the analysis of proteins expressed by the microbiota, providing information on the functional capacity of the microbial community [60]. Together with metabolomic profiling, these omics approaches can provide complementary information on the taxonomic composition, function, and metabolic capacity of the gut microbiota. Their integration can serve as a comprehensive set of tools with which to explore the complex set of interactions between the gut microbiota and the host.
Several studies in the last decade have found relationships between the gut microbiome and major depressive disorder (MDD) in humans [61,62]. Despite these significant findings, there is no consensus on which bacterial taxa, if any, are specific to depressive symptoms [61]. Interestingly, a meta-analysis of microbiome studies across a spectrum of psychiatric disorders found no real specificity between the relative abundance of different microbial taxa and a given disease, and no decrease in alpha-diversity (within-patient diversity or “richness”) [62]. Rather, the most consistent observation were similar patterns of microbial changes between each disorder compared to the healthy control group, including a reduction in the abundance of butyrate-producing bacteria in patients with MDD, bipolar disorder, schizophrenia, and anxiety [62]. A high level of inter-study variation was observed. These discrepancies are likely due in part to methodological differences between studies, but also may be due to the substantial interindividual variation in gut microbiome composition [11]. Given that functional metabolic processes are conserved across bacterial taxa [11], variable gut microbial taxonomy between depressed individuals is likely to exist, but may still give rise to highly convergent downstream functional outcomes. As a result, analysis of faecal and/or gut mucosal metabolites, or functional analysis of retrieved genomic data, may be more informative than describing the relative abundance of bacteria in the gut [63]. This concept is supported by a 2019 study investigating the inter-individual variation between the faecal microbiome and the faecal metabolome [64]. It was found that the participants (>1000 unrelated twins recruited from the UK twin registry) shared approximately double the number of metabolic pathways (82%) than microbial species (43%). Fundamentally, the study revealed that a variable microbial taxonomic signature does not necessarily lead to equivalent volatility in the downstream biochemistry. It is therefore more intuitive to explore the microbiome by using integrative, complementary approaches to microbiota profiling, for example faecal metabolomic and annotated metagenomic methods that can report on function, as opposed to the classical 16S ribosomal RNA (rRNA) amplicon sequencing, which is comparatively information-poor.
4.2. Animal Studies
Metabolites produced by the gut microbiota play a role in host pathways related to energy metabolism, immunity, and inflammation that connect the gut, liver, and brain [65]. Direct evidence has been provided by studies that have demonstrated neurobehavioural changes associated with faecal microbiota transplantation (FMT) from humans with MDD into rodents. In comparison to germ-free (GF) mice colonised with microbes from healthy (control) individuals, GF mice receiving microbes from patients with MDD showed increased depressive-like behaviours after 2 weeks [66]. This included a reduction in the time that the mice spent in the centre of an open field test (OFT) and increased time spent immobile in the FST. Depressive-like behaviour was linked to changes in carbohydrate and amino acid metabolism in faecal and serum samples, as well as in the hippocampus [66]. However, different metabolites within these pathways were either increased or decreased in mice receiving FMT from depressed individuals. Hippocampal glycine levels were reduced in mice who received the “depressed microbiota” compared to mice who received the “healthy microbiota”. Given that glycine is required to facilitate glutamatergic neurotransmission at N-methyl-D-aspartate receptors (NMDARs), this particular metabolic alteration may have contributed to the altered behaviours that were observed [67]. For example, hypofunction of NMDARs by alterations to the glycine binding site are thought to be implicated in the pathophysiology of schizophrenia in humans [68]. FMT in mice from depressed patients mediated the neuroimmune and neuroendocrine pathophysiology of depressive-like behaviour [69]. Depressed patients showed an increase in plasma pro-inflammatory cytokines, cortisol, and the kynurenine/tryptophan ratio. Following FMT, similar changes in plasma tryptophan metabolism were observed in recipient rats, but no changes in the level of pro-inflammatory cytokines or cortisol were observed. The rats also showed increased depressive-like behaviour. Both depressed individuals and the recipient rats showed reduced richness and diversity in the composition of the gut microbiome. From these studies, it can be concluded that microbe composition associated with depression can induce depressive-like behaviours in rodents, which may be mediated by host changes in energy metabolism and the levels of endogenous neuromodulatory metabolites. However, it remains uncertain from these studies whether microbiota that are associated with MDD in humans also directly contribute to inflammation. A more comprehensive assessment of host immunity post-FMT, for example the measurement of intestinal barrier integrity, circulating endotoxin levels, quantification of specific immune cell populations, and host response to immune challenges, is warranted.
A recent systematic review evaluated 241 metabolomic studies in animal models of depression [70]. Individual metabolites were investigated by a simple vote-counting method. In the brain, levels of serotonin, dopamine, and GABA amongst others were significantly downregulated, whereas kynurenine and myo-inositol were consistently upregulated. In studies that specifically investigated the metabolite composition of the prefrontal cortex (PFC), only glutamate was significant, which was significantly downregulated. In the blood, the most consistently upregulated metabolite in animal models of depression was N-acetyl glycoproteins. These results will have been influenced by the underlying methodologies of each study, including the analysis technique (nuclear magnetic resonance [NMR] spectroscopy or MS) and how depressive-like behaviour was induced and measured. Chronic mild stress (CMS) was the most common model of depression in the systematic review [71]. This may have influenced the prevalence of inflammation-related metabolites present as CMS has been associated with the induction of peripheral inflammation [72,73]. Additionally, MS was employed in the reviewed literature more often than NMR spectroscopy or magnetic resonance spectroscopy (MRS). While high inter-study variability was noted, trends towards decreased neurotransmitter levels in the brain, including reduced glutamate levels, and increased markers of inflammation in the brain and periphery were found to be the prominent features [70].
It is worth noting that substantially less research has focused on anxiety-like behaviours in relation to the microbiota–gut–brain axis. In humans, however, anxiety and depressive symptoms are highly comorbid [74], and in a large proportion of animal models for depression, anxiety-like behaviour is often assayed as part of a battery of behavioural tests by virtue of the OFT or EPM [66,75]. In one study [75], anxiety-like behaviour was related to the gut-derived metabolite 4-ethylphenyl sulphate (4EPS). 4-ethyl phenol is derived from dietary tyrosine via gut microbiota metabolism before sulphation to 4EPS by the host. When gnotobiotic animals were colonised with single strains that either did or did not produce 4EPS, 4EPS was shown to accumulate in the brain after injection of probenecid, which prevents small molecule efflux across the blood–brain barrier (BBB). It was therefore concluded that this gut-derived molecule was able to access the brain parenchyma. 4EPS was found to increase anxiety-like behaviour, but not cognition (Y-maze) or motor function (beam traversal, pole descent, wire hang tests) by disrupting oligodendrocyte maturation and myelination [75].
To conclude, there is substantial evidence for altered gut and brain metabolism in preclinical models of mood disorders—both in terms of the levels of endogenous neurotransmitters and metabolites that are exclusively microbially derived. Much of this evidence has accumulated recently in the last few years, and further research will be required to corroborate these findings in individuals with MDD and/or anxiety to assess the translational validity of candidate metabolites.
4.3. Human Studies
Clinical studies have confirmed that there is perturbation of the microbiota–gut–brain axis, including alterations to individual metabolites, in patients with mood disorders. In two independent studies, the abundance of Alistipes spp. has been shown to be elevated in individuals with MDD compared to controls [76,77]. Mechanistically, Alistipes spp. may be related to metabolic pathways associated with increased inflammation, as another study showed correlations with intensity of abdominal pain in irritable bowel syndrome [78]. Through the use of gut microbiota sequencing in tandem with gut metabolomics analysis, changes in gut microbiota composition between depressed and non-depressed individuals have been related to changes in host metabolism [79]. Bacteroides spp. were enriched in individuals with MDD and correlated with reduced faecal amino acid levels (leucine and proline) and increased lipid levels (2-indolecarboxylic acid and itaconic acid). Separately, the authors found that GABA and related metabolites were reduced in the gut in those with MDD compared to healthy controls, and the authors speculated that these findings may influence brain GABA levels in MDD. In 2022, it was also elegantly demonstrated that a significant portion of the variation in serum GABA levels (up to 22%), which were reduced in individuals with depressed bipolar disorder compared to healthy controls, could be explained by whether or not the gut microbiota of a given individual contained genes essential for GABA synthesis [80]. Moreover, GABA levels have been shown to be increased in the blood of obese individuals after faecal microbiota transplant from lean individuals, which coincided with increased insulin sensitivity [81]. These studies provide further evidence that changes to the gut microbiota can affect the level of circulating neurotransmitters. Nevertheless, it remains unclear to what extent microbially produced neurotransmitters, for example GABA, directly contribute to central neurotransmitter levels [82].
Two large (n > 1000) cohorts with microbiome metagenomic data, alongside quality of life (QoL) or depression scores, have shed further light on the possible links between gut microbial metabolism and MDD in humans [83]. In the first cohort, two metabolites were positively associated with QoL scores, including synthesis of 3,4-dihydroxyphenylacetic acid (DOPAC, a dopamine metabolite) and isovaleric acid (a SCFA). The correlation of QoL scores with DOPAC was reproduced in the second cohort. Butyrate-producing bacteria were positively associated with QoL in both cohorts [83]. Conversely, in a study of 113 children, chronic psychosocial stress showed a significant positive correlation with the faecal levels of several SCFAs, including butyrate and isovalerate [84]. Overall, these correlational studies in humans support the preclinical evidence for a causal role of the gut microbiota and associated microbial metabolites in the pathogenesis of depressive symptoms. Many discrepancies remain in terms of the directionality of these metabolites, and how the composition of a “depressed” or “healthy” microbiome should be defined.
