Protection of Inonotus hispidus (Bull.) P. Karst. against Chronic Alcohol-Induced Liver Injury in Mice via Its Relieving Inflammation Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. IH Preparation
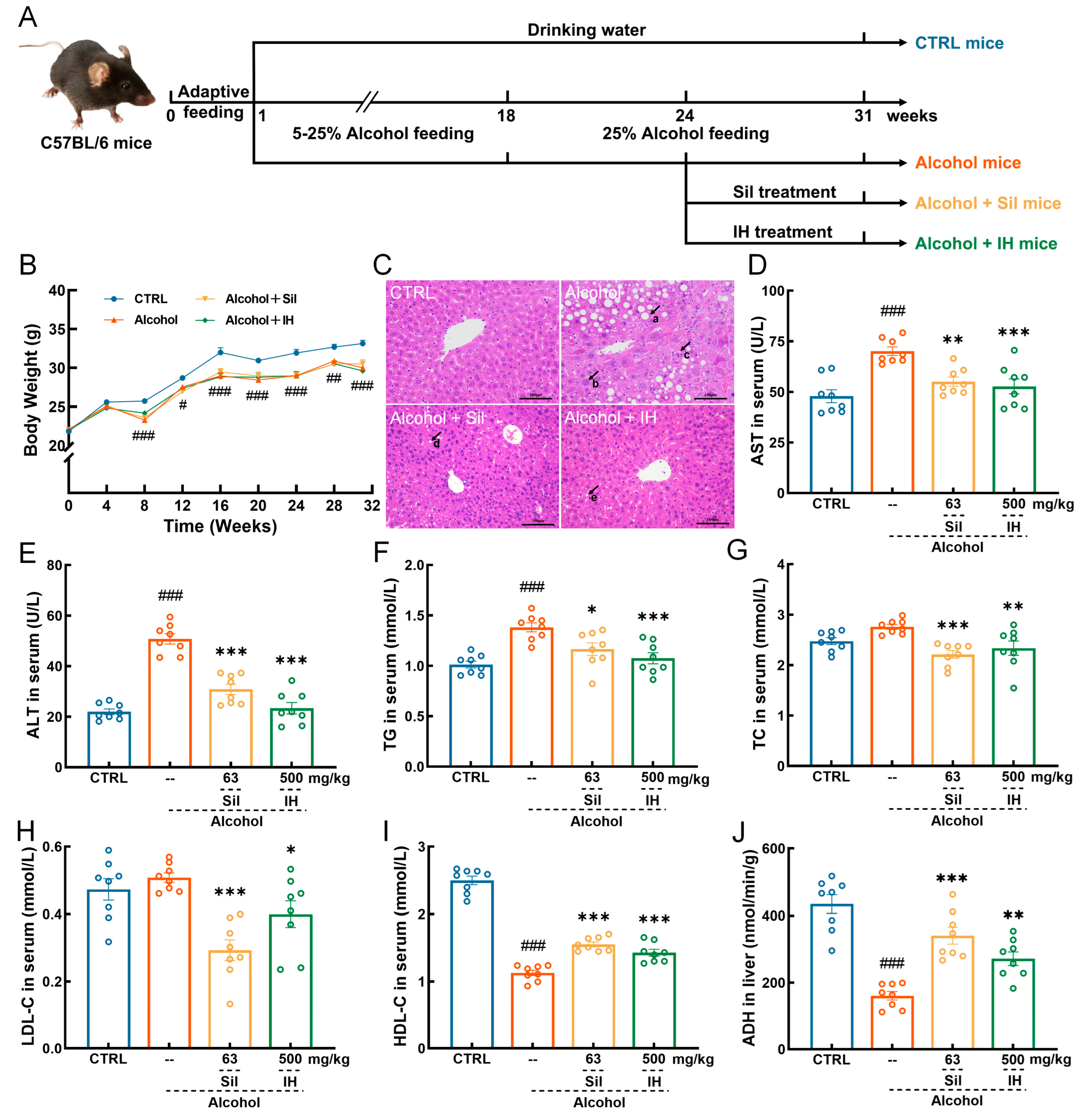
2.2. Animal Experimental Procedures
2.3. Hematoxylin and Eosin (H&E) Staining
2.4. Biochemical Detection
2.5. Intestinal Microbiota Analysis
2.6. Non-Targeted Metabolomics Analysis
2.7. Western Blot Analysis
2.8. Immunohistochemistry (IHC) Analysis
2.9. Statistical Analysis
3. Results
3.1. IH Protected against Chronic Alcohol Injury in Mice
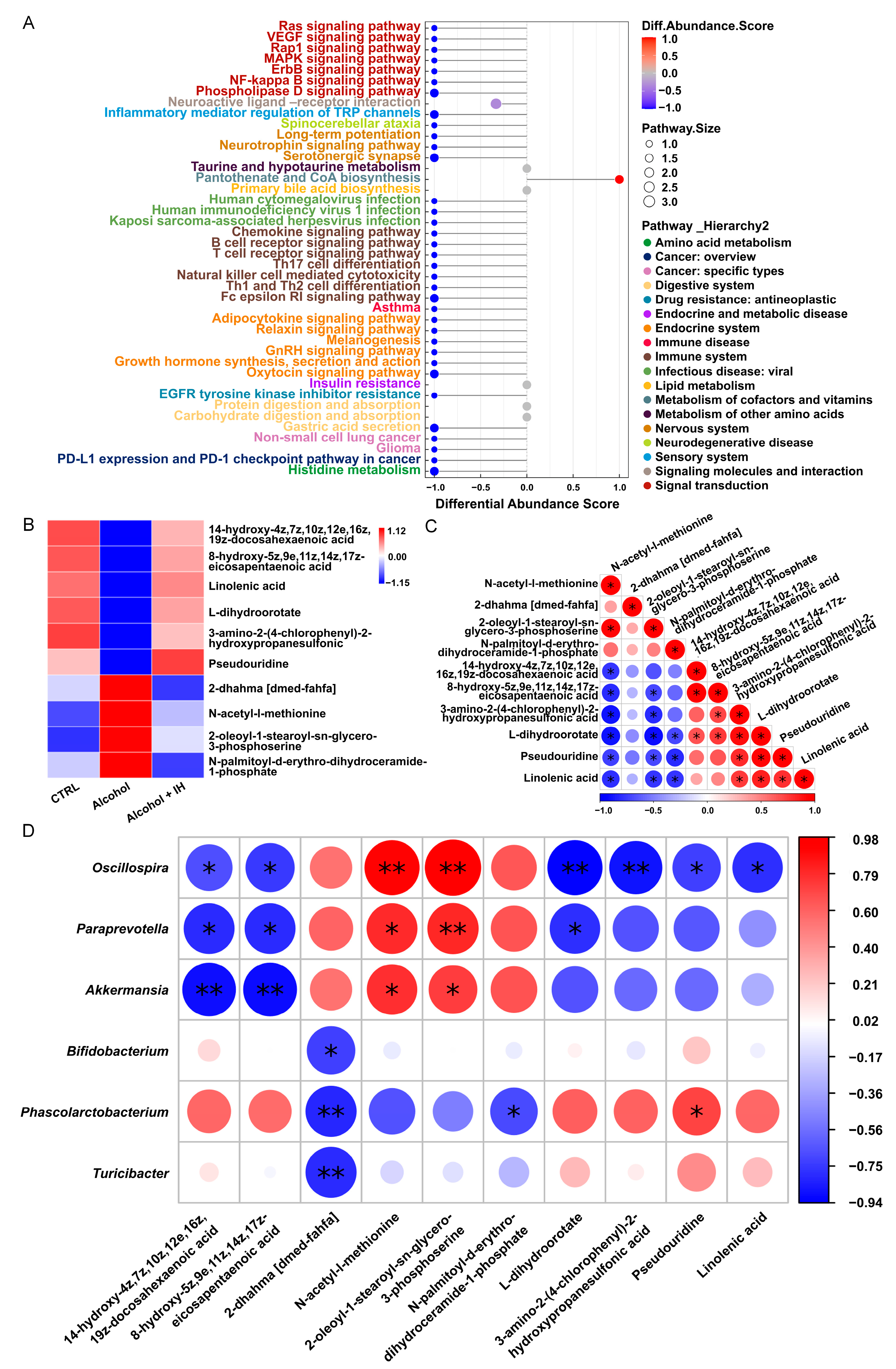
3.2. IH Regulated the Intestinal Microbiota in Chronic ALD Mice
3.3. IH Altered n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) among Serum Metabolites in Chronic ALD Mice
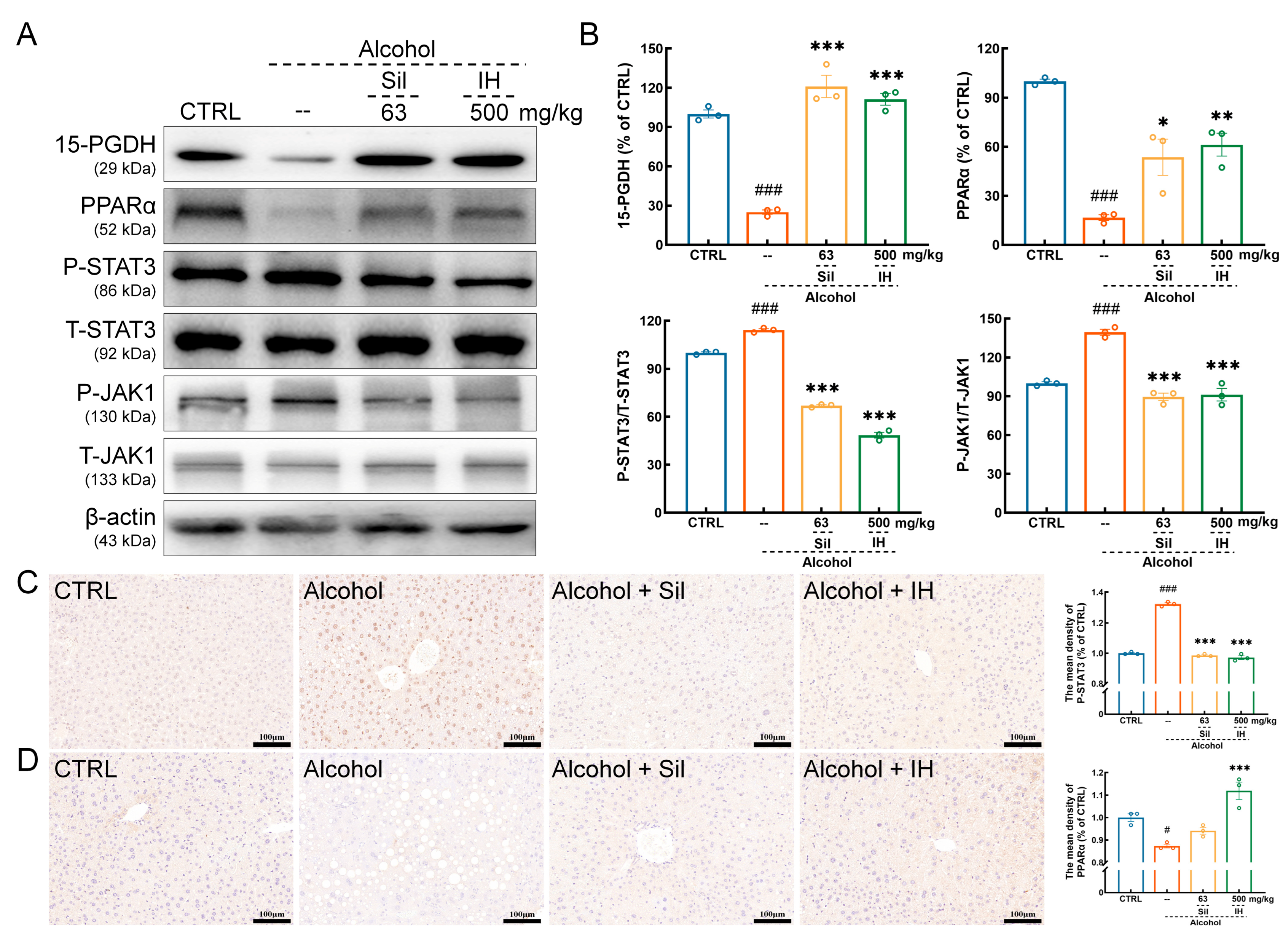
3.4. IH Suppressed Inflammation in Chronic ALD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singal, A.K.; Mathurin, P. Diagnosis and Treatment of Alcohol-Associated Liver Disease A Review. Jama-J. Am. Med. Assoc. 2021, 326, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xue, F.; Wu, X.-N.; Zhang, W.; Hou, J.-J.; Xiang, J.-X.; Lv, Y.; Zhang, X.-F. The global burden of alcoholic liver disease: A systematic analysis of the global burden of disease study 2019. Alcohol Alcohol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Xie, Z.Y.; Zhang, Y.; Liu, Y.; Niu, A.J.; Liu, Y.Y.; Zhang, L.B.; Guan, L.L. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Biosci. 2021, 44, 101385. [Google Scholar] [CrossRef]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk Thistle in Liver Diseases: Past, Present, Future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohl, K.; Moodley, P.; Dhanda, A.D. Alcohol’s Impact on the Gut and Liver. Nutrients 2021, 13, 3170. [Google Scholar] [CrossRef]
- Jew, M.H.; Hsu, C.L. Alcohol, the gut microbiome, and liver disease. J. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef]
- Ribeiro, M.D.; Szabo, G. Role of the Inflammasome in Liver Disease. Annu. Rev. Pathol. -Mech. Dis. 2022, 17, 345–365. [Google Scholar] [CrossRef]
