Rosmarinic Acid Improves Cognitive Abilities and Glucose Metabolism in Aged C57Bl/6N Mice While Disrupting Lipid Profile in Young Adults in a Sex-Dependent Fashion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Subjects
2.2. Treatment Administration
2.3. Experimental Procedures
2.4. Emotional Profile and Cognitive Abilities
2.4.1. Elevated Plus Maze (EPM)
2.4.2. Morris Water Maze (MWM)
2.5. Glucose Homeostasis
2.5.1. Glucose Tolerance Test (GTT)
2.5.2. Insulin Sensitivity Test (IST)
2.6. Biochemical Analysis on Trunk Blood for the Evaluation of the Glucose and Fat Metabolism
2.7. Biochemical Analysis on the Hippocampus for the Evaluation of BDNF Levels
2.8. Statistics
3. Results
3.1. Emotional Profile and Cognitive Abilities
3.1.1. Elevated Plus Maze (EPM)
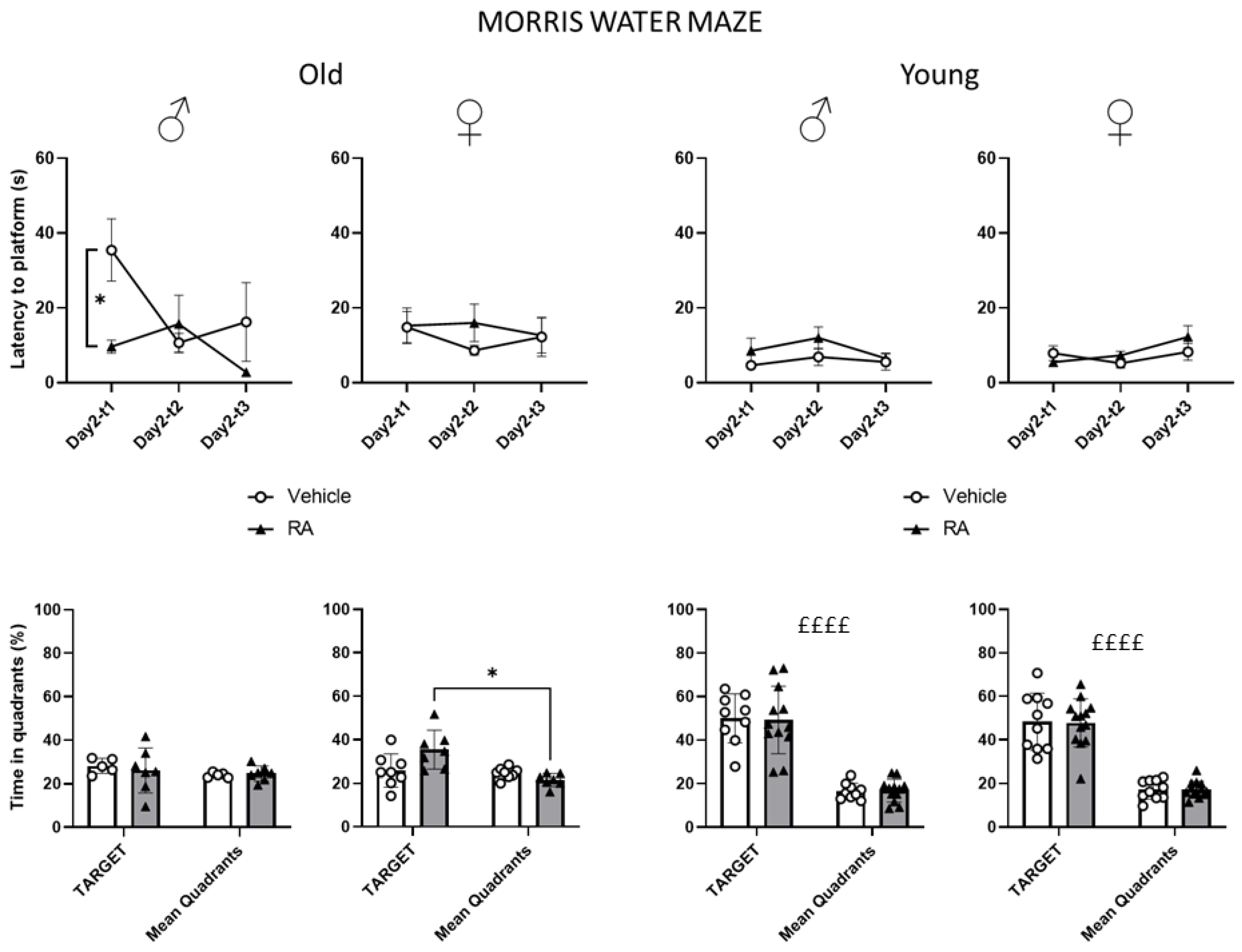
3.1.2. Morris Water Maze (MWM)
3.2. Hippocampal BDNF Levels
3.3. Metabolic Characterisation
3.3.1. Body Weight
3.3.2. Glucose Tolerance Test (GTT)
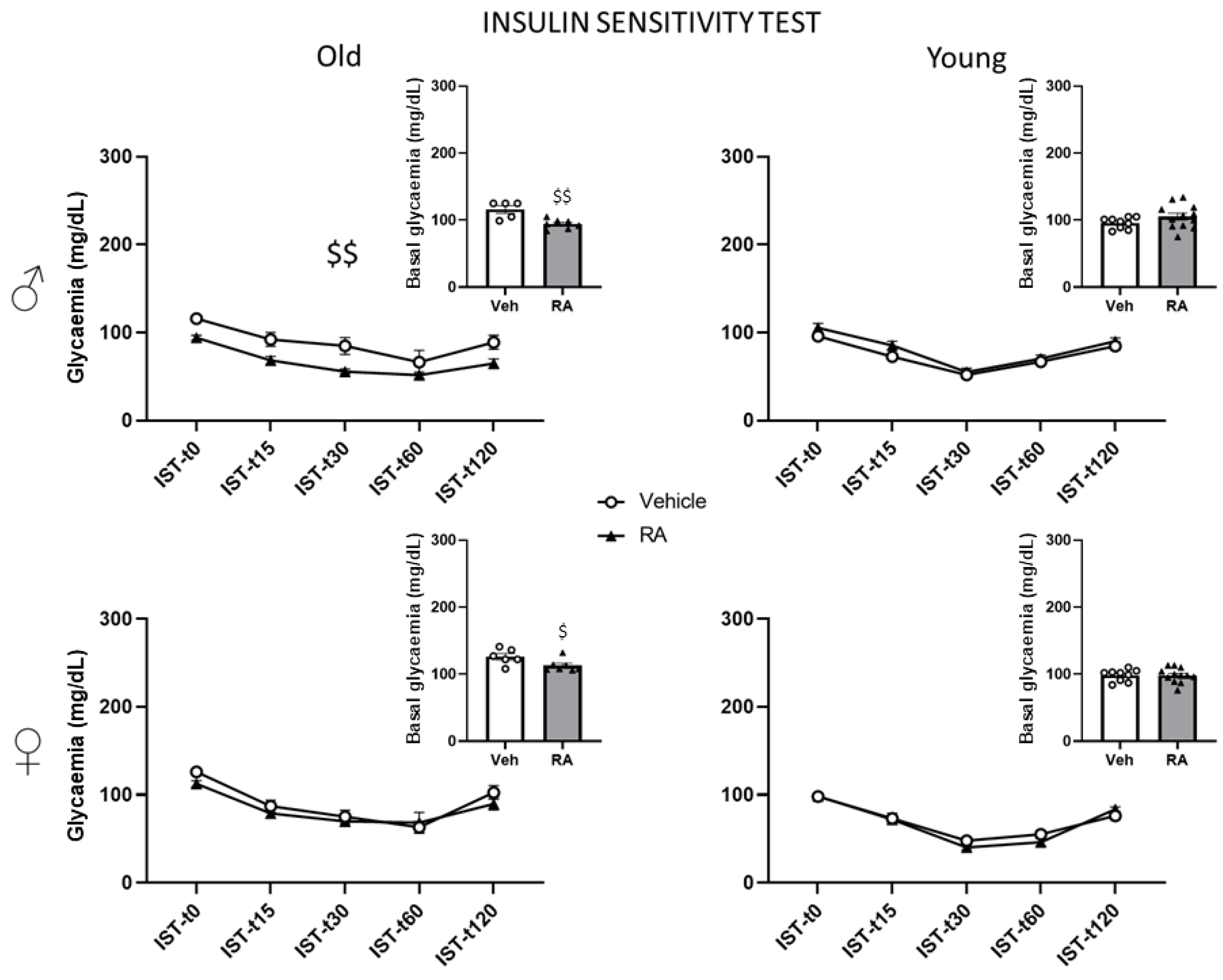
3.3.3. Insulin Sensitivity Test (IST)
3.3.4. Serum Parameters
3.3.5. Plasma Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musillo, C.; Borgi, M.; Saul, N.; Möller, S.; Luyten, W.; Berry, A.; Cirulli, F. Natural products improve healthspan in aged mice and rats: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 121, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Cirulli, F. The p66Shc gene paves the way for healthspan: Evolutionary and mechanistic perspectives. Neurosci. Biobehav. Rev. 2013, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Fuellen, G.; Jansen, L.; Cohen, A.A.; Luyten, W.; Gogol, M.; Simm, A.; Saul, N.; Cirulli, F.; Berry, A.; Antal, P.; et al. Health and aging: Unifying concepts, scores, biomarkers and pathways. Aging Dis. 2019, 10, 883–900. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P.; Cheng, A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006, 29, 632–639. [Google Scholar] [CrossRef]
- Panickar, K.S. Beneficial Effects of Herbs, Spices and Medicinal Plants on the Metabolic Syndrome, Brain and Cognitive Function. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 13–29. [Google Scholar] [CrossRef]
