Plasminogen Activator Inhibitor-1 and Vitamin D Association in the Overweight and Obese Pediatric Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Type
- -
- Written informed consent from the parties who were legally able to do so, or from the parent(s) or legal guardian(s) of the child in accordance with local legislation;
- -
- Consent for school-age subjects or subjects younger than 6 years old;
- -
- Male or female subjects between the ages of 2 and 18;
- -
- Overweight (i.e., a BMI between the 85th and 95th percentiles (1.064 SDS and 1.645 SDS, respectively) based on the Italian growth curves;
- -
- Non-syndromic obesity (defined as a BMI below the 95th percentile and a BMI z-score for both age and sex below 1.064 SDS);
- -
- Absence of endocrine or systemic disease (apart from obesity/overweight).
- -
- No documented informed consent from the patient or parents or, if appropriate, no consent from the juvenile;
- -
- Hereditary disorders such as genetic obesity and others;
- -
- Any condition that is linked to a rise in inflammatory markers, especially values of erythrocyte sedimentation rate (ESR) ≥ 15 mm, C-reactive protein (CRP) ≥ 0.50 mg/dL, or a white blood cell count ≥ 16.00 × 103/µL;
- -
- Presence of cognitive deficiencies;
- -
- Past or present thromboembolic or hemorrhagic episodes;
- -
- The use of anticoagulant medication;
- -
- The use of long-term corticosteroid treatment;
- -
- A positive lupus anticoagulant (LA) test result;
- -
- Previous bariatric surgery;
- -
- Presence of allergies (i.e., total immunoglobulin E (IgE) ≥ 100.0 kU/L).
- -
- Written informed consent from the parties who were legally able to do so or from the parent(s) or legal guardian(s) of the child in accordance with local legislation;
- -
- Consent for school-age subjects or subjects younger than 6 years old;
- -
- Male or female subjects between the ages of 2 and 18;
- -
- A body mass index between the 10th and 84th percentiles was considered to be normal weight (BMI z-score for age and sex 1.064 SDS);
- -
- No documented informed consent from the patient or parents or, if appropriate, no consent from the juvenile;
- -
- Presence of inflammatory processes in the lower or upper respiratory tract, erythrocyte sedimentation rate values (ESR) ≥ 15 mm, C-Reactive Protein (CRP) ≥ 0.50 mg/dL, and number of white blood cells ≥ 16.00 × 103/µL;
- -
- Suspected celiac disease (anti-transglutaminase antibodies ≥ 20 CU);
- -
- Past or present thromboembolic or hemorrhagic episodes;
- -
- The use of anticoagulant medication;
- -
- The use of long-term corticosteroid treatment;
- -
- A positive lupus anticoagulant (LA) test result;
- -
- Previous bariatric surgery;
- -
- Presence of allergies (i.e., total immunoglobulin E (IgE) ≥ 100.0 kU/L).
2.2. PAI-1 Determinations
2.3. Insulin, Glucose, ALT, AST, CRP, Total Cholesterol, HDL, Triglycerides, and VD
2.4. HbA1c, Leptin
2.5. Platelets Count