5. Modulation of the Microbiota–Gut–Brain Axis by Prebiotics and Probiotics
5.1. Prebiotics Modulate Brain Function and Behaviour in Animal Models
In rodents, galacto-oligosaccharide (GOS) prebiotics have been shown to elevate brain BDNF in the hippocampus. BDNF expression is thought to be important for ongoing neurogenesis in the adult hippocampus [85], and its levels in human blood have been shown to be negatively correlated with depression severity [86]. BDNF protein was also shown to be elevated in the hippocampus of rats after five-week supplementation with the human milk oligosaccharide (HMO) 2′-fucosyllactose [87]. In this case, associative learning behaviour was also assessed, and rats supplemented with the HMO tended to acquire the operant conditioning task in fewer sessions than the control group [87]. Prebiotic supplementation has also been shown to modulate anxiety and depressive-like behaviour in rodents. Mice fed GOS for three weeks were shown to be less anxious than controls (drinking water only) in the LDB 24 h after LPS administration [88]. The anxiolytic effect of the prebiotic was associated with an increase in gut Bifidobacterium spp. and an attenuation of IL-1B and 5-HT2A receptor proteins in the frontal cortex after LPS administration [88]. Prebiotics have also been shown to mitigate the adverse behavioural effects of both early life stress by maternal separation [89] and chronic mild stress [90] in rodents. This included reduced anxiety and depressive-like behaviour [90] and an increased rate of spatial learning in the Morris water maze which was independent of early life stress [89]. Lastly, postnatal supplementation of rats with GOS has been shown to reduce anxiety-like behaviour in the EPM at juvenile (3-week-old), adolescent (8-week-old), and adult (4–6-month-old) timepoints [91]. Interestingly, this effect was only observed if the rats were supplemented prior to weaning, and not when supplemented in adulthood, which suggests that the anxiolytic effects of GOS supplementation in unchallenged animals may depend on the modulation of brain function during a specific neurodevelopmental window. No overall effect of postnatal prebiotic supplementation was observed on the gut microbiome in any age group, although GOS supplementation was found to modify the relationship between branched-chain amino acid levels and age in the hippocampus [91]. Overall, there is substantial preclinical evidence to support a neuromodulatory effect of prebiotics at both juvenile and adult timepoints, despite conflicting evidence in some cases. The mechanisms largely remain unclear due to inconsistent effects of prebiotics on the microbiome—some studies have reported a bifidogenic effect whereas others report no clear effects. A proportion of this variation may be due to inconsistencies in the age at which animals were treated, the length of treatment, the type of prebiotic, and the amount of prebiotic administered. Comparing the effects of prebiotics in GF and SPF animals would allow confirmation of prebiotics’ action by modification of the microbiome in vivo. This would be important, as GOSs have been shown to have microbiota-independent anti-inflammatory effects, possibly via TLR4-mediated IL-10 production [92]. While the effects of prebiotics have not been assessed in GF animals, prebiotics have been shown to be effective in gnotobiotic animals colonised with just a single strain [93].
5.2. Probiotics Modulate Brain Function and Behaviour in Animal Models
It is well-established that commensal bacteria contribute to host physiology, including brain function and behaviour [1]. The contributions of individual bacterial strains to the developmental and homeostatic mechanisms involving the gut–brain axis in gnotobiotic models has been discussed in Section 3. Here, studies involving probiotic supplementation as a potential therapeutic will be discussed (Table 1).
In BALB/c mice, which exhibit an innately anxious phenotype relative to other mouse strains [94], Bifidobacterium longum 1714 reduced anxiety-like behaviour in the OFT and reduced depressive-like behaviour in the FST, while Bifidobacterium breve 1205 reduced anxiety-like behaviour in the EPM [95]. Both probiotics were administered daily for three weeks prior to the start of behavioural testing as 1 × 109 colony forming units (CFU)/mL by oral gavage. In each case, a three-week treatment with escitalopram (daily oral gavage 20 mg/kg) in the positive control group did not alter the behaviour in each of these tests, relative to phosphate-buffered saline controls. It is possible that, as the BALB/c mice were healthy, the behavioural assays used may not have been sensitive enough to detect subtle changes in behaviour when the mice were unchallenged. Escitalopram treatment did reduce stereotypic behaviour in the marble burying test (MBT), as did both Bifidobacterium probiotics. While no insight was provided into how the probiotics elicited these behavioural changes, and why the behavioural changes appeared to be strain-dependent, a previous study in BALB/c mice using the probiotic L. rhamnosus (JB-1) showed that behavioural effects were dependent on the vagus nerve [35].
Since these early studies that pioneered the field of probiotics as novel therapeutics for mood disorders, further efforts have been made to understand how certain strains exert their effects via the microbiota–gut–brain axis. In a brain in vivo MRS study, BALB/c mice were treated with L. rhamnosus (JB-1) daily for four weeks, and brain imaging was performed weekly [96]. A significant increase in brain glutamate + glutamine (Glx) levels was identified after two weeks of treatment. Brain Glx remained elevated for 4 weeks after cessation of probiotic treatment. In contrast, brain NAA and GABA were elevated after four weeks of probiotic supplementation but returned to baseline levels immediately after treatment cessation. The study suggests that central levels of Glx levels may be of relevance to the enduring effects of L. rhamnosus (JB-1) on behaviour in mice, though the effects of probiotic supplementation on levels of glutamate and glutamine need to be individually resolved and quantified in future work.


Table 1.
Summary of probiotic studies in rodent models on anxiety and depressive-like behaviours and brain metabolism. ↑ increase, ↓ decrease.
Table 1.
Summary of probiotic studies in rodent models on anxiety and depressive-like behaviours and brain metabolism. ↑ increase, ↓ decrease.
| Model Organism (Paradigm) | Probiotic (Dose) | Duration of Treatment | Behavioural Effects | Neurochemical Effects | Reference |
|---|---|---|---|---|---|
| 7-week-old male BALB/c mice (healthy) | B. longum 1714 or B. breve 1205 (1 × 109 CFU, daily) | 3 weeks prior to start of behavioural tests, 3 weeks during behavioural testing (6 weeks total) | ↓ anxiety (OFT, MBT) and ↓ depressive-like behaviour (FST) (B. longum 1714) ↓ anxiety (EPM and MBT) (B. breve 1205) | Not applicable | [95] |
| 6-week-old male BALB/c mice (vagotomy) | L. rhamnosus JB-1 (1 × 109 CFU, daily) | 4 weeks (behavioural assays started during final week of treatment) | ↓ anxiety (EPM, OFT) ↓ depressive-like behaviour (FST) | Altered hippocampal GABAA receptor subunit expression, effects dependent on vagus nerve | [35] |
| 4-week-old male BALB/c mice (healthy MRI and MRS brain imaging) | L. rhamnosus JB-1 (1 × 109 CFU, daily) | 4 weeks (imaging performed throughout duration of treatment at weeks 0, 1, 2, 3, 4, and 4 weeks post-treatment cessation) | Not applicable | ↑ brain Glx at weeks 2, 3, 4, 8 ↑ brain NAA at weeks 1, 2, 3, 4 ↑ brain GABA at week 4 only | [96] |
| Male Swiss albino mice 25–30 g (28 days CMS) | L. plantarum MTCC 9510 (2 × 1010 CFU, daily) | 4 weeks (duration of CMS paradigm) | CMS ↓ locomotion (OFT), ↑ depressive-like behaviour (FST, TST), reversed by L. plantarum MTCC 9510 | CMS ↑ BBB permeability, ↑ brain NF-kB, reversed by L. plantarum MTCC 9510 | [97] |
| Male neonatal C57Bl/6 mice (maternal separation) | B. pseudocatenulatum CECT 7765 (1 × 108 CFU, daily) | 20 days (PND2 to PND21) | MS ↑ anxiety (EPM). B. pseudocatenulatum CECT 7765 ↓ anxiety (EPM) | Not applicable | [98] |
| 4-week-old male Sprague Dawley rats (chronic HFD, 60% of kilocalories from fat) | B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lc. Lactis W19, Lc. Lactis W58 (3.75 × 108 CFU/mL in drinking water, daily) | 5 weeks prior to start of behavioural tests, 10 weeks total | Probiotic mixture ↓ depressive-like behaviour (FST) irrespective of diet. | Not applicable | [99] |
Probiotics have also been shown to exert remedial effects in stressed rodents. In one study [97], male Swiss albino mice were subject to 28 days of CMS or left undisturbed. Half of the CMS group and half of the control group received Lactobacillus plantarum MTCC 9510 by oral gavage during the same period. Chronic treatment with L. plantarum MTCC 9510 normalised locomotor activity in the OFT, which had been significantly reduced by CMS [97]. Immobility in the FST and tail suspension test were also reduced by the CMS/probiotic compared to CMS/vehicle, but it was not discussed as to whether activity levels in the preceding OFT may have confounded these results. CMS increased serum TNF and brain NF-kB, which were prevented by the probiotic. Increased peripheral inflammation may have occurred through increased intestinal permeability and increased circulating endotoxin levels after CMS, which were also attenuated by L. plantarum MTCC 9510 treatment [97]. BBB integrity was also reduced by CMS and increased by the probiotic. Interestingly, the microbial metabolite butyrate has been shown to increase both gut barrier integrity [100] and BBB integrity [101], possibly via increased HIF-1 expression [100]. In this study, caecal butyrate was significantly increased in the CMS/probiotic mice relative to the CMS/vehicle mice, although probiotic treatment alone did not significantly increase butyrate levels. Overall, this study suggests that chronic probiotic treatment can attenuate stress-induced inflammation and depressive-like behaviours in mice by increasing intestinal and brain barrier integrity.