- Fan, J.; Chen, Q.S.; Wei, L.W.; Zhou, X.M.; Wang, R.; Zhang, H. Asiatic acid ameliorates CCl4-induced liver fibrosis in rats: Involvement of Nrf2/ARE, NF-kappa B/I kappa B alpha, and JAK1/STAT3 signaling pathways. Drug Des. Dev. Ther. 2018, 12, 3595–3605. [Google Scholar] [CrossRef] [Green Version]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol. Res. 2017, 38, 147–161. [Google Scholar]
- Adekunle, A.D.; Adejumo, A.; Singal, A.K. Therapeutic targets in alcohol-associated liver disease: Progress and challenges. Therap. Adv. Gastroenterol. 2023, 16, 17562848231170946. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPAR alpha in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, A.A.; De Sá-Nakanishi, A.B.; Bracht, A.; Da Costa, S.M.G.; Koehnlein, E.A.; De Souza, C.G.M.; Peralta, R.M. Hepatoprotective Effects of Mushrooms. Molecules 2013, 18, 7609–7630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Hou, R.L.; Yan, J.J.; Xu, K.Q.; Wu, X.P.; Lin, W.X.; Zheng, M.F.; Fu, J.S. Purification and characterization of Inonotus hispidus exopolysaccharide and its protective effect on acute alcoholic liver injury in mice. Int. J. Biol. Macromol. 2019, 129, 41–49. [Google Scholar] [CrossRef]
- Zhang, F.P.; Xue, F.Z.; Xu, H.; Yuan, Y.; Wu, X.P.; Zhang, J.L.; Fu, J.S. Optimization of Solid-State Fermentation Extraction of Inonotus hispidus Fruiting Body Melanin. Foods 2021, 10, 2893. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-J.; Shao, C.-X.; Yang, Y.; Ren, R.; Jin, L.; Hu, D.; Wu, S.-L.; Lei, P.; He, Y.-L.; Xu, J. The antitumor effect of mycelia extract of the medicinal macrofungus Inonotus hispidus on HeLa cells via the mitochondrial-mediated pathway. J. Ethnopharmacol. 2023, 311, 116407. [Google Scholar] [CrossRef]
- Huo, Y.H.; Liu, D.C.; Yang, Q.; Sun, C.Y.; Wang, Z.B.; Li, D.H. Transcriptional Responses for Biosynthesis of Triterpenoids in Exogenous Inducers Treated Inonotus Hispidus Using RNA-Seq. Molecules 2022, 27, 8541. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Z.G.; Tan, Y.Y.; Wang, X.X.; Wang, C.X.; Dong, M.Y.; Liu, H.H.; Chen, H.; Li, Y.; Li, L.Z.; et al. Anti-Gouty Arthritis and Anti-Hyperuricemia Properties of Sanghuangporus vaninii and Inonotus hispidus in Rodent Models. Nutrients 2022, 14, 4421. [Google Scholar] [CrossRef]