- Tewari, D.; Stankiewicz, A.M.; Mocan, A.; Sah, A.N.; Tzvetkov, N.T.; Huminiecki, L.; Horbanczuk, J.O.; Atanasov, A.G. Ethnopharmacological Approaches for Dementia Therapy and Significance of Natural Products and Herbal Drugs. Front. Aging Neurosci. 2018, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Sodagam, L.; Lewinska, A.; Kwasniewicz, E.; Kokhanovska, S.; Wnuk, M.; Siems, K.; Rattan, S.I.S. Phytochemicals Rosmarinic Acid, Ampelopsin, and Amorfrutin-A Can Modulate Age-Related Phenotype of Serially Passaged Human Skin Fibroblasts in vitro. Front. Genet. 2019, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Siracusa, L.; Ruberto, G. New Tricks for Old Guys: Recent Developments in the Chemistry, Biochemistry, Applications and Exploitation of Selected Species from the Lamiaceae Family. Chem. Biodivers. 2020, 17, e1900677. [Google Scholar] [CrossRef]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E.W. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef]
- Topal, M.; Gulcin, İ. Evaluation of the in vitro antioxidant, antidiabetic and anticholinergic properties of rosmarinic acid from rosemary (Rosmarinus officinalis L.). Biocatal. Agric. Biotechnol. 2022, 43, 102417. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kayama, T.; Noguchi-Shinohara, M.; Hamaguchi, T.; Yamada, M.; Abe, K.; Kobayashi, S. Rosmarinic acid suppresses tau phosphorylation and cognitive decline by downregulating the JNK signaling pathway. NPJ Sci. Food 2021, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Nyandwi, J.B.; Ko, Y.S.; Jin, H.; Yun, S.P.; Park, S.W.; Kim, H.J. Rosmarinic Acid Exhibits a Lipid-Lowering Effect by Modulating the Expression of Reverse Cholesterol Transporters and Lipid Metabolism in High-Fat Diet-Fed Mice. Biomolecules 2021, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Liu, G.A.; Perez, E.; Rainer, R.D.; Febo, M.; Cruz-Almeida, Y.; Ebner, N.C. Systemic inflammation mediates age-related cognitive deficits. Front. Aging Neurosci. 2018, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Komleva, Y.; Chernykh, A.; Lopatina, O.; Gorina, Y.; Lokteva, I.; Salmina, A.; Gollasch, M. Inflamm-Aging and Brain Insulin Resistance: New Insights and Role of Life-style Strategies on Cognitive and Social Determinants in Aging and Neurodegeneration. Front. Neurosci. 2021, 14, 618395. [Google Scholar] [CrossRef]
- Guicciardi, M.; Crisafulli, A.; Doneddu, A.; Fadda, D.; Lecis, R. Effects of metabolic syndrome on cognitive performance of adults during exercise. Front. Psychol. 2019, 10, 1845. [Google Scholar] [CrossRef] [Green Version]