In a maternal separation model of early life stress, male offspring were treated with Bifidobacterium pseudocatenulatum CECT 7765 or vehicle between postnatal day (PND)2 and PND21 [98]. In the small intestine, maternal separation led to increased interferon gamma and interleukin (IL)-10, suggesting the induction of a Th1-type inflammatory response—interferon gamma being the archetypal Th1 cytokine [102]. Increased IL-10 may represent the homeostatic response to attenuate the pro-inflammatory response to early life stress. B. pseudocatenulatum CECT 7765 supplementation significantly reduced interferon gamma levels in the intestine, both after early life stress and in non-stressed control mice. At PND41, approximately three weeks after cessation of probiotic treatment and early life stress, mice showed increased anxiety-like behaviour in the EPM, which was attenuated by probiotic treatment [98]. This suggests that concurrent probiotic treatment is sufficient to prevent emotional dysfunction induced by early life stress, though it was not tested whether probiotic treatment after early life stress could also prevent early life stress. On the whole, single-species probiotics have been shown to attenuate inflammation and anxiety-like behaviour in response to stress paradigms, although it remains unclear as to why a given probiotic strain is preferable over another.
The effects of multi-species probiotics have been less well investigated in rodents. The efficacy of eight bacterial strains (Bifidobacterium spp., Lactobacillus spp., Lactococcus spp. altogether) on affecting depression-like behaviour were assessed after HFD-induced obesity in rats [99]. Interestingly, probiotic intake was found to have a clear, independent effect on depressive-like behaviour in the FST. Total immobility time was significantly reduced in both lean and obese rats given the probiotic, whereas obesity with or without probiotic supplementation had no effect on depressive-like behaviour [99]. As expected, chronic HFD feeding elevated circulating levels of LPS, although in this case, probiotics had no effect. Plasma metabolomics was performed by mass spectrometry. HFD-induced obesity led to many (209) metabolite changes compared to lean rats, including a highly significant increase in the tryptophan catabolite and NMDA agonist [103] quinolinic acid. Following FDR correction, probiotic treatment increased the levels of two tryptophan metabolites, indole-propionic acid and indole-acrylic acid, as well as acetylornithine. While no significant diet x probiotic treatment interactions were found, indole-propionic acid has been shown to exert anti-inflammatory effects, attenuating astrocytic CCL2 cytokine expression in a mouse model of experimental autoimmune encephalitis [104]. By exerting this central anti-inflammatory effect, indole-propionic acid may contribute to the antidepressant-like effects of the probiotic mixture.
In the above studies, only male rodents were used as test subjects. While the studies discussed here are not an exhaustive review of the current literature, the vast majority have reported the effects on males only. Further studies exploring the possible interaction between probiotic treatment and sex, in healthy subjects and in disease models, are urgently required to identify, validate, and translate the therapeutic effects of microbiota modulation in mood disorders.
5.3. Prebiotics and Probiotics in Human Studies
The psychotropic effects of probiotics, and to a lesser extent prebiotics, have been assessed in humans, although the composition, timeframe, and nature of the human subjects varies greatly between studies (Table 2). In healthy subjects, a GOS prebiotic has been shown to significantly reduce the waking cortisol response after three weeks of treatment [105]. This effect was associated with reduced attentional vigilance to negative stimuli and increased vigilance to positive stimuli, which was significantly different from the placebo. Whilst this suggests some possibly anxiolytic effects of the prebiotic at a subconscious level, there were no significant changes in self-reported measures of anxiety or negative affect pre- and post-treatment. One possible reason for this was because the subjects were not clinically anxious or depressed at baseline. In fact, previous history of a psychiatric disorder was an exclusion criterion of the study [105]. Another study stratified healthy individuals into high and low trait anxiety at recruitment [106] and found that four weeks GOS treatment reduced self-reported anxiety scores in highly anxious participants, but not in individuals who had low levels of anxiety to begin with. This substantiates the notion that modifying the microbiota to improve mood may be most efficacious in people with mood disorders.
In people with irritable bowel syndrome (IBS), where anxiety is highly comorbid [107], GOS prebiotic treatment both increased levels of faecal Bifidobacteria and reduced anxiety scores using the Hospital Anxiety and Depression (HAD) scale at baseline and follow-up [108]. Despite some evidence pointing to greater efficacy of prebiotics in people with greater symptom severity, the one study that has tested (GOS) prebiotic treatment in people with MDD found no significant effect on Beck Depression Inventory (BDI) scores after an eight-week treatment regime [109]. The study power was comparable with other studies (n = 36–38 per group). Anxiety scores were not measured. Notably, about 10% of individuals taking the prebiotic experienced mild adverse effects including gastrointestinal complaints. Overall, there is limited evidence for the efficacy of prebiotics for improving mood, which is supported by a recent meta-analysis [110]. The evidence is especially sparce in people with clinically significant levels of anxiety and depression, and further studies are needed to determine whether prebiotics are effective in people with clinically significant anxiety disorders.
Probiotics have thus far been more extensively tested as a potential therapeutic for low mood and MDD. In healthy subjects, 30-day probiotic treatment with Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduced anxiety and depressive symptoms using the HAD scale [111]. Conversely, BDI and Beck Anxiety Inventory (BAI) scores were not significantly different between pre- and post-treatment. Lactobacillus rhamnosus (JB-1), which showed promising preclinical results in reducing basal levels of anxiety-like behaviour in healthy mice [35], failed to improve mood or reduce anxiety in healthy volunteers after eight weeks of treatment [112].
Probiotics have also been trialled in individuals with subclinical levels of anxiety and depression. In individuals with mild to moderate levels of depression (Patient Health Questionnaire [PHQ]9 score 5–19), a four-week treatment with a multi-species probiotic (Bio-Kult Advanced®) significantly reduced PHQ9 scores, suggesting an antidepressant effect [113]. In contrast with previous research in prebiotics [105], salivary cortisol levels were unaffected by probiotic treatment, and no changes in blood C-reactive protein (CRP) levels were identified [113]. In individuals with moderate to severe depression, a probiotic mixture of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 did not improve psychological outcomes after eight weeks of treatment, including depression or anxiety [114]. The sample size was greater and the duration of treatment longer than a previously successful trial of the same probiotic mixture in healthy subjects [111].
Lastly, probiotics have been trialled in individuals with a clinical diagnosis of MDD. In the two studies that have sought to assess the antidepressant efficacy of probiotics for clinically significant depression, both showed an improvement in BDI score at follow-up with the probiotics compared to those given the placebo [109,115]. In both studies, both placebo and probiotic groups were receiving SSRI treatment—the use of the probiotic was therefore an adjunct therapeutic. One study trialled Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 for eight weeks [109] and the other trialled Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum for eight weeks [115]. In both cases, circulating inflammatory markers such as CRP and the kynurenine/tryptophan ratio were downregulated after probiotic supplementation compared to placebo.


Table 2.
Summary of probiotic studies on anxiety and/or depressive symptoms in humans. ↑ increase, ↓ decrease.
Table 2.
Summary of probiotic studies on anxiety and/or depressive symptoms in humans. ↑ increase, ↓ decrease.
| Paradigm | Probiotic (Dose) | Duration of Treatment | Effects | Reference |
|---|---|---|---|---|
| Adult males and females (healthy) | L. helveticus R0052 and B. longum R0175 (3 × 109 CFU, daily) | 30 days | ↓ anxiety and depression HAD scores No effect on BDI or BAI scores | [111] |
| Adult males (healthy) | L. rhamnosus JB-1 (1 × 109 CFU, daily) | 4 weeks | No differences between probiotic and placebo for BDI, BDAI, or serum cytokine levels | [112] |
| Adult males and females (mild to moderate depression: PHQ9 score 5–19) | Bacillus subtilis, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium infantis, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus delbrueckii ssp. Bulgaricus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus helveticus, Lactobacillus salivarius, Lactococcus lactis ssp. Lactis, Streptococcus thermophilus. (2 × 109 CFU, daily) | 4 weeks | No effect of probiotic on salivary cortisol or serum CRP Probiotic ↓ PHQ9 scores | [113] |
| Adult males and females (moderate or severe depression: ≥11 QIDS-SR16 or ≥14 DASS-42) | L. helveticus R0052 and B. longum R0175 (3 × 109 CFU, daily) | 8 weeks | No effects of probiotic treatment on self-reported depression or anxiety scales, and no effect on serum CRP, IL-1B, IL-6, TNF, BDNF, or vitamin D | [114] |
| Adult males and females (MDD diagnosis, moderate-severe symptoms) | L. acidophilus, L. casei, and B. bifidum (6 × 109 CFU, daily) | 8 weeks | Probiotic ↓ BDI score compared to placebo Probiotic ↓ serum CRP level compared to placebo | [115] |
| Adult males and females (MDD diagnosis, mild-moderate symptoms) | L. helveticus R0052 and B. longum R0175 (1 × 1010 CFU, daily) | 8 weeks | Probiotic ↓ BDI score compared to placebo Probiotic ↓ serum kynurenine/tryptophan ratio compared to placebo | [109] |
| Adult pregnant women (at least mild anxiety or depression: ≥10 EPDS or ≥40 STAI) | B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lc. Lactis W19, Lc. Lactis W58 (5 × 109 CFU, daily) | From 26 to 30 weeks gestation until delivery | No effect of probiotic on perinatal maternal depression (EPDS) or anxiety (STAI) scores | [116] |
| Adult pregnant women (healthy) | L. rhamnosus HN001 (6 × 109 CFU, daily) | From 14 to 16 weeks gestation until 6 months postpartum | Probiotic significantly ↓ depression (EPDS) and anxiety (STAI) scores compared to placebo | [117] |
| Adult pregnant women (obese: BMI ≥ 30 kg/m2) | L. rhamnosus GG and B. lactis BB12 (6.5 × 109 CFU, daily) | From 12 to 18 weeks gestation until 36 weeks gestation | No effect of probiotic on depression (EPDS) or anxiety (STAI-6) scores compared to placebo | [118] |
5.4. Prebiotics and Probiotics during the Perinatal Period
Preclinical studies have investigated the effects of prebiotic and probiotic treatments during the perinatal period on offspring health outcomes, whereas clinical studies are lacking. There is currently no evidence for the consumption of prebiotics on maternal wellbeing outcomes—a recent systematic review did not identify any relevant studies using prebiotics [119]. A feature of recent and ongoing clinical trials is the effect on pregnancy outcomes [120] and the prevention of autoimmune-related disease in infants with conflicting results of both beneficial and no effects reported [121,122,123,124].