- Yang, H.X.; Li, S.Y.; Qu, Y.D.; Li, L.Z.; Li, Y.; Wang, D. Anti-Colorectal Cancer Effects of Inonotus hispidus (Bull.: Fr.) P. Karst. Spore Powder through Regulation of Gut Microbiota-Mediated JAK/STAT Signaling. Nutrients 2022, 14, 3299. [Google Scholar] [CrossRef]
- Teng, S.; Zhang, Y.; Jin, X.; Zhu, Y.; Li, L.; Huang, X.; Wang, D.; Lin, Z. Structure and hepatoprotective activity of Usp10/NF-κB/Nrf2 pathway-related Morchella esculenta polysaccharide. Carbohydr. Polym. 2023, 303, 120453. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Hao, J.; Liu, Z.J.; Li, Z.G.; Teng, L.R.; Wang, D. Inonotus hispidus Protects against Hyperlipidemia by Inhibiting Oxidative Stress and Inflammation through Nrf2/NF-kappa B Signaling in High Fat Diet Fed Mice. Nutrients 2022, 14, 3477. [Google Scholar] [CrossRef]
- Song, J.Y.; Zhang, Y.F.; Zhu, Y.F.; Jin, X.H.; Li, L.Z.; Wang, C.Y.; Zhou, Y.; Li, Y.T.; Wang, D.; Hu, M. Structural characterization and anti-osteoporosis effects of polysaccharide purified from Eucommia ulmoides Oliver cortex based on its modulation on bone metabolism. Carbohydr. Polym. 2023, 306, 120601. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Hu, X.; Zhao, C.; Zhang, X.; Zhang, H. Effect of an Antibacterial Polysaccharide Produced by Chaetomium globosum CGMCC 6882 on the Gut Microbiota of Mice. Foods 2021, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Wendell, S.G.; Golin-Bisello, F.; Wenzel, S.; Sobol, R.W.; Holguin, F.; Freeman, B.A. 15-Hydroxyprostaglandin Dehydrogenase Generation of Electrophilic Lipid Signaling Mediators from Hydroxy Omega-3 Fatty Acids. J. Biol. Chem. 2015, 290, 5868–5880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, H.; Kikuchi, S.; Hakozaki, M.; Motodate, K.; Nagahora, N.; Hirose, M. 8-Hydroxyeicosapentaenoic Acid Decreases Plasma and Hepatic Triglycerides via Activation of Peroxisome Proliferator-Activated Receptor Alpha in High-Fat Diet-Induced Obese Mice. J. Lipids 2016, 2016, 