Serum Iodine as a Potential Individual Iodine Status Biomarker: A Cohort Study of Mild Iodine Deficient Pregnant Women in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Analysis
2.2.1. Sample Collection and Processing
2.2.2. Testing and Analysis
2.3. Assessment of Covariates
2.4. Statistical Analysis
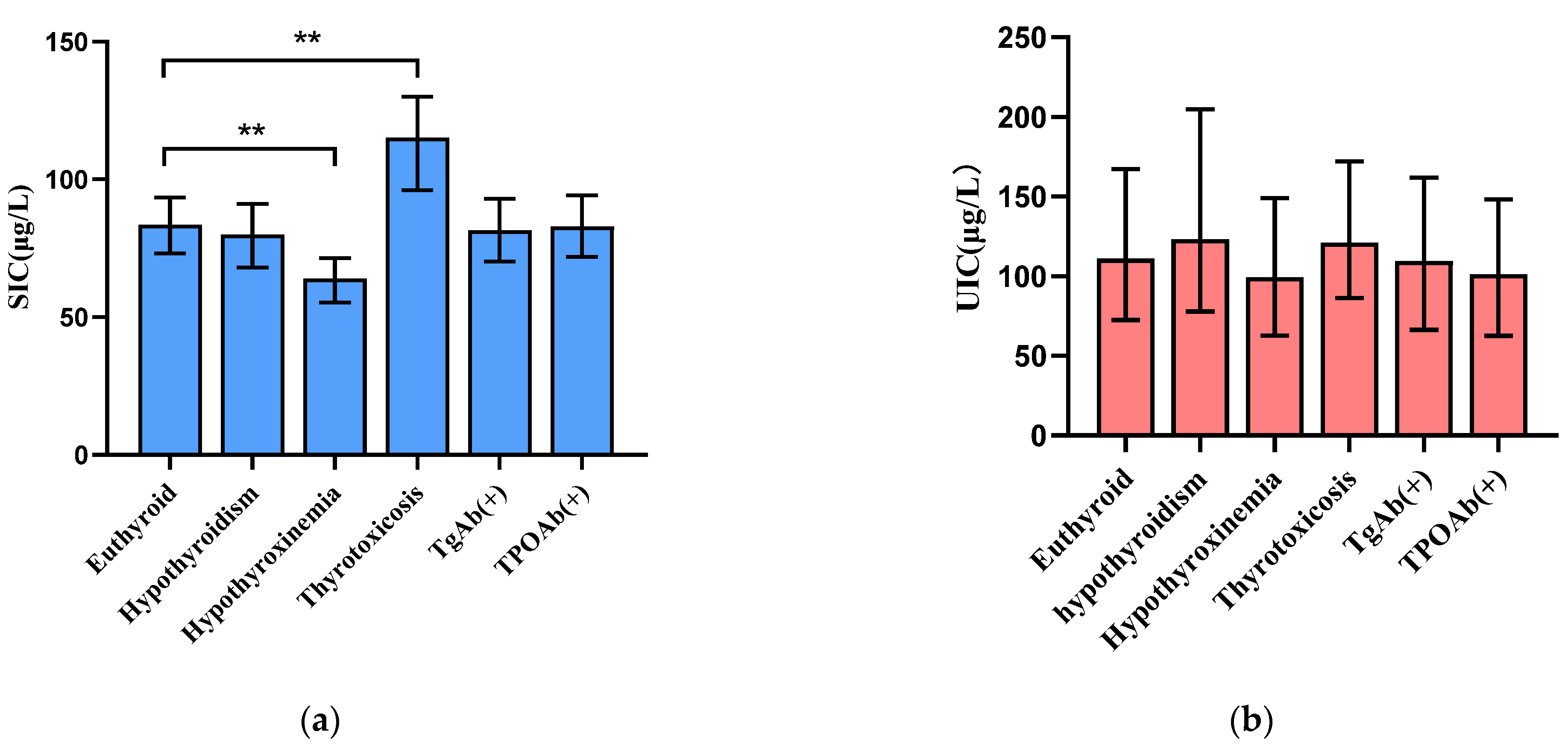
3. Results
3.1. Participant Characteristics
3.2. Distribution of Thyroid Function, Urinary Iodine, and Serum Iodine in Pregnant Women by Trimester
3.3. Correlation between Iodine Levels and Thyroid Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M.B. The Importance of Adequate Iodine during Pregnancy and Infancy. World Rev. Nutr. Diet. 2016, 115, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B.; Gizak, M.; Abbott, K.; Andersson, M.; Lazarus, J.H. Iodine deficiency in pregnant women in Europe. Lancet Diabetes Endocrinol. 2015, 3, 672–674. [Google Scholar] [CrossRef]
- Hess, S.Y.; Ouedraogo, C.T.; Young, R.R.; Bamba, I.F.; Stinca, S.; Zimmermann, M.B.; Wessells, K.R. Urinary iodine concentration identifies pregnant women as iodine deficient yet school-aged children as iodine sufficient in rural Niger. Public Health Nutr. 2017, 20, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, P.; Su, X.; Zou, S.; Song, J.; Liu, S. A Comparison of Iodine Status in Children and Pregnant Women After a Policy Change in the Iodized Salt Standard in Shanghai, China. Biol. Trace Elem. Res. 2018, 185, 275–281. [Google Scholar] [CrossRef]
- Fan, L.; Su, X.; Shen, H.; Liu, P.; Meng, F.; Yan, J.; Lei, Z.; Zhang, S.; Gu, Y.; Liu, S.; et al. Iodized salt consumption and iodine deficiency status in China: A cross-sectional study. Global Health J. 2017, 1, 23–37. [Google Scholar] [CrossRef]
- Andersson, M.; de Benoist, B.; Delange, F.; Zupan, J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: Conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007, 10, 1606–1611. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B.; Andersson, M. Assessment of iodine nutrition in populations: Past, present, and future. Nutr. Rev. 2012, 70, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Assessing iodine status and monitoring progress of iodized salt programs. J. Nutr. 2004, 134, 1673–1677. [Google Scholar] [CrossRef]
- Ma, Z.F.; Skeaff, S.A. Thyroglobulin as a biomarker of iodine deficiency: A review. Thyroid 2014, 24, 1195–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Yin, Y.; Cheng, Q.; Han, J.; Cheng, X.; Guo, Y.; Sun, D.; Xie, S.; Qiu, L. Validation of a simple inductively coupled plasma mass spectrometry method for detecting urine and serum iodine and evaluation of iodine status of pregnant women in Beijing. Scand. J. Clin. Lab. Investig. 2018, 78, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Michalke, B.; Witte, H. Characterization of a rapid and reliable method for iodide biomonitoring in serum and urine based on ion chromatography-ICP-mass spectrometry. J. Trace Elem. Med. Biol. 2015, 29, 63–68. [Google Scholar] [CrossRef]