- Cirulli, F.; Alleva, E. The NGF saga: From animal models of psychosocial stress to stress-related psychopathology. Front. Neuroendocrinol. 2009, 30, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef] [Green Version]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Saghaei, M. An Overview of Randomization Minimization Programs for Randomized Clinical Trials. J. Med. Signals Sens. 2011, 1, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Berry, A.; Capone, F.; Giorgio, M.; Pelicci, P.G.; de Kloet, E.R.; Alleva, E.; Minghetti, L.; Cirulli, F. Deletion of the life span determinant p66Shc prevents age-dependent increases in emotionality and pain sensitivity in mice. Exp. Gerontol. 2007, 42, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Gulinello, M.; Gertner, M.; Mendoza, G.; Schoenfeld, B.P.; Oddo, S.; LaFerla, F.; Choi, C.H.; McBride, S.M.J.; Faber, D.S. Validation of a 2-day water maze protocol in mice. Behav. Brain Res. 2009, 196, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Berry, A.; Bellisario, V.; Panetta, P.; Raggi, C.; Magnifico, M.C.; Arese, M.; Cirulli, F. Administration of the Antioxidant N-Acetyl-Cysteine in Pregnant Mice Has Long-Term Positive Effects on Metabolic and Behavioral Endpoints of Male and Female Offspring Prenatally Exposed to a High-Fat Diet. Front. Behav. Neurosci. 2018, 12, 48. [Google Scholar] [CrossRef] [Green Version]
- Grubbs, F.E. Sample Criteria for Testing Outlying Observations. Ann. Math. Stat. 1950, 21, 27–58. [Google Scholar] [CrossRef]
- Olejnik, S.; Wilcox, R.R. New Statistical Procedures for the Social Sciences: Modern Solutions to Basic Problems. J. Educ. Stat. 1990, 15, 78. [Google Scholar] [CrossRef]
- You, G.; Yao, J.; Liu, Q.; Li, N. The Strategies for Treating “Alzheimer’s Disease”: Insulin Signaling May Be a Feasible Target. Curr. Issues Mol. Biol. 2022, 44, 6172–6188. [Google Scholar] [CrossRef]
- Huang, J.; Huang, N.; Mao, Q.; Shi, J.; Qiu, Y. Natural bioactive compounds in Alzheimer’s disease: From the perspective of type 3 diabetes mellitus. Front. Aging Neurosci. 2023, 15, 1130253. [Google Scholar] [CrossRef]
- Janoutová, J.; Machaczka, O.; Zatloukalová, A.; Janout, V. Is Alzheimer’s Disease a Type 3 Diabetes? A Review. Cent. Eur. J. Public Health 2022, 30, 139–143. [Google Scholar] [CrossRef]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef]