Maternal prebiotic intake has been shown to alter the behaviour of juvenile and adult offspring in mice [125]. Specifically, maternal GOS intake increased exploratory behaviour in young offspring compared to dams fed normal drinking water only. In adult offspring, anxiety-like behaviour was reduced and social behaviour was increased by maternal GOS intake. Age-dependent changes in NMDA subunit receptor expression was noted alongside increases in offspring faecal butyrate and propionate [125]. Direct stimulation of early life gut microbes through neonatal prebiotic administration from PND3 to PND21 has also been shown to increase NMDA receptor subunit GluN2A, synaptophysin, and brain-derived neurotrophic factor protein levels in the hippocampus at PND22 [126]. These effects persisted from weaning into early adulthood, at 8 weeks of age, despite the cessation of prebiotic supplementation. While gut microbiota analysis was not performed in this study, the same galacto-oligosaccharide prebiotic has been shown to be Bifidogenic in adult rodents [85].
A meta-analysis on the effect of probiotics on maternal metabolic health indicates that probiotic interventions improve glucose and lipid metabolism, including reducing insulin resistance [127]. Recently, probiotics have been investigated in clinical trials for their effects on maternal mental health during pregnancy and the postpartum period [119]. Completed clinical studies have shown mixed results. A 2021 study aimed to assess the effect of prenatal probiotic (n = 20) or placebo (n = 20) consumption from mid- to late gestation until delivery on maternal depression and anxiety symptoms during pregnancy [116]. Both Edinburgh Postnatal Depression Scale and State-Trait Anxiety Inventory scores decreased between the baseline and the eight-week follow-up in both probiotic and placebo groups. Thus, while there was a significant effect of time, there was no significant difference between the intervention and control groups. No biomarkers of treatment response were used, and faecal microbiome sequencing was not performed. A study of 380 women who received either Lactobacillus rhamnosus HN001 (193) or placebo (187) from 14 to 16 weeks’ gestation until six months postpartum were assessed for anxiety and depressive symptoms during pregnancy and the postpartum period [117]. This study found a reduction in these symptoms in the probiotic group both during and after pregnancy compared to those given a placebo. The large sample size and long duration of treatment of this study is a strength that should be replicated in future studies, particularly those that will assess offspring development. On the other hand, supplementation of Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12, at a similar dose, did not affect depression and anxiety scores in an obese (BMI > 30.0 kg/m2) cohort of 164 women [118]. Probiotics were taken starting from a similar timepoint, from mid-gestation until birth rather than post-weaning. Hence, this was an assessment of mental wellbeing during pregnancy only, rather than postpartum anxiety and depression.
The effects of maternal probiotic consumption on offspring health outcomes have also thus far only been investigated preclinically, and the scope of research here is limited. Mouse prenatal probiotic supplementation has been suggested to increase serum IgG and intestinal secretory IgA [128] in the adult offspring, which may have immunoprotective roles [129]. In line with this, antibody responses to hepatitis-B surface antigen were shown to be higher in offspring from BGOS-supplemented dams compared to controls [128]. Similarly, supplementation of rat dams with Lactobacillus fermentum CECT5716 during pregnancy and the first two weeks of lactation increased IgA in offspring pups at postnatal day 14 [130]. L. fermentum supplementation also had a positive effect on plasma fatty acid profiles in both dams and offspring. The same experiment demonstrated that these effects may have been mediated by changes to the mammary milk composition, which showed increased polyunsaturated fatty acid contents, reduced palmitic acid, and increased IgA in probiotic-supplemented dams compared to controls [131]. Despite these changes, L. fermentum CECT5716 was only detected in half of the supplemented dams (3/6) suggesting that the presence of the probiotic in the milk was not a prerequisite for imparting positive immune effects on the offspring. Rather, changes to the intestinal microbiota, with subsequent systemic effects, may have mediated the beneficial effects on the rat milk composition [131].
Our laboratory has shown that faecal SCFA levels, specifically butyrate and propionate, are elevated in adult offspring of dams supplemented with a multispecies probiotic [132]. Maternal probiotic intake was also associated with reduced anxiety and depressive-like behaviour in these offspring, and faecal butyrate levels correlated positively with brain mRNA levels of PFKFB3, a marker of astrocytic metabolic activity. This suggests that early life exposure to the probiotic, possibly by maternal coprophagia or simply cohabitation, allows for long-term colonisation of the probiotic species that promote increased butyrate and propionate production and altered brain energetics. Likewise, another study [133] explored the effects of maternal CD, HFD, and HFD/probiotic during gestation and nursing in male rat offspring at 16 weeks of age (eight animals per group). In support of the results of our laboratory, a non-significant trend towards increased faecal butyrate levels was observed in the offspring of HFD/probiotic dams compared to CD dams (22% increase) and HFD dams (35% increase).
Overall, there is some evidence for a beneficial role of early life exposure, prenatal and postnatal, on maternal intake of prebiotics and/or probiotics to support normal brain and immune development. Further research in this area should be encouraged, particularly as to whether supplementation during pregnancy and lactation mitigates the consequences of maternal obesity on offspring neurobiology and behaviour [134]. This is because there is some evidence that offspring microbial reconstitution has been suggested to reverse neurological and behavioural deficits induced by a maternal high-fat diet [135], while other work suggests that microbial reconstitution post-weaning is not sufficient to rescue neurological adaptations that arise during development [25]. Studies that include maternal diet are also needed because the type of diet consumed by the host, and the subsequent metabolic and inflammatory profiles associated with the diet, may significantly impact the efficacy of probiotic administration in terms of its anti-inflammatory properties and ability to modify the brain and behaviour [136].
6. Gastrointestinal Engraftment of Probiotic Supplements
In humans, conflicting evidence exists as to whether probiotic supplementation exerts a lasting effect on gut microbiota composition. Supplementation of adults with Bifidobacterium infantis 35624 for eight weeks increased B. infantis 35624 in faeces compared to placebo; however, the levels of the probiotic declined to baseline levels at the follow-up timepoint, two weeks after ceasing the daily probiotic treatment [137]. In another study, Bifidobacterium spp. have been shown to colonise the adult human gut only transiently, for eight days [138]. Conversely, supplementation of healthy individuals with Bifidobacterium longum AH1206 remained detectable in stool for up to six months after discontinuation of the probiotic [139]. Human studies investigating the effect of maternal perinatal probiotic supplementation on the offspring faecal microbiota have also exhibited conflicting results. One study investigated the effect of a Lactobacillus-containing probiotic supplement, between the last month of gestation and the first 3 months of lactation, on the longitudinal microbiota of the infants. Sequencing of 16S rRNA revealed that the infants had increased levels of faecal Lactobacillus rhamnosus GG (contained in the probiotic) relative to control infants at 3 months of age, but not at 12 months [140]. However, in mothers supplemented with Lactobacillus rhamnosus GG during late pregnancy (but not lactation), children have been shown to have detectable faecal levels of L. rhamnosus GG until at least 12 months of age [141]. The infants of mothers who did not receive the probiotic had no detectable levels of L. rhamnosus GG at this timepoint.
There are two somewhat opposing mechanisms to explain why probiotics persistently colonise the gut in some individuals but not others. On the one hand, colonisation success may depend on the pre-existing level of conspecific strains in the gut. A 2016 study found that only probiotic strains of species already existing in the gut were able to be detected three months after probiotic treatment, the reason being that the species will already have the appropriate microenvironment with which to colonise successfully [142]. On the other hand, low levels of B. longum predict B. longum AH1206 engraftment success [139]. Further metagenomic analysis suggested that colonisation success was related to the under-exploitation of certain carbohydrates produced in the gut, providing a window of opportunity for the probiotic strain to establish itself.
While an indigenous “mature” microbiome is generally quite resistant to colonisation by probiotics, a microbe-deficient GF gut is readily colonised by probiotic species [143]. Accordingly, the neonatal gut may be much more readily colonisable with probiotic species, especially if they are obtained from the maternal milk or faecal matter. In humans, bacterial strains that colonise the infant gut in the first six months of life have been shown to persist for at least the next six years, even if they are not the dominant strain of a given species [144]. Similarly, multi-strain probiotic administration to pre-term infants resulted in the increased abundance of probiotic Bifidobacterium spp. strains up to five months later [145]. This is evidence for the notion that early engraftment may be an important factor in the longevity of colonisation and may contribute to early life prebiotic and probiotic interventions may be more efficacious than in adulthood.
7. Depression and Obesity: A Bidirectional Relationship
Depression and obesity are both common, multifactorial disorders that impart severe global health and economic burdens [4,146]. At an individual level, they can reduce the quality of life of those affected. Substantial epidemiological evidence exists for a bidirectional relationship between depression and obesity [147,148,149,150]. A meta-analysis of longitudinal studies found that obese individuals had a 55% increased risk of developing depression over time, while depressed persons were 58% more likely to become obese [147]. Reciprocally, weight loss has been shown to improve mood in people with depressive symptoms [151]. While antidepressant medication usage can cause short-term weight gain, medication status is not thought to mediate the relationship between obesity and depression [152].