- Yu, S.; Wang, D.; Cheng, X.; Zhang, Q.; Wang, M.; Guo, H.; Yu, B.; Zhang, X.; Xia, L.; Sun, D.; et al. Establishing reference intervals for urine and serum iodine levels: A nationwide multicenter study of a euthyroid Chinese population. Clin. Chim. Acta 2020, 502, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Wang, W.; Chen, W.; Pan, Z.; Gao, S.; Tan, L.; Pearce, E.N.; Zimmermann, M.B.; Shen, J.; Zhang, W. Serum Iodine Is Correlated with Iodine Intake and Thyroid Function in School-Age Children from a Sufficient-to-Excessive Iodine Intake Area. J. Nutr. 2019, 149, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, P.; Liu, L.; Jia, Q.; Liu, P.; Meng, F.; Zhang, X.; Guan, Y.; Pang, Y.; Lu, Z.; et al. The application of serum iodine in assessing individual iodine status. Clin. Endocrinol. 2017, 87, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Cui, T.; Chen, W.; Gao, S.; Pearce, E.N.; Wang, W.; Chen, Y.; Guo, W.; Tan, L.; Shen, J.; et al. Serum iodine concentration in pregnant women and its association with urinary iodine concentration and thyroid function. Clin. Endocrinol. 2019, 90, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Konig, F.; Andersson, M.; Hotz, K.; Aeberli, I.; Zimmermann, M.B. Ten repeat collections for urinary iodine from spot samples or 24-h samples are needed to reliably estimate individual iodine status in women. J. Nutr. 2011, 141, 2049–2054. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Peng, S.; Zhang, X.; Xie, X.; Wang, D.; Mao, J.; Teng, X.; Shan, Z.; Teng, W. The Urine Iodine to Creatinine as an Optimal Index of Iodine During Pregnancy in an Iodine Adequate Area in China. J. Clin. Endocrinol. Metab. 2016, 101, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, X.; Guo, X.; Shen, J.; Tan, L.; Lin, L.; Wu, Y.; Wang, W.; Wang, W.; Bian, J.; et al. Urinary iodine excretion (UIE) estimated by iodine/creatinine ratio from spot urine in Chinese school-age children. Clin. Endocrinol. 2017, 86, 628–633. [Google Scholar] [CrossRef]