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morandi, A.; Maffeis, C. Predictors of Metabolic Risk in Childhood Obesity. Horm. Res. Paediatr. 2014, 82, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Gallistl, S.; Sudi, K.; Borkenstein, M.; Troebinger, M.; Weinhandl, G.; Muntean, W. Determinants of haemostatic risk factors for coronary heart disease in obese children and adolescents. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1459–1464. [Google Scholar] [CrossRef]
- Freedman, D.S.; Khan, L.K.; Serdula, M.K.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. The relation of childhood BMI to adult adiposity: The Bogalusa Heart Study. Pediatrics 2005, 115, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Blokhin, I.O.; Lentz, S.R. Mechanisms of thrombosis in obesity. Curr. Opin. Hematol. 2013, 20, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; van der Poll, T.; Schultz, M. Infection and inflammation as risk factors for thrombosis and atherosclerosis. Semin. Thromb. Hemost. 2012, 38, 506–514. [Google Scholar] [CrossRef]
- Mertens, I.; Van Gaal, L.F. Obesity, haemostasis and the fibrinolytic system. Obes. Rev. 2002, 3, 85–101. [Google Scholar] [CrossRef]
- Rosito, G.A.; D’agostino, R.B.; Massaro, J.; Lipinska, I.; Mittleman, M.A.; Sutherland, P.; Wilson, P.W.F.; Levy, D.; Muller, J.E.; Tofler, G.H. Association between obesity and a prothrombotic state: The Framingham Offspring Study. Thromb. Haemost. 2004, 91, 683–689. [Google Scholar] [CrossRef]
- Samad, F.; Pandey, M.; Loskutoff, D.J. Regulation of tissue factor gene expression in obesity. Blood 2001, 98, 3353–3358. [Google Scholar] [CrossRef]
- Langer, F.; Spath, B.; Fischer, C.; Stolz, M.; Ayuk, F.A.; Kröger, N.; Bokemeyer, C.; Ruf, W. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood 2013, 121, 2324–2335. [Google Scholar] [CrossRef]
- Sprengers, E.D.; Kluft, C. Plasminogen activator inhibitors. Blood 1987, 69, 381–387. [Google Scholar] [CrossRef]
- Declerck, P.J.; Alessi, M.C.; Verstreken, M.; Kruithof, E.K.; Juhan-Vague, I.; Collen, D. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood 1988, 71, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R.A.; Grant, P.J. Role of clotting factors and fibrin structure in predisposition to atherothrombotic disease. Expert Rev. Cardiovasc. Ther. 2005, 3, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Mussoni, L.; Tremoli, E. PAI-1, the primary plasmatic inhibitor of fibrinolysis. Physiopathologic role and molecular mechanisms. Minerva Endocrinol. 2002, 27, 181–191. [Google Scholar]
- Yao, Y.; Zhu, L.; He, L.; Duan, Y.; Liang, W.; Nie, Z.; Jin, Y.; Wu, X.; Fang, Y. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int. J. Clin. Exp. Med. 2015, 8, 14977–14984. [Google Scholar]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. 2019, 10, 103. [Google Scholar] [CrossRef]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of Vitamin D and its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Polack, B.; Schved, J.F.; Boneu, B. Preanalytical recommendations of the ‘Groupe d’Etude sur l’Hémostase et la Thrombose’ (GEHT) for venous blood testing in hemostasis laboratories. Haemostasis 2001, 31, 61–68. [Google Scholar] [CrossRef]