Obesity and depression share genetic risk factors. Genome-wide association studies (GWAS) have identified an overlap between the genetic risk for depression [153] and obesity [154] through single-nucleotide polymorphisms near or in OLFM4 and NEGR1. The association between NEGR1, obesity, and depression has been validated in independent human studies that cumulatively exceed 1.5 million individuals [155,156,157,158,159,160]. Transcriptomic analysis has mapped NEGR1 expression in the CNS predominately to the hypothalamus in both humans [160] and rodents [161]. NEGR1−/− mice exhibit reduced brain volume, as well as reduced relative hippocampal volume [162]. This is thought to be due to the fact that NEGR1 is required for ongoing neurogenesis in the adult hippocampus in mice [163]. NEGR1−/− mice also demonstrate increased anxiety-like behaviour in the OFT and EPM, and increased depressive-like behaviour in the FST and tail suspension test compared to wildtype controls [163]. In the hypothalamus, NEGR1 function may be related to the central regulation of feeding behaviours and energy balance [164].
Chronic, low-grade inflammation is a hallmark of both obesity and depression [165] (Figure 2). In a large, case-control study of depression in the UK Biobank [38], low-grade inflammation was identified in 21% of patients (defined as serum CRP > 3 mg/L). In the same study, the genetic risk for lifetime depression was found to be positively correlated with serum CRP levels. Interestingly, while this signature of inflammation in depression was found to be independent of BMI and other clinical and demographic features, the correlation with genetic risk was entirely dependent on BMI and smoking status. BMI was higher in individuals with depression. Thus, it was concluded that the genetic contribution to an inflammatory phenotype in depression is entirely dependent on these two factors [38].
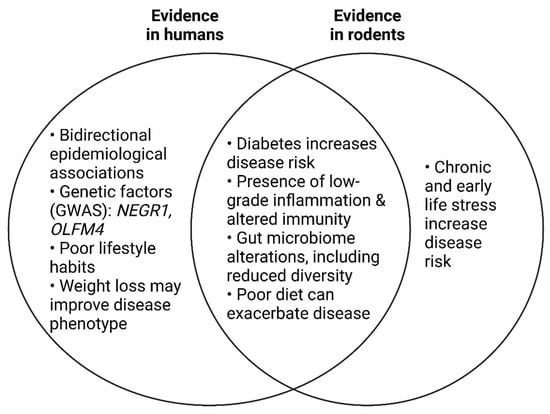
Figure 2.
Summary of the evidence from human and/or rodent studies that demonstrate overlapping pathophysiology between obesity and major depressive disorder.
Limited preclinical evidence exists to support a role for the microbiota in the bidirectional relationship between obesity and depression. Transplanted gut microbiota from mice subject to HFD-induced obesity increased anxiety-like and stereotypic behaviour in recipient mice [166]. Recipient mice were first given broad-spectrum antibiotics to deplete the microbiome. Given that antibiotic treatment alone can alter behaviour [167], this may have confounded the results. Nevertheless, the obese-type gut microbiota were also found to increase intestinal permeability as well as peripheral and central inflammation without a significant change in body weight [166].
Isoleucine is a metabolite that may link depression and obesity pathophysiology. Increased isoleucine has previously been related to depression in both humans [168] and rodents [169,170]. Circulating isoleucine levels can affect the brain bioavailability of tryptophan, the key substrate for serotonin synthesis. Tryptophan and isoleucine (as well as other large, neutral amino acids such as leucine and valine) compete for uptake by the transporter LAT1 into the brain. Therefore, increased circulating isoleucine levels are thought to competitively reduce brain tryptophan uptake, thereby reducing brain serotonin levels and potentially altering behaviour [109,171] (Figure 3). Our laboratory has previously shown that, in an LPS model of post-inflammatory depressive-like behaviour (as assessed by the FST), increased plasma isoleucine levels also coincided with increased glutamine levels and reduced glutamate levels in the brain [170]. This may suggest that different models of depressive-like behaviour in rodents may converge on a similar mechanism involving altered circulating isoleucine and glutamate/glutamine ratios in the brain.
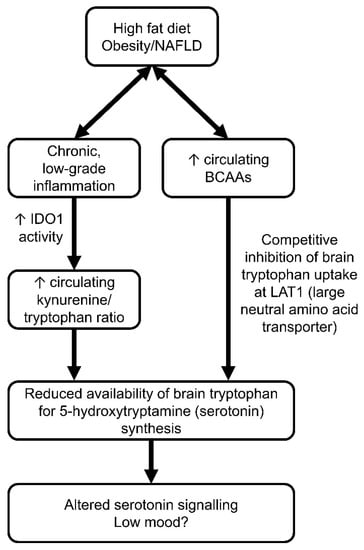
Figure 3.
Potential mechanisms describing how diet-induced metabolic syndrome (obesity/NAFLD) may lead to altered brain plasticity and low mood. On the left side of the flowchart, chronic, low-grade inflammation is thought to upregulate peripheral indoleamine 2,3-dioxygenase (IDO1) activity, leading to increased kynurenine production from tryptophan and reduced brain tryptophan uptake for central serotonin synthesis. On the right, elevated circulating branched-chain amino acids may arise after prolonged high-fat diet feeding. BCAAs compete with tryptophan for the large neutral amino acid transporter (LAT1), reducing brain uptake of tryptophan and central serotonin synthesis. This may lead to altered neurotransmission and low mood. ↑ increase.
Increased isoleucine may arise due to an excessive level of dietary fat. Mice fed a HFD displayed elevated circulating BCAA levels alongside depressive-like behaviour and reduced extracellular hippocampal levels of serotonin [169]. In the same study, metformin reduced circulating BCAA levels and showed antidepressant-like effects in an HFD model of depression, suggesting that BCAA levels may indeed play a causal role in the pathophysiology of depression, especially in the context of metabolic dysfunction. Despite an apparent connection between diet-induced obesity and elevated circulating BCAAs, it is unlikely to be the diet itself that directly contributes to elevated BCAA levels. In the aforementioned study, BCAA levels were matched between the HFD and CD, whereby increased energy consumption was derived exclusively from the increased lipid content [169]. Rather than through diet, elevated fasting BCAAs are thought to be due to impaired catabolic pathways through the branched-chain keto acid and acyl-CoA pathways [172]. To date, no study has tested whether enzymes responsible for BCAA catabolism are altered in expression or activity in individuals with depression, so it remains unclear whether this mechanism—which likely underlies the association with elevated BCAAs in obesity [173]—may also account for the observation of elevated isoleucine in individuals with depression.
Overall, there is substantial epidemiological evidence for a bidirectional relationship between obesity and depression, which may be mediated by chronic low-grade inflammation or other genetic factors associated with the regulation of food intake. Isoleucine may be important to this concept, because existing evidence suggests that its elevation may be a consequence of altered metabolism in obesity [173] and a contributory cause of depression by depleting brain bioavailability of tryptophan. Studies in humans are required to determine the putative role for obese-type gut microbiota in peripheral inflammation and depression.
8. Conclusion and Perspectives for Future Research
The microbiota–gut–brain axis is mediated by a complex network of neural, endocrine, and immune signalling pathways, as well as by the release of various metabolites and neurotransmitters that can influence both gut function and brain function [1]. In both healthy [57] and diseased rodent models [37], the gut microbiota and associated metabolites have been demonstrated to regulate brain development, plasticity, and behaviour. In humans, dysfunction of the microbiota–gut–brain axis through obesity and metabolic disease has been implicated in a range of psychiatric and neurological disorders, including depression [83].
Prebiotics and probiotics, through their modulation of the gut microbiome, have emerged as potential therapeutic options for the management of depression [2]. While more research is needed to fully understand the complex mechanisms underlying this relationship, the evidence so far suggests that interventions targeting the gut microbiota have not been convincingly translated in clinical trials, though more studies are required in patients with moderate to severe symptoms. Ultimately, a comprehensive approach to mental health should take into account the bidirectional relationship between the gut and brain, and consider the potential role of prebiotics and probiotics in promoting optimal gut health and overall well-being.
Moreover, the use of prebiotics and probiotics is relatively safe and well-tolerated, making them an attractive option for individuals who are looking for natural and non-invasive interventions for depression. It is important to note, however, that not all prebiotics and probiotics are created equal, and their efficacy and safety may vary depending on the specific strain, dose, and formulation.
Moving forward, a key area of research in the field of prebiotics and probiotics for depression is to better understand the specific mechanisms by which these interventions modulate the gut microbiome and ultimately affect mental health outcomes. In terms of uncovering key molecular and metabolic intermediates in microbe-to-brain communication, isotopically labelled tracers (e.g., 13C-labelled glucose, acetate, or lactate) should be considered. Depending on the positioning of the labelling at each carbon atom, the kinetics of different metabolic pathways can be inferred following prebiotic and probiotic treatments [174].
Placebo-controlled clinical trials that explore the potential use of prebiotics and probiotics to target specific subtypes of depression, such as treatment-resistant depression or postpartum depression, are also needed. By shedding light on the precise mechanisms by which prebiotics and probiotics impact mental health, future research in this field has the potential to uncover novel therapeutic targets and ultimately lead to more effective treatments for depression.
Author Contributions
Conceptualization, D.E.R.-S. and D.C.A.; writing—original draft preparation, D.E.R.-S.; writing—review and editing, D.E.R.-S. and D.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Jorm, A.F.; Patten, S.B.; Brugha, T.S.; Mojtabai, R. Has increased provision of treatment reduced the prevalence of common mental disorders? Review of the evidence from four countries. World Psychiatry 2017, 16, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Moncrieff, J. Trends in prescriptions and costs of drugs for mental disorders in England, 1998–2010. Br. J. Psychiatry 2012, 200, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, G. NHS prescribed record number of antidepressants last year. BMJ 2019, 364, l1508. [Google Scholar] [CrossRef] [PubMed]
- Baker, C. Mental Health Statistics: Prevalence, Services and Funding in England. 2021. Available online: https://commonslibrary.parliament.uk/research-briefings/sn06988/ (accessed on 28 April 2022).