7498508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Wahyudi, L.D.; Gonzalez, F.J.; Kim, J.H. Nuclear Receptor PPAR alpha Agonist Wy-14,643 Ameliorates Hepatic Cell Death in Hepatic IKK beta-Deficient Mice. Biomol. Ther. 2017, 25, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.F.; Li, W.Z.; Zhang, Y.Y.; Li, J.J.; He, F.T.; Dong, K.; Hong, Z.H.; Luo, R.C.; Pei, X.F. alpha-Linolenic Acid Inhibits RANKL-Induced Osteoclastogenesis In Vitro and Prevents Inflammation In Vivo. Foods 2023, 12, 682. [Google Scholar] [CrossRef]
- Borsini, A.; Nicolaou, A.; Camacho-Munoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.P.; Pariante, C.M. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: Relevance for major depression and for human hippocampal neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Song, Z.; Saari, J.T.; McClain, C.J.; Kang, Y.J. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am. J. Pathol. 2005, 166, 1681–1690. [Google Scholar] [CrossRef] [Green Version]
- Ciuclan, L.; Ehnert, S.; Ilkavets, I.; Weng, H.L.; Gaitantzi, H.; Tsukamoto, H.; Ueberham, E.; Meindl-Beinker, N.M.; Singer, M.V.; Breitkopf, K.; et al. TGF-beta enhances alcohol dependent hepatocyte damage via down-regulation of alcohol dehydrogenase I. J. Hepatol. 2010, 52, 407–416. [Google Scholar] [CrossRef]
- Sarin, S.K.; Pande, A.; Schnabl, B. Microbiome as a therapeutic target in alcohol-related liver disease. J. Hepatol. 2019, 70, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Budden, K.F.; Gellatly, S.L.; Vaughan, A.; Amorim, N.; Horvat, J.C.; Hansbro, N.G.; Wood, D.L.A.; Hugenholtz, P.; Dennis, P.G.; Wark, P.A.B.; et al. Probiotic Bifidobacterium longum subsp. longum Protects against Cigarette Smoke-Induced Inflammation in Mice. Int. J. Mol. Sci. 2023, 24, 252. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.C.; Cao, F.W.; Lai, S.L.; Zhuge, H.; Chang, K.X.; Valencak, T.G.; Liu, J.X.; Li, S.T.; Ren, D.X. Lactobacillus plantarum ZY08 relieves chronic alcohol-induced hepatic steatosis and liver injury in mice via restoring intestinal flora homeostasis. Food Res. Int. 2022, 157, 111259. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Hwang, J.H.; Park, E.O.; Lee, S.O.; Chung, Y.J.; Chung, M.J.; Lim, S.; Lim, T.J.; Ha, Y.; Park, B.H.; et al. Regulation of Alcohol and Acetaldehyde Metabolism by a Mixture of Lactobacillus and Bifidobacterium Species in Human. Nutrients 2021, 13, 1875. [Google Scholar] [CrossRef]