- Di Felice, G.; Vidali, M.; Parisi, G.; Pezzi, S.; Di Pede, A.; Deidda, G.; D’agostini, M.; Carletti, M.; Ceccarelli, S.; Porzio, O. Reference Intervals for Coagulation Parameters in Developmental Hemostasis from Infancy to Adolescence. Diagnostics 2022, 12, 2552. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef]
- Tobisch, B.; Blatniczky, L.; Barkai, L. Cardiometabolic risk factors and insulin resistance in obese children and adolescents: Relation to puberty. Pediatr. Obes. 2015, 10, 37–44. [Google Scholar] [CrossRef]
- González-Gil, E.M.; Anguita-Ruiz, A.; Kalén, A.; Perez, C.D.L.L.; Rupérez, A.I.; Vázquez-Cobela, R.; Flores, K.; Gil, A.; Gil-Campos, M.; Bueno, G.; et al. Longitudinal associations between cardiovascular biomarkers and metabolic syndrome during puberty: The PUBMEP study. Eur. J. Pediatr. 2023, 182, 419–429. [Google Scholar] [CrossRef]
- Kalyoncu, D. Platelet indices in overweight and obese children. Eur. J. Pediatr. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Purdy, J.C.; Shatzel, J.J. The hematologic consequences of obesity. Eur. J. Haematol. 2021, 106, 306–319. [Google Scholar] [CrossRef]
- He, H.-M.; Zhang, L.; Qiu, N.; Zhou, Z.-T.; Zhang, K.; Li, Y.; Chen, H.-B.; Xu, J.-N. Insulin resistance in school-aged girls with overweight and obesity is strongly associated with elevated white blood cell count and absolute neutrophil count. Front. Endocrinol. 2022, 13, 1041761. [Google Scholar] [CrossRef]
- Khan, A.H.; Fatima, S.S.; Raheem, A.; Jafri, L. Are serum leptin levels predicted by lipoproteins, vitamin D and body composition? World J. Diabetes 2019, 10, 260–268. [Google Scholar] [CrossRef]
- Gangloff, A.; Bergeron, J.; Lemieux, I.; Tremblay, A.; Poirier, P.; Alméras, N.; Després, J.-P. Relationships between circulating 25(OH) vitamin D, leptin levels and visceral adipose tissue volume: Results from a 1-year lifestyle intervention program in men with visceral obesity. Int. J. Obes. 2020, 44, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Madhu, S.; Aslam, M.; Mishra, B.; Gupta, A.; Jhamb, R. Association of 25 (OH) Vitamin D and Leptin in Individuals with Insulin Resistance. Indian J. Endocrinol. Metab. 2022, 26, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Makariou, S.E.; Challa, A.; Siomou, E.; Tellis, C.; Tselepis, A.; Elisaf, M.; Liberopoulos, E. Vitamin D status and cardiometabolic risk factors in Greek adolescents with obesity—The effect of vitamin D supplementation: A pilot study. Arch. Med. Sci.-Atheroscler. Dis. 2020, 5, e64–e71. [Google Scholar] [CrossRef]
- Menendez, C.; Lage, M.; Peino, R.; Baldelli, R.; Concheiro, P.; Dieguez, C.; Casanueva, F. Retinoic acid and vitamin D(3) powerfully inhibit in vitro leptin secretion by human adipose tissue. J. Endocrinol. 2001, 170, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Breslavsky, A.; Frand, J.; Matas, Z.; Boaz, M.; Barnea, Z.; Shargorodsky, M. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin. Nutr. 2013, 32, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev. 2009, 23, 225–229. [Google Scholar] [CrossRef]
- Previtali, E.; Bucciarelli, P.; Passamonti, S.M.; Martinelli, I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011, 9, 120–138. [Google Scholar]