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2015, 486, 207–214. [Google Scholar] [CrossRef]
- After the Integrative Human Microbiome Project, what’s next for the microbiome community? Nature 2019, 569, 599. [CrossRef]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Sailani, M.R.; Contrepois, K.; Zhou, Y.; Ahadi, S.; Leopold, S.R.; Zhang, M.J.; Rao, V.; Avina, M.; Mishra, T.; et al. Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature 2019, 569, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Gaulke, C.A.; Sharpton, T.J. The influence of ethnicity and geography on human gut microbiome composition. Nat. Med. 2018, 24, 1495–1496. [Google Scholar] [CrossRef]
- Finding diversity in the microbiome. Nat. Med. 2019, 25, 863. [CrossRef]
- Hegstrand, L.R.; Hine, R.J. Variations of brain histamine levels in germ-free and nephrectomized rats. Neurochem. Res. 1986, 11, 185–191. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; MacQueen, G.M.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255-e119. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 2014, 817, 115–133. [Google Scholar] [CrossRef]
- Sinclair-Gieben, A.H.C.; Clark, C.G.; Dean, A.C.B. Psychiatric Illness following Surgery for Duodenal Ulcer. Scott. Med. J. 1962, 7, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Kral, J. Vagotomy for treatment of severe obesity. Lancet 1978, 311, 307–308. [Google Scholar] [CrossRef]
- Kral, J.G. Behavioral effects of vagotomy in humans. J. Auton. Nerv. Syst. 1983, 9, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, S.T.; Sears, P.; Ruvuna, F.; Bunker, M.; Conway, C.R.; Dougherty, D.D.; Reimherr, F.W.; Schwartz, T.L.; Zajecka, J.M. A 5-Year Observational Study of Patients with Treatment-Resistant Depression Treated with Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am. J. Psychiatry 2017, 174, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Layé, S.; Bluthé, R.M.; Kent, S.; Combe, C.; Medina, C.; Parnet, P.; Kelley, K.; Dantzer, R. Subdiaphragmatic vagotomy blocks induction of IL-1 beta mRNA in mice brain in response to peripheral LPS. Am. J. Physiol. Integr. Comp. Physiol. 1995, 268, R1327–R1331. [Google Scholar] [CrossRef]
- Bluthé, R.M.; Walter, V.; Parnet, P.; Layé, S.; Lestage, J.; Verrier, D.; Poole, S.; Stenning, B.E.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. Comptes Rendus Acad. Sci. III 1994, 317, 499–503. [Google Scholar]
- Bretdibat, J.; Bluthe, R.; Kent, S.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide and Interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav. Immun. 1995, 9, 242–246. [Google Scholar] [CrossRef]
- Van Dam, A.-M.; Bol, J.G.; Gaykema, R.P.; Goehler, L.E.; Maier, S.F.; Watkins, L.R.; Tilders, F.J. Vagotomy does not inhibit high dose lipopolysaccharide-induced interleukin-1β immunoreactivity in rat brain and pituitary gland. Neurosci. Lett. 2000, 285, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef] [PubMed]
- Pitharouli, M.C.; Hagenaars, S.P.; Glanville, K.P.; Coleman, J.R.; Hotopf, M.; Lewis, C.M.; Pariante, C.M. Elevated C-Reactive Protein in Patients with Depression, Independent of Genetic, Health, and Psychosocial Factors: Results from the UK Biobank. Am. J. Psychiatry 2021, 178, 522–529. [Google Scholar] [CrossRef]
- Boeschoten, R.E.; Braamse, A.M.J.; Beekman, A.T.F.; Cuijpers, P.; van Oppen, P.; Dekker, J.; Uitdehaag, B.M. Prevalence of depression and anxiety in Multiple Sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2017, 372, 331–341. [Google Scholar] [CrossRef]
- Keefer, L.; Kane, S.V. Considering the Bidirectional Pathways Between Depression and IBD: Recommendations for Comprehensive IBD Care. Gastroenterol. Hepatol. 2017, 13, 164–169. [Google Scholar]
- Labenz, C.; Huber, Y.; Michel, M.; Nagel, M.; Galle, P.R.; Kostev, K.; Schattenberg, J.M. Nonalcoholic Fatty Liver Disease Increases the Risk of Anxiety and Depression. Hepatol. Commun. 2020, 4, 1293–1301. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Walker, M.M.; Talley, N.J. The mucosal immune system: Master regulator of bidirectional gut–brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004, 303, 1662–1665. [Google Scholar] [CrossRef]
- Peterson, D.A.; McNulty, N.P.; Guruge, J.L.; Gordon, J.I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2007, 2, 328–339. [Google Scholar] [CrossRef]
- Faria, A.M.C.; Weiner, H.L. Oral tolerance. Immunol. Rev. 2005, 206, 232–259. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Mossad, O.; Batut, B.; Yilmaz, B.; Dokalis, N.; Mezö, C.; Nent, E.; Nabavi, L.S.; Mayer, M.; Maron, F.J.M.; Buescher, J.M.; et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat. Neurosci. 2022, 25, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Liu, C.-W.; Yang, Y.; Hsiao, Y.-C.; Ru, H.; Lu, K. High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice. Nat. Commun. 2021, 12, 6000. [Google Scholar] [CrossRef]
- Chu, C.; Murdock, M.H.; Jing, D.; Won, T.H.; Chung, H.; Kressel, A.M.; Tsaava, T.; Addorisio, M.E.; Putzel, G.G.; Zhou, L.; et al. The microbiota regulate neuronal function and fear extinction learning. Nature 2019, 574, 543–548. [Google Scholar] [CrossRef]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef]
- Valle-Gough, R.E.; Samaniego-Gámez, B.Y.; Apodaca-Hernández, J.E.; Chiappa-Carrara, F.X.; Rodríguez-Dorantes, M.; Arena-Ortiz, M.L. RNA-Seq Analysis on the Microbiota Associated with the White Shrimp (Litopenaeus vannamei) in Different Stages of Development. Appl. Sci. 2022, 12, 2483. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Rizk, G.; Cattoir, V.; Sassi, M.; Thibault, V.; Del Giudice, J.; Gangneux, J.-P. Efficient and Quality-Optimized Metagenomic Pipeline Designed for Taxonomic Classification in Routine Microbiological Clinical Tests. Microorganisms 2022, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Karaduta, O.; Dvanajscak, Z.; Zybailov, B. Metaproteomics—An Advantageous Option in Studies of Host-Microbiota Interaction. Microorganisms 2021, 9, 980. [Google Scholar] [CrossRef]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.-C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders. JAMA Psychiatry 2021, 78, 1343. [Google Scholar] [CrossRef] [PubMed]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.-J.; Fan, S.-H.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Cummings, K.A.; Popescu, G.K. Glycine-dependent activation of NMDA receptors. J. Gen. Physiol. 2015, 145, 513–527. [Google Scholar] [CrossRef]
- Labrie, V.; Roder, J.C. The involvement of the NMDA receptor d-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci. Biobehav. Rev. 2010, 34, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Pu, J.; Liu, Y.; Gui, S.; Tian, L.; Yu, Y.; Song, X.; Zhong, X.; Chen, X.; Chen, W.; Zheng, P.; et al. Metabolomic changes in animal models of depression: A systematic analysis. Mol. Psychiatry 2021, 26, 7328–7336. [Google Scholar] [CrossRef]
- Pu, J.; Yu, Y.; Liu, Y.; Tian, L.; Gui, S.; Zhong, X.; Fan, C.; Xu, S.; Song, X.; Liu, L.; et al. MENDA: A comprehensive curated resource of metabolic characterization in depression. Briefings Bioinform. 2020, 21, 1455–1464. [Google Scholar] [CrossRef]
- López-López, A.L.; Jaime, H.B.; Villanueva, M.D.C.E.; Padilla, M.B.; Palacios, G.V.; Aguilar, F.J.A. Chronic unpredictable mild stress generates oxidative stress and systemic inflammation in rats. Physiol. Behav. 2016, 161, 15–23. [Google Scholar] [CrossRef]
- Pavlov, D.; Bettendorff, L.; Gorlova, A.; Olkhovik, A.; Kalueff, A.V.; Ponomarev, E.D.; Inozemtsev, A.; Chekhonin, V.; Lesch, K.-P.; Anthony, D.C.; et al. Neuroinflammation and aberrant hippocampal plasticity in a mouse model of emotional stress evoked by exposure to ultrasound of alternating frequencies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 90, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Charney, D. Comorbidity of mood and anxiety disorders. Depress. Anxiety 2000, 12, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Funabashi, M.; Adame, M.D.; Wang, Z.; Boktor, J.C.; Haney, J.; Wu, W.-L.; Rabut, C.; Ladinsky, M.S.; Hwang, S.-J.; et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 602, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, D.M.; Riehle, K.; Mistretta, T.A.; Diaz, M.A.; Mandal, D.; Raza, S.; Weidler, E.M.; Qin, X.; Coarfa, C.; Milosavljevic, A.; et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1782–1791. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Li, Z.; Lai, J.; Zhang, P.; Ding, J.; Jiang, J.; Liu, C.; Huang, H.; Zhen, H.; Xi, C.; Sun, Y.; et al. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota-gut-brain axis in bipolar depression. Mol. Psychiatry 2022, 27, 4123–4135. [Google Scholar] [CrossRef] [PubMed]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; Van de Wiele, T.; De Henauw, S. Chronic Psychosocial Stress and Gut Health in Children: Associations with Calprotectin and Fecal Short-Chain Fatty Acids. Psychosom. Med. 2017, 79, 927–935. [Google Scholar] [CrossRef]