- Yang, Z.D.; Su, H.H.; Lv, Y.J.; Tao, H.Q.; Jiang, Y.H.; Ni, Z.Y.; Peng, L.; Chen, X.Q. Inulin intervention attenuates hepatic steatosis in rats via modulating gut microbiota and maintaining intestinal barrier function. Food Res. Int. 2023, 163, 112309. [Google Scholar] [CrossRef]
- Grouls, M.; Janssen, A.W.F.; Duivenvoorde, L.P.M.; Hooiveld, G.; Bouwmeester, H.; van der Zande, M. Differential gene expression in iPSC-derived human intestinal epithelial cell layers following exposure to two concentrations of butyrate, propionate and acetate. Sci. Rep. 2022, 12, 13988. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.M.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Wen, S.T.; He, L.; Zhong, Z.T.; Zhao, R.Y.; Weng, S.H.; Mi, H.; Liu, F.B. Stigmasterol Restores the Balance of Treg/Th17 Cells by Activating the Butyrate-PPAR gamma Axis in Colitis. Front. Immunol. 2021, 12, 741934. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Liu, C.P.; Guo, M.; Gao, C.L.; Zhou, L.P.; Long, Y.; Xu, Y. Butyrate ameliorates alcoholic fatty liver disease via reducing endotoxemia and inhibiting liver gasdermin D-mediated pyroptosis. Ann. Transl. Med. 2021, 9, 873. [Google Scholar] [CrossRef]
- Li, W.D.; Li, L.S.; Yang, F.J.; Hu, Q.Y.; Xiong, D.Q. Correlation between gut bacteria Phascolarctobacterium and exogenous metabolite alpha-linolenic acid in T2DM: A case-control study. Ann. Transl. Med. 2022, 10, 1056. [Google Scholar] [CrossRef]
- Fan, H.J.; Huang, W.Y.; Guo, Y.; Ma, X.F.; Yang, J.H. alpha-Linolenic Acid Suppresses Proliferation and Invasion in Osteosarcoma Cells via Inhibiting Fatty Acid Synthase. Molecules 2022, 27, 2741. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.J.; Yang, Y.; Xiao, Z.Y.; Wan, J.B. n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, S116–S129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, L.; Bian, C.; Bai, M.J.; Yu, H.T.; Gao, H.; Zhao, J.X.; Zhang, C.J.; Zhao, R.J. Alterations and correlations of gut microbiota, fecal, and serum metabolome characteristics in a rat model of alcohol use disorder. Front. Microbiol. 