- Iacone, R.; D’Elia, L.; Guida, B.; Barbato, A.; Scanzano, C.; Strazzullo, P. Validation of daily urinary creatinine excretion measurement by muscle-creatinine equivalence. J. Clin. Lab. Anal. 2018, 32, e22407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilderink, J.M.; van der Linden, N.; Kimenai, D.M.; Litjens, E.; Klinkenberg, L.; Aref, B.M.; Aziz, F.; Kooman, J.P.; Rennenberg, R.; Bekers, O.; et al. Biological Variation of Creatinine, Cystatin C, and eGFR over 24 Hours. Clin. Chem. 2018, 64, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Blazewicz, A.; Klatka, M.; Dolliver, W.; Kocjan, R. Determination of total iodine in serum and urine samples by ion chromatography with pulsed amperometric detection—Studies on analyte loss, optimization of sample preparation procedures, and validation of analytical method. J. Chromatogr. B 2014, 962, 141–146. [Google Scholar] [CrossRef]
- Haddow, J.E.; Palomaki, G.E.; Allan, W.C.; Williams, J.R.; Knight, G.J.; Gagnon, J.; O’Heir, C.E.; Mitchell, M.L.; Hermos, R.J.; Waisbren, S.E.; et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 1999, 341, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M.B. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: A review. Am. J. Clin. Nutr. 2009, 89, 668S–672S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dosiou, C.; Medici, M. Management of endocrine disease: Isolated maternal hypothyroxinemia during pregnancy: Knowns and unknowns. Eur. J. Endocrinol. 2017, 176, R21–R38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Yang, J.; Su, Q.; Gu, H.; Qin, L. Urinary iodine concentration and its associations with thyroid function in pregnant women of Shanghai. Front. Endocrinol. 2023, 14, 1184747. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L.; Sorensen, L.K.; Krejbjerg, A.; Moller, M.; Laurberg, P. Challenges in the evaluation of urinary iodine status in pregnancy: The importance of iodine supplement intake and time of sampling. Eur. Thyroid J. 2014, 3, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.L. Iodine status in pregnant and breastfeeding women: A Danish regional investigation. Dan. Med. J. 2015, 62, B5074. [Google Scholar]
- Ainy, E.; Ordookhani, A.; Hedayati, M.; Azizi, F. Assessment of intertrimester and seasonal variations of urinary iodine concentration during pregnancy in an iodine-replete area. Clin. Endocrinol. 2007, 67, 577–581. [Google Scholar] [CrossRef]
- Kung, A.W. Iodine nutrition of pregnant and lactating women in Hong Kong, where intake is of borderline sufficiency. Public Health Nutr. 2007, 10, 1600–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezvanian, H.; Aminorroaya, A.; Majlesi, M.; Amini, A.; Hekmatnia, A.; Kachoie, A.; Amini, M.; Emami, J. Thyroid size and iodine intake in iodine-repleted pregnant women in Isfahan, Iran. Endocr. Pract. 2002, 8, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Arrizabalaga, J.J.; Larranaga, N.; Espada, M.; Amiano, P.; Bidaurrazaga, J.; Latorre, K.; Gorostiza, E. Changes in iodine nutrition status in schoolchildren from the Basque Country. Endocrinol. Nutr. 2012, 59, 474–484. [Google Scholar] [CrossRef] [PubMed]