- Martinelli, I.; Bucciarelli, P.; Mannucci, P.M. Thrombotic risk factors: Basic pathophysiology. Crit. Care Med. 2010, 38, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mannucci, P.M. Association between venous and arterial thrombosis: Clinical implications. Eur. J. Intern. Med. 2012, 23, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Blank, M.; Zandman-Goddard, G.; Orbach, H.; Meroni, P.L.; Tincani, A.; Doria, A.; Cervera, R.; Miesbach, W.; Stojanovich, L.; et al. Vitamin D: An instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann. Rheum. Dis. 2011, 70, 145–150. [Google Scholar] [CrossRef]
- Khademvatani, K.; Seyyed-Mohammadzad, M.H.; Akbari, M.; Rezaei, Y.; Eskandari, R.; Rostamzadeh, A. The relationship between vitamin D status and idiopathic lower-extremity deep vein thrombosis. Int. J. Gen. Med. 2014, 7, 303–309. [Google Scholar]
- Wu, W.X.; He, D.R. Low Vitamin D Levels Are Associated with the Development of Deep Venous Thromboembolic Events in Patients with Ischemic Stroke. Clin. Appl. Thromb./Hemost. 2018, 24 (Suppl. S9), 69s–75s. [Google Scholar] [CrossRef]
- Cura-Esquivel, I.; Perales-Quintana, M.M.; Torres-González, L.; Guzmán-Avilán, K.; Muñoz-Espinosa, L.; Cordero-Pérez, P. Metabolic, inflammatory and adipokine differences on overweight/obese children with and without metabolic syndrome: A cross-sectional study. PLoS ONE 2023, 18, e0281381. [Google Scholar] [CrossRef]
- Corsello, A.; Macchi, M.; D’oria, V.; Pigazzi, C.; Alberti, I.; Treglia, G.; De Cosmi, V.; Mazzocchi, A.; Agostoni, C.; Milani, G.P. Effects of vitamin D supplementation in obese and overweight children and adolescents: A systematic review and meta-analysis. Pharmacol. Res. 2023, 192, 106793. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, W.; Li, M.; Wang, W.; Sun, C.; Liu, L.; Fang, Y.; Liu, L.; Yang, X.; Zhang, X.; et al. Association between the Children’s Dietary Inflammatory Index (C-DII) and Markers of Inflammation and Oxidative Stress among Children and Adolescents: NHANES 2015–2018. Front. Nutr. 2022, 9, 894966. [Google Scholar] [CrossRef]
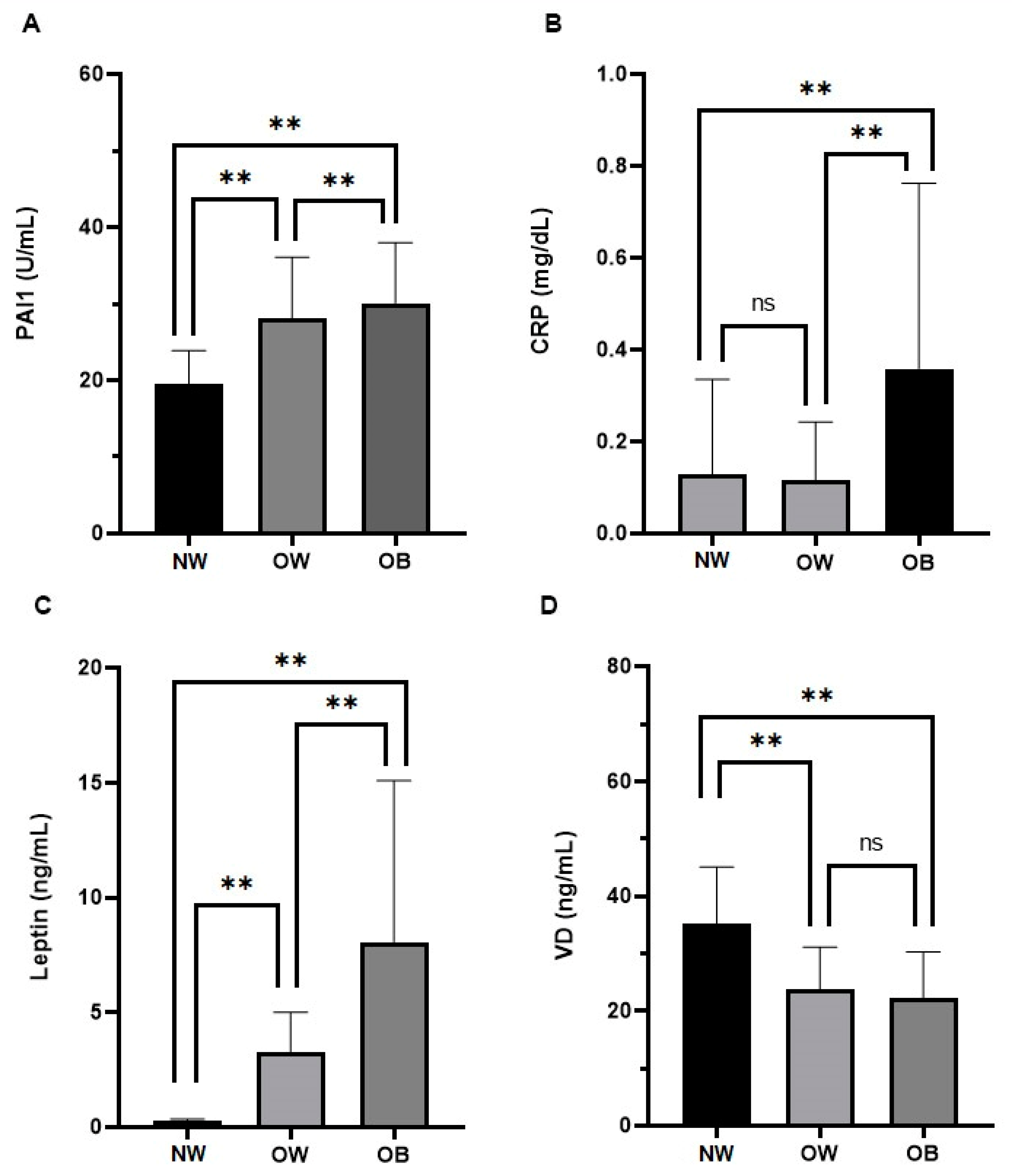

| Normal Weight | Overweight | Obese | p | p | p | |
|---|---|---|---|---|---|---|
| n = 80 | n = 32 | n = 227 | (NW vs. OW) | (NW vs. OB) | (OW vs. OB) | |
| Age, y, mean (standard deviation) | 13.4 (3.2) | 13.4 (2.7) | 12.5 (3.2) | 0.882 | <0.05 | <0.05 |
| BMI, mean (standard deviation) | 18.1 (2.9) | 24.0 (2.6) | 32.2 (6.4) | <0.001 | <0.001 | <0.001 |
| SBP, mm Hg, mean (standard deviation) | 109.1 (10.2) | 115.9 (6.6) | 120.6 (8.8) | <0.001 | <0.001 | <0.001 |
| DBP, mm Hg, mean (standard deviation) | 64.9 (9.3) | 66.5 (5.3) | 69.7 (8.8) | 0.26 | <0.001 | 0.001 |
| Cholesterol, mg/dL, mean (standard deviation) | 138.2 (23.7) | 148.2 (25.0) | 155.2 (27.5) | 0.08 | <0.01 | 0.093 |
| HDL, mg/dL, mean (standard deviation) | 62.7 (16.8) | 54.0 (11.8) | 47.3 (10.2) | 0.051 | <0.01 | <0.01 |
| LDL, mg/dL, mean (standard deviation) | 68.1 (16.4) | 85.3 (22.0) | 95.4 (25.7) | <0.01 | <0.001 | <0.01 |