- Falach-Malik, A.; Rozenfeld, H.; Chetboun, M.; Rozenberg, K.; Elyasiyan, U.; Sampson, S.R. Rosenzweig TN-Acetyl-L-Cysteine inhibits the development of glucose intolerance hepatic steatosis in diabetes-prone mice. Am. J. Transl. Res. 2016, 8, 3744. [Google Scholar]
- Pellizzon, M.A.; Ricci, M.R. Choice of Laboratory Rodent Diet May Confound Data Interpretation and Reproducibility. Curr. Dev. Nutr. 2020, 4, nzaa031. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Martínez, M.; González-González, M.; Martagón, A.J.; Hlavinka, V.; Willson, R.C.; Rito-Palomares, M. Recent Developments in Biomarkers for Diagnosis and Screening of Type 2 Diabetes Mellitus. Curr. Diabetes Rep. 2022, 22, 95–115. [Google Scholar] [CrossRef]
- Robertson, R.; Zhou, H.; Zhang, T.; Harmon, J.S. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem. Biophys. 2007, 48, 139–146. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058. [Google Scholar] [CrossRef] [PubMed]
- Link, J.C.; Chen, X.; Arnold, A.P.; Reue, K. Metabolic impact of sex chromosomes. Adipocyte 2013, 2, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T., Jr. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 2014, 5, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lephart, E.D. Modulation of Aromatase by Phytoestrogens. Enzyme Res. 2015, 2015, 594656. [Google Scholar] [CrossRef] [Green Version]
- Zych, M.; Kaczmarczyk-Sedlak, I.; Wojnar, W.; Folwarczna, J. Effect of Rosmarinic Acid on the Serum Parameters of Glucose and Lipid Metabolism and Oxidative Stress in Estrogen-Deficient Rats. Nutrients 2019, 11, 267. [Google Scholar] [CrossRef] [Green Version]
- Loeb, J.; Northrop, J.H. What Determines the Duration of Life in Metazoa? Proc. Natl. Acad. Sci. USA 1917, 3, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Strader, A.D.; Sorrell, J.E.; Chambers, J.B.; Woods, S.C.; Seeley, R.J. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E630–E639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapauw, B.; Mahmoud, A.; Kaufman, J.M.; Ruige, J.B. Short-term aromatase inhibition: Effects on glucose metabolism and serum leptin levels in young and elderly men. Eur. J. Endocrinol. 2009, 160, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Meng, F.; Lei, Y.; Liu, J.; Liu, J.; Zhang, J.; Liu, F.; Liu, C.; Guo, M.; Lu, X.