| First Trimester | Second Trimester | Third Trimester | |
|---|---|---|---|
| Clinical hypothyroidism | TSH > 4.52 mU/L and FT4 < 13.15 pmol/L | TSH > 4.32 mU/L and FT4 < 9.77 pmol/L | TSH > 4.98 mU/L and FT4 < 9.04 pmol/L |
| Subclinical hypothyroidism | TSH > 4.52 mU/L and 13.15 < FT4 < 20.78 pmol/L | TSH > 4.32 mU/L and 9.77 < FT4 < 18.89 pmol/L | TSH > 4.98 mU/L and 9.04 < FT4 < 15.22 pmol/L |
| Hypothyroxinemia | 0.09 < TSH < 4.52 mU/L and FT4 < 13.15 pmol/L | 0.45 < TSH < 4.32 mU/L and FT4 < 9.77 pmol/L | 0.30 < TSH < 4.98 mU/L and FT4 < 9.04 pmol/L |
| Thyrotoxicosis | TSH < 0.09 mU/L and FT4 > 20.78 pmol/L | TSH < 0.45 mU/L and FT4 > 18.89 pmol/L | TSH < 0.30 mU/L and FT4 > 15.22 pmol/L |
| Characteristic 1 | Mean ± SD/N(%) |
|---|---|
| Age, years | 29.0 ± 4.6 |
| BMI, Kg/m2 | 21.7 ± 3.3 |
| Education | |
| Junior high school or below | 271 (17.6) |
| High school | 423 (27.5) |
| College or above | 846 (54.9) |
| Alcohol use during pregnancy | |
| Yes | 69 (4.5) |
| No | 1471 (95.5) |
| Smoking during pregnancy | |
| Yes | 18 (1.2) |
| No | 1522 (98.8) |
| Passive smoking during pregnancy | |
| Yes | 313 (20.3) |
| No | 1227 (79.7) |
| First Trimester (n = 1540) | Second Trimester (n = 1487) | Third Trimester (n = 1306) | p | |
|---|---|---|---|---|
| Gestational age (week) a | 10 (6, 13) | 17 (16, 27) | 32 (28, 36) | - |
| TSH (mIU/L) b | 1.1 (0.6, 1.8) ** | 1.7 (1.2, 2.3) ** | 1.8 (1.3, 2.4) ** | <0.001 |
| FT3 (pmol/L) b | 4.8 (4.4, 5.3) ** | 4.3 (4.0, 4.7) ** | 3.8 (3.5, 4.2) ** | <0.001 |
| FT4 (pmol/L) b | 16.7 (15.2, 18.5) ** | 13.9 (12.7, 15.2) ** | 12.1 (11.0, 13.3) ** | <0.001 |
| Tg (µg/L) b | 11.1 (6.6, 17.9) | 10.8 (6.2, 17.5) * | 11.8 (6.7, 18.3) | 0.019 |
| Thyroid dysfunction n(%) | ||||
| Overt hypothyroidism | 7 (0.5) | 1 (0.1) | 0 (0.0) | - |
| Subclinical hypothyroidism | 22 (1.4) | 31 (2.1) | 16 (1.2) | 0.158 |
| Hypothyroxinemia | 51 (3.3) | 16 (1.1) # | 24 (2.4) # | <0.001 |
| Thyrotoxicosis | 76 (4.9) | 10 (0.7) # | 9 (0.7) # | <0.001 |
| TgAb(+) n(%) | 114 (7.4) * | 82 (5.5) * | 21 (1.6) | <0.001 |
| TPOAb(+) n(%) | 137 (8.9) * | 118 (7.9) * | 68 (5.2) | 0.001 |
| UIC (µg/L) b | 112.9 (73.5, 172.2) * | 114.0 (73.7, 168.0) * | 105.0 (68.2, 156.6) | 0.007 |
| SIC (µg/L) b | 79.6 (68.1, 91.5) ** | 86.5 (76.2, 96.5) ** | 83.7 (73.5, 93.3) ** | <0.001 |
| TSH | FT3 | FT4 | Tg | |
|---|---|---|---|---|
| First trimester | ||||
| UIC | −0.036 | −0.049 | 0.038 | −0.018 |
| SIC | −0.282 ** | 0.178 ** | 0.449 ** | 0.108 ** |
| Second trimester | ||||
| UIC | 0.030 | −0.018 | 0.013 | −0.020 |
| SIC | 0.024 | 0.165 ** | 0.550 ** | 0.040 |
| Third trimester | ||||
| UIC | 0.075 ** | 0.010 | 0.045 | −0.096 ** |