2023, 13, 1068825. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.G.; Zhang, X.N.; Liu, S.X.; Wang, Y.R.; Zeng, T. Roles of peroxisome proliferator-activated receptor alpha in the pathogenesis of ethanol-induced liver disease. Chem. -Biol. Interact. 2020, 327, 109176. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Yamada, H.; Hirose, M. Euphausia pacifica (North Pacific Krill): Review of Chemical Features and Potential Benefits of 8-HEPE against Metabolic Syndrome, Dyslipidemia, NAFLD, and Atherosclerosis. Nutrients 2021, 13, 3765. [Google Scholar] [CrossRef]
- Coskun, M.; Salem, M.; Pedersen, J.; Nielsen, O.H. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol. Res. 2013, 76, 1–8. [Google Scholar] [CrossRef]
- Hu, Q.; Bian, Q.H.; Rong, D.C.; Wang, L.Y.; Song, J.A.; Huang, H.S.; Zeng, J.; Mei, J.; Wang, P.Y. JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens. Front. Bioeng. Biotechnol. 2023, 11, 1110765. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.P.; Luo, F.M. The Role of JAK/STAT Pathway in Fibrotic Diseases: Molecular and Cellular Mechanisms. Biomolecules 2023, 13, 119. [Google Scholar] [CrossRef]
- Hu, X.Y.; Li, J.; Fu, M.R.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Makino, Y.; Hikita, H.; Kato, S.; Sugiyama, M.; Shigekawa, M.; Sakamoto, T.; Sasaki, Y.; Murai, K.; Sakane, S.; Kodama, T.; et al. STAT3 is Activated by CTGF-mediated Tumor-stroma Cross Talk to Promote HCC Progression. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 99–119. [Google Scholar] [CrossRef]
- Meng, H.; Niu, R.; You, H.; Wang, L.; Feng, R.; Huang, C.; Li, J. Interleukin-9 attenuates inflammatory response and hepatocyte apoptosis in alcoholic liver injury. Life Sci. 2022, 288, 120180. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-M.; Li, Z.-q.; Zhou, Q.; Liu, C.; Wang, M.-J.; Wu, H.-X.; Mu, Y.-Z.; He, Y.-F.; Zhang, Y.; Wu, X.