- Savignac, H.M.; Corona, G.; Mills, H.; Chen, L.; Spencer, J.P.; Tzortzis, G.; Burnet, P.W. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-d-aspartate receptor subunits and d-serine. Neurochem. Int. 2013, 63, 756–764. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Lan, Y.; Miao, J.; Pan, C.; Sun, W.; Li, G.; Wang, Y.; Zhao, X.; Zhu, Z.; et al. Blood biomarkers of post-stroke depression after minor stroke at three months in males and females. BMC Psychiatry 2022, 22, 162. [Google Scholar] [CrossRef]
- Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Martínez-Lara, E.; Blanco, S.; Martín, M.J.; Castanys, E.; Buck, R.; et al. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J. Nutr. Biochem. 2015, 26, 455–465. [Google Scholar] [CrossRef]
- Savignac, H.M.; Couch, Y.; Stratford, M.; Bannerman, D.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav. Immun. 2016, 52, 120–131. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Neufeld, K.-A.M.; Waworuntu, R.V.; Pusceddu, M.M.; Manurung, S.; Murphy, K.; Strain, C.; Laguna, M.C.; Peterson, V.L.; Stanton, C.; et al. The enduring effects of early-life stress on the microbiota–gut–brain axis are buffered by dietary supplementation with milk fat globule membrane and a prebiotic blend. Eur. J. Neurosci. 2020, 51, 1042–1058. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Spitzer, S.O.; Tkacz, A.; Savignac, H.M.; Cooper, M.; Giallourou, N.; Mann, E.O.; Bannerman, D.M.; Swann, J.R.; Anthony, D.C.; Poole, P.S.; et al. Postnatal prebiotic supplementation in rats affects adult anxious behaviour, hippocampus, electrophysiology, metabolomics, and gut microbiota. iScience 2021, 24, 103113. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Hiller, J.; Van Bergenhenegouwen, J.; Knippels, L.M.J.; Garssen, J.; Traidl-Hoffmann, C. In Vitro Evidence for Immune-Modulatory Properties of Non-Digestible Oligosaccharides: Direct Effect on Human Monocyte Derived Dendritic Cells. PLoS ONE 2015, 10, e0132304. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Onuki, M.; Hattori, K.; Ito, M.; Yamada, T.; Kamikado, K.; Kim, Y.-G.; Nakamoto, N.; Kimura, I.; Clarke, J.M.; et al. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome 2021, 9, 188. [Google Scholar] [CrossRef]
- Belzung, C.; Griebel, G. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behav. Brain Res. 2001, 125, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteriaexert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Janik, R.; Thomason, L.A.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 2016, 125, 988–995. [Google Scholar] [CrossRef]
- Dhaliwal, J.; Singh, D.P.; Singh, S.; Pinnaka, A.; Boparai, R.K.; Bishnoi, M.; Kondepudi, K.K.; Chopra, K. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J. Appl. Microbiol. 2018, 125, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Moya-Pérez, A.; Perez-Villalba, A.; Benítez-Páez, A.; Campillo, I.; Sanz, Y. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav. Immun. 2017, 65, 43–56. [Google Scholar] [CrossRef]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 2017, 79, 40–48. [Google Scholar] [CrossRef]
- Fachi, J.L.; Felipe, J.D.S.; Pral, L.P.; da Silva, B.K.; Corrêa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Câmara, N.O.S.; Souza, L.D.S.E.; et al. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019, 27, 750–761.e7. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Wang, F.; Ding, G.; Chen, W.; Fang, R.; Yao, Y.; Pang, M.; Lu, Z.-Q.; Liu, J. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. 2016, 1642, 70–78. [Google Scholar] [CrossRef]
- Berger, A. Science commentary: Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de La Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxidative Med. Cell. Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.-C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Cowen, P.; Harmer, C.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, N.; Milesi, C.; Burn, O.; van den Bogert, B.; Nauta, A.; Hart, K.; Sowden, P.; Burnet, P.W.J.; Kadosh, K.C. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy females (18–25 years) with corresponding changes in gut bacterial composition. Sci. Rep. 2021, 11, 8302. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Park, J.M.; Lim, C.H.; Cho, Y.K.; Lee, I.S.; Kim, S.W.; Choi, M.-G.; Chung, I.-S.; Chung, Y.K. Anxiety, depression and quality of life in patients with irritable bowel syndrome. Gut Liver 2011, 5, 29–36. [Google Scholar] [CrossRef]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs. placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Baião, R.; Capitão, L.P.; Higgins, C.; Browning, M.; Harmer, C.J.; Burnet, P.W.J. Multispecies probiotic administration reduces emotional salience and improves mood in subjects with moderate depression: A randomised, double-blind, placebo-controlled study. Psychol. Med. 2022, 1–11. [Google Scholar] [CrossRef]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.D.; Bolte, A.C.; der Vaart, I.B.-V.; Claassen, E.; de Weerth, C. Probiotics as a treatment for prenatal maternal anxiety and depression: A double-blind randomized pilot trial. Sci. Rep. 2021, 11, 3051. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Probiotic in Pregnancy Study Group. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Dawe, J.P.; McCowan, L.M.E.; Wilson, J.; Okesene-Gafa, K.A.M.; Serlachius, A.S. Probiotics and Maternal Mental Health: A Randomised Controlled Trial among Pregnant Women with Obesity. Sci. Rep. 2020, 10, 1291. [Google Scholar] [CrossRef]
- Desai, V.; Kozyrskyj, A.L.; Lau, S.; Sanni, O.; Dennett, L.; Walter, J.; Ospina, M.B. Effectiveness of Probiotic, Prebiotic, and Synbiotic Supplementation to Improve Perinatal Mental Health in Mothers: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 622181. [Google Scholar] [CrossRef]
- Sohn, K.; Underwood, M.A. Prenatal and postnatal administration of prebiotics and probiotics. Semin. Fetal Neonatal Med. 2017, 22, 284–289. [Google Scholar] [CrossRef]
- Wickens, K.; Stanley, T.V.; Mitchell, E.A.; Barthow, C.; Fitzharris, P.; Purdie, G.; Siebers, R.; Black, P.N.; Crane, J. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: Does it also reduce atopic sensitization? Clin. Exp. Allergy 2013, 43, 1048–1057. [Google Scholar] [CrossRef]
- Wickens, K.; Barthow, C.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.; Rowden, J.; Kang, J.; Hood, F.; van den Elsen, L.; Forbes-Blom, E.; et al. Maternal supplementation alone with Lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr. Allergy Immunol. 2018, 29, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Barthow, C.; Wickens, K.; Stanley, T.; Mitchell, E.A.; Maude, R.; Abels, P.; Purdie, G.; Murphy, R.; Stone, P.; Kang, J.; et al. The Probiotics in Pregnancy Study (PiP Study): Rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth 2016, 16, 133. [Google Scholar] [CrossRef]
- Baldassarre, M.E.; Palladino, V.; Amoruso, A.; Pindinelli, S.; Mastromarino, P.; Fanelli, M.; Di Mauro, A.; Laforgia, N. Rationale of Probiotic Supplementation during Pregnancy and Neonatal Period. Nutrients 2018, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.C.; Radford-Smith, D.E.; Probert, F.; Ilott, N.; Chan, K.W.; Anthony, D.C.; Burnet, P.W.J. Mom’s diet matters: Maternal prebiotic intake in mice reduces anxiety and alters brain gene expression and the fecal microbiome in offspring. Brain Behav. Immun. 2021, 91, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Chen, L.; Savignac, H.M.; Tzortzis, G.; Anthony, D.; Burnet, P.W. Neonatal prebiotic (BGOS) supplementation increases the levels of synaptophysin, GluN2A-subunits and BDNF proteins in the adult rat hippocampus. Synapse 2016, 70, 121–124. [Google Scholar] [CrossRef]
- Han, M.-M.; Sun, J.-F.; Su, X.-H.; Peng, Y.-F.; Goyal, H.; Wu, C.-H.; Zhu, X.-Y.; Li, L. Probiotics improve glucose and lipid metabolism in pregnant women: A meta-analysis. Ann. Transl. Med. 2019, 7, 99. [Google Scholar] [CrossRef]
- Himaja, N.; Hemalatha, R.; Babu, K.N.; Shujauddin, M. Lactobacillus rhamnosus GG supplementation during critical windows of gestation influences immune phenotype in Swiss albino mice offspring. Benef. Microbes 2016, 7, 195–204. [Google Scholar] [CrossRef]
- Di Benedetto, M.G.; Bottanelli, C.; Cattaneo, A.; Pariante, C.M.; Borsini, A. Nutritional and immunological factors in breast milk: A role in the intergenerational transmission from maternal psychopathology to child development. Brain Behav. Immun. 2020, 85, 57–68. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, À.; Castell, M.; Guardiola, F.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation affects mammary milk composition. J. Dairy Sci. 2020, 103, 2982–2992. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, À.; Castell, M.; Guardiola, F.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J. Lactobacillus fermentum CECT5716 Supplementation in Rats during Pregnancy and Lactation Impacts Maternal and Offspring Lipid Profile, Immune System and Microbiota. Cells 2020, 9, 575. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Probert, F.; Burnet, P.W.J.; Anthony, D.C. Modifying the maternal microbiota alters the gut–brain metabolome and prevents emotional dysfunction in the adult offspring of obese dams. Proc. Natl. Acad. Sci. USA 2022, 119, e2108581119. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Hou, C.-Y.; Chan, J.Y.; Lee, C.-T.; Tain, Y.-L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, 2908. [Google Scholar] [CrossRef] [PubMed]