| SIC | −0.041 | 0.022 | 0.544 ** | 0.065 * |
| Hypothyroxinemia | Thyrotoxicosis | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted 1 | Unadjusted | Adjusted 1 | |||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| SIC (µg/L) | ||||||||
| <72.4 (Quartile 1) | 9.619 (5.567–16.619) | 0.000 | 8.911 (5.141–15.447) | 0.000 | 0.400 (0.116–1.386) | 0.149 | 0.415 (0.120–1.440) | 0.166 |
| 72.4–93.9 (Quartile 2 + Quartile 3) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | ||||
| >93.9 (Quartile 4) | 0.379 (0.110–1.302) | 0.123 | 0.388 (0.113–1.338) | 0.134 | 11.143 (6.376–19.473) | 0.000 | 11.064 (6.324–19.357) | 0.000 |
| UIC (µg/L) | ||||||||
| <150 (Iodine deficiency) | 1.319 (0.800–2.175) | 0.277 | 1.405 (0.856–2.306) | 0.179 | 1.001 (0.637–1.572) | 0.997 | 0.979 (0.629–1.526) | 0.927 |
| 150–500 (Iodine sufficiency) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | ||||
| >500 (Iodine excess) | -- | -- | -- | -- | 0.546 (0.071–4.167) | 0.559 | 0.849 (0.113–6.361) | 0.873 |
| SIC (µg/L) | UIC < 150 µg/L | UIC > 500 µg/L | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted 1 | Unadjusted | Adjusted 1 | |||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| <72.4 (Quartile 1) | 1.259 (1.044–1.519) | 0.016 | 1.244 (1.031–1.502) | 0.023 | 0.694 (0.294–1.637) | 0.404 | 0.675 (0.286–1.596) | 0.371 |
| 72.4–93.9 (Quartile 2 + Quartile 3) | 1(referent) | 1 (referent) | 1 (referent) | 1 (referent) | ||||
| >93.9 (Quartile 4) | 1.001 (0.835–1.199) | 0.992 | 1.000 (0.834–1.198) | 0.997 | 1.869 (1.026–3.404) | 0.041 | 1.866 (1.024–3.399) | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tu, P.; Gu, S.; Mo, Z.; Wu, L.; Xing, M.; Chen, Z.; Wang, X. Serum Iodine as a Potential Individual Iodine Status Biomarker: A Cohort Study of Mild Iodine Deficient Pregnant Women in China. Nutrients 2023, 15, 3555. https://doi.org/10.3390/nu15163555
Li X, Tu P, Gu S, Mo Z, Wu L, Xing M, Chen Z, Wang X. Serum Iodine as a Potential Individual Iodine Status Biomarker: A Cohort Study of Mild Iodine Deficient Pregnant Women in China. Nutrients. 2023; 15(16):3555. https://doi.org/10.3390/nu15163555
Chicago/Turabian StyleLi, Xueqing, Pengcheng Tu, Simeng Gu, Zhe Mo, Lizhi Wu, Mingluan Xing, Zhijian Chen, and Xiaofeng Wang. 2023. "Serum Iodine as a Potential Individual Iodine Status Biomarker: A Cohort Study of Mild Iodine Deficient Pregnant Women in China" Nutrients 15, no. 16: 3555. https://doi.org/10.3390/nu15163555
APA StyleLi, X., Tu, P., Gu, S., Mo, Z., Wu, L., Xing, M., Chen, Z., & Wang, X. (2023). Serum Iodine as a Potential Individual Iodine Status Biomarker: A Cohort Study of Mild Iodine Deficient Pregnant Women in China. Nutrients, 15(16), 3555. https://doi.org/10.3390/nu15163555