-N.; et al. Mesenchymal stem cells alleviate liver injury induced by chronic-binge ethanol feeding in mice via release of TSG6 and suppression of STAT3 activation. Stem Cell Res. Ther. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.W.; Sun, Y.M. The IL-6/JAK/STAT3 pathway: Potential therapeutic strategies in treating colorectal cancer (Review). Int. J. Oncol. 2014, 44, 1032–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, K.-C.; Chang, K.-S.; Hsu, S.-Y.; Sung, H.-C.; Feng, T.-H.; Chao, M.; Juang, H.-H. Human Heme Oxygenase-1 Induced by Interleukin-6 via JAK/STAT3 Pathways Is a Tumor Suppressor Gene in Hepatoma Cells. Antioxidants 2020, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.F.; Ansari, M.A.; Zoheir, K.M.; Bakheet, S.A.; Korashy, H.M.; Nadeem, A.; Ashour, A.E.; Attia, S.M. Regulation of TNF-α and NF-κB activation through the JAK/STAT signaling pathway downstream of histamine 4 receptor in a rat model of LPS-induced joint inflammation. Immunobiology 2015, 220, 889–898. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Li, Z.; Zhang, Y.; Zhu, Y.; Su, L.; Song, J.; Hao, J.; Wang, D. Protection of Inonotus hispidus (Bull.) P. Karst. against Chronic Alcohol-Induced Liver Injury in Mice via Its Relieving Inflammation Response. Nutrients 2023, 15, 3530. https://doi.org/10.3390/nu15163530
Jin X, Li Z, Zhang Y, Zhu Y, Su L, Song J, Hao J, Wang D. Protection of Inonotus hispidus (Bull.) P. Karst. against Chronic Alcohol-Induced Liver Injury in Mice via Its Relieving Inflammation Response. Nutrients. 2023; 15(16):3530. https://doi.org/10.3390/nu15163530
Chicago/Turabian StyleJin, Xinghui, Zhige Li, Yongfeng Zhang, Yanfeng Zhu, Ling Su, Jiyu Song, Jie Hao, and Di Wang. 2023. "Protection of Inonotus hispidus (Bull.) P. Karst. against Chronic Alcohol-Induced Liver Injury in Mice via Its Relieving Inflammation Response" Nutrients 15, no. 16: 3530. https://doi.org/10.3390/nu15163530
APA StyleJin, X., Li, Z., Zhang, Y., Zhu, Y., Su, L., Song, J., Hao, J., & Wang, D. (2023). Protection of Inonotus hispidus (Bull.) P. Karst. against Chronic Alcohol-Induced Liver Injury in Mice via Its Relieving Inflammation Response. Nutrients, 15(16), 3530. https://doi.org/10.3390/nu15163530