| Triglycerides, mg/dL, median (5th and 95th percentile) | 32.0 (30.0–112.5) | 56.5 (32.0–111.5) | 82.0 (45.0–196.4) | 0.578 | <0.001 | <0.001 |
| AST, U/L, median (5th and 95th percentile) | 18.0 (8.1–34.0) | 15.0 (8.0–33.5) | 20.5 (11.0–61.0) | 0.311 | <0.001 | <0.001 |
| ALT, U/L, median (5th and 95th percentile) | 44.0 (10.0–44.0) | 20.0 (12.0–44.0) | 21.0 (14.0–40.9) | 0.132 | 0.197 | <0.01 |
| GGT, U/L, median (5th and 95th percentile) | 7.0 (6.0–25.5) | 11.0 (6.0–20.0) | 15.0 (4.9–34.2) | 0.611 | <0.001 | <0.001 |
| HbA1c, mmol/mol, mean (standard deviation) | 29.0 (1.5) | 33.0 (3.1) | 33.8 (3.8) | <0.001 | <0.001 | 0.229 |
| Glucose, mg/dL, mean (standard deviation) | 85.3 (9.1) | 83.7 (9.3) | 87.5 (7.7) | 0.394 | 0.074 | <0.05 |
| Insulin, U/mL, median (5th and 95th percentile) | 9.9 (9.6–9.9) | 11.4 (6.1–32.5) | 20.4 (5.4–50.7) | <0.01 | <0.001 | <0.001 |
| HOMA-IR, median (5th and 95th percentile) | 1.9 (1.0–2.0) | 2.1 (0.7–6.6) | 4.3 (1.1–11.8) | <0.01 | <0.001 | <0.001 |
| Leptin, ng/mL, median (5th and 95th percentile) | 0.2 (0.2–0.3) | 2.1 (0.2–7.5) | 5.9 (2.0–23.0) | <0.001 | <0.001 | <0.001 |
| CRP, mg/dL, median (5th and 95th percentile) | 0.1 (0.0–0.8) | 0.04 (0.03–0.79) | 0.25 (0.34–1.09) | 0.321 | <0.01 | <0.001 |
| WBC, 103/mL mean (standard deviation) | 6.0 (2.2) | 6.6 (1.4) | 7.6 (1.8) | 0.067 | <0.001 | <0.001 |
| PLT, 103/mL mean (standard deviation) | 244.9 (79.9) | 276.5 (55.7) | 297.0 (68.8) | <0.05 | <0.001 | <0.05 |
| PAI-1, U/mL, mean (standard deviation) | 15.7 (0.9) | 22.9 (5.5) | 30.4 (7.9) | <0.001 | <0.001 | <0.001 |
| VD, ng/mL, mean (standard deviation) | 35.1 (9.9) | 23.7 (7.4) | 22.3 (8.0) | <0.001 | <0.001 | =0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Felice, G.; D’Alessandro, A.; Pastore, A.; Mariani, M.; Fintini, D.; Aureli, A.; Pezzi, S.; Montemari, A.L.; Rocco, B.B.; Borseti, A.; et al. Plasminogen Activator Inhibitor-1 and Vitamin D Association in the Overweight and Obese Pediatric Population. Nutrients 2023, 15, 3717. https://doi.org/10.3390/nu15173717
Di Felice G, D’Alessandro A, Pastore A, Mariani M, Fintini D, Aureli A, Pezzi S, Montemari AL, Rocco BB, Borseti A, et al. Plasminogen Activator Inhibitor-1 and Vitamin D Association in the Overweight and Obese Pediatric Population. Nutrients. 2023; 15(17):3717. https://doi.org/10.3390/nu15173717
Chicago/Turabian StyleDi Felice, Giovina, Annamaria D’Alessandro, Anna Pastore, Michela Mariani, Danilo Fintini, Alessia Aureli, Simona Pezzi, Anna Lisa Montemari, Beatrice Barbara Rocco, Andrea Borseti, and et al. 2023. "Plasminogen Activator Inhibitor-1 and Vitamin D Association in the Overweight and Obese Pediatric Population" Nutrients 15, no. 17: 3717. https://doi.org/10.3390/nu15173717
APA StyleDi Felice, G., D’Alessandro, A., Pastore, A., Mariani, M., Fintini, D., Aureli, A., Pezzi, S., Montemari, A. L., Rocco, B. B., Borseti, A., Onetti Muda, A., Manco, M., & Porzio, O. (2023). Plasminogen Activator Inhibitor-1 and Vitamin D Association in the Overweight and Obese Pediatric Population. Nutrients, 15(17), 3717. https://doi.org/10.3390/nu15173717