-Y. Leptin regulates exon-specific transcription of the Bdnf gene via epigenetic modifications mediated by an AKT/p300 HAT cascade. Mol. Psychiatry 2021, 26, 3701–3722. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Collacchi, B.; Capoccia, S.; D’Urso, M.T.; Cecchetti, S.; Raggi, C.; Sestili, P.; Aricò, E.; Pontecorvi, G.; Puglisi, R.; et al. Chronic Isolation Stress Affects Central Neuroendocrine Signaling Leading to a Metabolically Active Microenvironment in a Mouse Model of Breast Cancer. Front. Behav. Neurosci. 2021, 15, 660738. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Bellisario, V.; Berry, A.; Capoccia, S.; Raggi, C.; Panetta, P.; Branchi, I.; Piccaro, G.; Giorgio, M.; Pelicci, P.G.; Cirulli, F. Gender-dependent resiliency to stressful and metabolic challenges following prenatal exposure to high-fat diet in the p66(Shc-/-) mouse. Front. Behav. Neurosci. 2014, 8, 285. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musillo, C.; Giona, L.; Ristow, M.; Zarse, K.; Siems, K.; Di Francesco, A.; Collacchi, B.; Raggi, C.; Cirulli, F.; Berry, A. Rosmarinic Acid Improves Cognitive Abilities and Glucose Metabolism in Aged C57Bl/6N Mice While Disrupting Lipid Profile in Young Adults in a Sex-Dependent Fashion. Nutrients 2023, 15, 3366. https://doi.org/10.3390/nu15153366
Musillo C, Giona L, Ristow M, Zarse K, Siems K, Di Francesco A, Collacchi B, Raggi C, Cirulli F, Berry A. Rosmarinic Acid Improves Cognitive Abilities and Glucose Metabolism in Aged C57Bl/6N Mice While Disrupting Lipid Profile in Young Adults in a Sex-Dependent Fashion. Nutrients. 2023; 15(15):3366. https://doi.org/10.3390/nu15153366
Chicago/Turabian StyleMusillo, Chiara, Letizia Giona, Michael Ristow, Kim Zarse, Karsten Siems, Alessia Di Francesco, Barbara Collacchi, Carla Raggi, Francesca Cirulli, and Alessandra Berry. 2023. "Rosmarinic Acid Improves Cognitive Abilities and Glucose Metabolism in Aged C57Bl/6N Mice While Disrupting Lipid Profile in Young Adults in a Sex-Dependent Fashion" Nutrients 15, no. 15: 3366. https://doi.org/10.3390/nu15153366
APA StyleMusillo, C., Giona, L., Ristow, M., Zarse, K., Siems, K., Di Francesco, A., Collacchi, B., Raggi, C., Cirulli, F., & Berry, A. (2023). Rosmarinic Acid Improves Cognitive Abilities and Glucose Metabolism in Aged C57Bl/6N Mice While Disrupting Lipid Profile in Young Adults in a Sex-Dependent Fashion. Nutrients, 15(15), 3366. https://doi.org/10.3390/nu15153366