- Radford-Smith, D.E.; Anthony, D.C. Mechanisms of Maternal Diet-Induced Obesity Affecting the Offspring Brain and Development of Affective Disorders. Metabolites 2023, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Ohland, C.L.; Kish, L.; Bell, H.; Thiesen, A.; Hotte, N.; Pankiv, E.; Madsen, K.L. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology 2013, 38, 1738–1747. [Google Scholar] [CrossRef]
- Charbonneau, D.; Gibb, R.D.; Quigley, E.M. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes 2013, 4, 201–211. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Pochart, P.; Marteau, P.; Arlet, G.; Goderel, I.; Rambaud, J.C. Fecal recovery in humans of viable Bifidobacterium sp ingested in fermented milk. Gastroenterology 1992, 102, 875–878. [Google Scholar] [CrossRef]
- Maldonado-Gómez, M.X.; Martínez, I.; Bottacini, F.; O’Callaghan, A.; Ventura, M.; van Sinderen, D.; Hillmann, B.; Vangay, P.; Knights, D.; Hutkins, R.W.; et al. Stable Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host Microbe 2016, 20, 515–526. [Google Scholar] [CrossRef]
- Dotterud, C.K.; Avershina, E.; Sekelja, M.; Simpson, M.; Rudi, K.; Storrø, O.; Johnsen, R.; Øien, T. Does Maternal Perinatal Probiotic Supplementation Alter the Intestinal Microbiota of Mother and Child? J. Pediatr. Gastroenterol. Nutr. 2015, 61, 200–207. [Google Scholar] [CrossRef]
- Schultz, M.; Göttl, C.; Young, R.J.; Iwen, P.; Vanderhoof, J.A. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J. Craniofacial Surg. 2004, 38, 293–297. [Google Scholar] [CrossRef]
- Li, S.S.; Zhu, A.; Benes, V.; Costea, P.I.; Hercog, R.; Hildebrand, F.; Huerta-Cepas, J.; Nieuwdorp, M.; Salojärvi, J.; Voigt, A.Y.; et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 2016, 352, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Akiyama, T.; Matsuda, K.; Gawad, A.; Makino, H.; Ishikawa, E.; Oishi, K.; Kushiro, A.; Fujimoto, J. Long-term colonization exceeding six years from early infancy of Bifidobacterium longum subsp. longum in human gut. BMC Microbiol. 2018, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, E.I.; Carvalho, M.; Dizzell, S.E.; Kim, S.; Gunn, E.; Twiss, J.; Giglia, L.; Stuart, C.; Hutton, E.K. Persistence of Suspected Probiotic Organisms in Preterm Infant Gut Microbiota Weeks after Probiotic Supplementation in the NICU. Front. Microbiol. 2020, 11, 574137. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Jung, S.J.; Woo, H.-T.; Cho, S.; Park, K.; Jeong, S.; Lee, Y.J.; Kang, D.; Shin, A. Association between body size, weight change and depression: Systematic review and meta-analysis. Br. J. Psychiatry 2017, 211, 14–21. [Google Scholar] [CrossRef]
- Pereira-Miranda, E.; Costa, P.R.; Queiroz, V.A.; Pereira-Santos, M.; Santana, M.L.P. Overweight and Obesity Associated with Higher Depression Prevalence in Adults: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2017, 36, 223–233. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Patel, P.J.; Irvine, K.M.; Russell, A.; Siskind, D.; Anthony, D.C.; Powell, E.E.; Probert, F. Depressive symptoms in non-alcoholic fatty liver disease are identified by perturbed lipid and lipoprotein metabolism. PLoS ONE 2022, 17, e0261555. [Google Scholar] [CrossRef]
- Fuller, N.R.; Burns, J.; Sainsbury, A.; Horsfield, S.; da Luz, F.; Zhang, S.; Denyer, G.; Markovic, T.P.; Caterson, I.D. Examining the association between depression and obesity during a weight management programme. Clin. Obes. 2017, 7, 354–359. [Google Scholar] [CrossRef]
- Gibson-Smith, D.; Bot, M.; Milaneschi, Y.; Twisk, J.W.; Visser, M.; Brouwer, I.; Penninx, B.W.J.H. Major depressive disorder, antidepressant use, and subsequent 2-Year weight change patterns in the Netherlands study of depression and anxiety. J. Clin. Psychiatry 2016, 77, e144–e151. [Google Scholar] [CrossRef] [PubMed]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Thorleifsson, G.; Walters, G.B.; Gudbjartsson, D.F.; Steinthorsdottir, V.; Sulem, P.; Helgadottir, A.; Styrkarsdottir, U.; Gretarsdottir, S.; Thorlacius, S.; Jonsdottir, I.; et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009, 41, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Willer, C.J.; Speliotes, E.K.; Loos, R.J.F.; Li, S.; Lindgren, C.M.; Heid, I.M.; Berndt, S.I.; Elliott, A.L.; Jackson, A.U.; Lamina, C.; et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 2009, 41, 25–34. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Allen, H.L.; Lindgren, C.M.; Luan, J.; Mägi, R.; et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef]
- The Early Growth Genetics (EGG) Consortium. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat. Genet. 2012, 44, 526–531. [Google Scholar] [CrossRef]
- Berndt, S.I.; Gustafsson, S.; Mägi, R.; Ganna, A.; Wheeler, E.; Feitosa, M.F.; Justice, A.E.; Monda, K.L.; Croteau-Chonka, D.C.; Day, F.R.; et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 2013, 45, 501–512. [Google Scholar] [CrossRef]
- Levey, D.F.; Stein, M.B.; Wendt, F.R.; Pathak, G.A.; Zhou, H.; Aslan, M.; Quaden, R.; Harrington, K.M.; Nuñez, Y.Z.; Overstreet, C.; et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 2021, 24, 954–963. [Google Scholar] [CrossRef]
- Lee, A.W.S.; Hengstler, H.; Schwald, K.; Diaz, M.B.; Loreth, D.; Kirsch, M.; Kretz, O.; Haas, C.A.; de Angelis, M.H.; Herzig, S.; et al. Functional inactivation of the genome-wide association study obesity gene neuronal growth regulator 1 in mice causes a body mass phenotype. PLoS ONE 2012, 7, e41537. [Google Scholar] [CrossRef]
- Singh, K.; Jayaram, M.; Kaare, M.; Leidmaa, E.; Jagomäe, T.; Heinla, I.; Hickey, M.A.; Kaasik, A.; Schäfer, M.K.; Innos, J.; et al. Neural cell adhesion molecule Negr1 deficiency in mouse results in structural brain endophenotypes and behavioral deviations related to psychiatric disorders. Sci. Rep. 2019, 9, 5457. [Google Scholar] [CrossRef]
- Noh, K.; Lee, H.; Choi, T.-Y.; Joo, Y.; Kim, S.-J.; Kim, H.; Kim, J.Y.; Jahng, J.W.; Lee, S.; Choi, S.-Y.; et al. Negr1 controls adult hippocampal neurogenesis and affective behaviors. Mol. Psychiatry 2019, 24, 1189–1205. [Google Scholar] [CrossRef]
- Boender, A.J.; van Gestel, M.A.; Garner, K.M.; Luijendijk, M.C.M.; Adan, R.A.H. The obesity-associated gene Negr1 regulates aspects of energy balance in rat hypothalamic areas. Physiol. Rep. 2014, 2, e12083. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; Van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard, E.; Taylor, C.M.; Welsh, D.A.; Berthoud, H.-R. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef]
- Vicentini, F.A.; Mathews, A.J.; Pittman, Q.J.; Swain, M.G.; Sharkey, K.A.; Hirota, S.A. Behavioural adaptations after antibiotic treatment in male mice are reversed by activation of the aryl hydrocarbon receptor. Brain Behav. Immun. 2021, 98, 317–329. [Google Scholar] [CrossRef]
- Bot, M.; Milaneschi, Y.; Al-Shehri, T.; Amin, N.; Garmaeva, S.; Onderwater, G.L.; Pool, R.; Thesing, C.S.; Vijfhuizen, L.S.; Vogelzangs, N.; et al. Metabolomics Profile in Depression: A Pooled Analysis of 230 Metabolic Markers in 5283 Cases with Depression and 10,145 Controls. Biol. Psychiatry 2020, 87, 409–418. [Google Scholar] [CrossRef]
- Zemdegs, J.; Martin, H.; Pintana, H.; Bullich, S.; Manta, S.; Marqués, M.A.; Moro, C.; Layé, S.; Ducrocq, F.; Chattipakorn, N.; et al. Metformin Promotes Anxiolytic and Antidepressant-Like Responses in Insulin-Resistant Mice by Decreasing Circulating Branched-Chain Amino Acids. J. Neurosci. 2019, 39, 5935–5948. [Google Scholar] [CrossRef]
- Chan, S.Y.; Probert, F.; Radford-Smith, D.E.; Hebert, J.C.; Claridge, T.D.W.; Anthony, D.C.; Burnet, P.W.J. Post-inflammatory behavioural despair in male mice is associated with reduced cortical glutamate-glutamine ratios, and circulating lipid and energy metabolites. Sci. Rep. 2020, 10, 16857. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Large neutral amino acids: Dietary effects on brain neurochemistry and function. Amino Acids 2013, 45, 419–430. [Google Scholar] [CrossRef]
- Siddik, A.B.; Shin, A.C. Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond. Endocrinol. Metab. 2019, 34, 234–246. [Google Scholar] [CrossRef]
- Pietiläinen, K.H.; Naukkarinen, J.; Rissanen, A.; Saharinen, J.; Ellonen, P.; Keränen, H.; Suomalainen-Wartiovaara, A.; Götz, A.; Suortti, T.; Yki-Jarvinen, H.; et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: Pathways behind acquired obesity. PLoS Med. 2008, 5, e51. [Google Scholar] [CrossRef]
- Mishra, P.K.; Kumar, A.; Behar, K.L.; Patel, A.B. Subanesthetic ketamine reverses neuronal and astroglial metabolic activity deficits in a social defeat model of depression. J. Neurochem. 2018, 146, 722–734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).