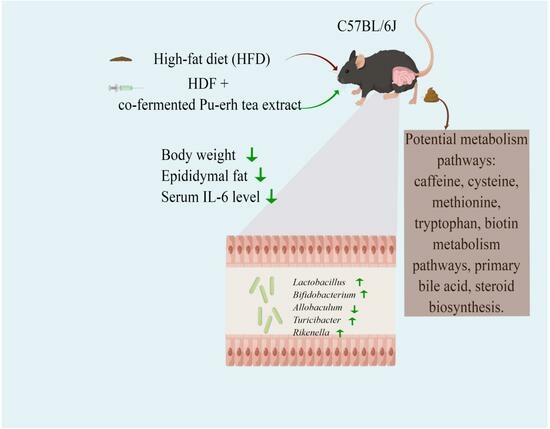
Modulatory Effects of Co-Fermented Pu-erh Tea with Aqueous Corn Silk Extract on Gut Microbes and Fecal Metabolites in Mice Fed High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of CPC Extract
2.3. Animals and Experimental Design
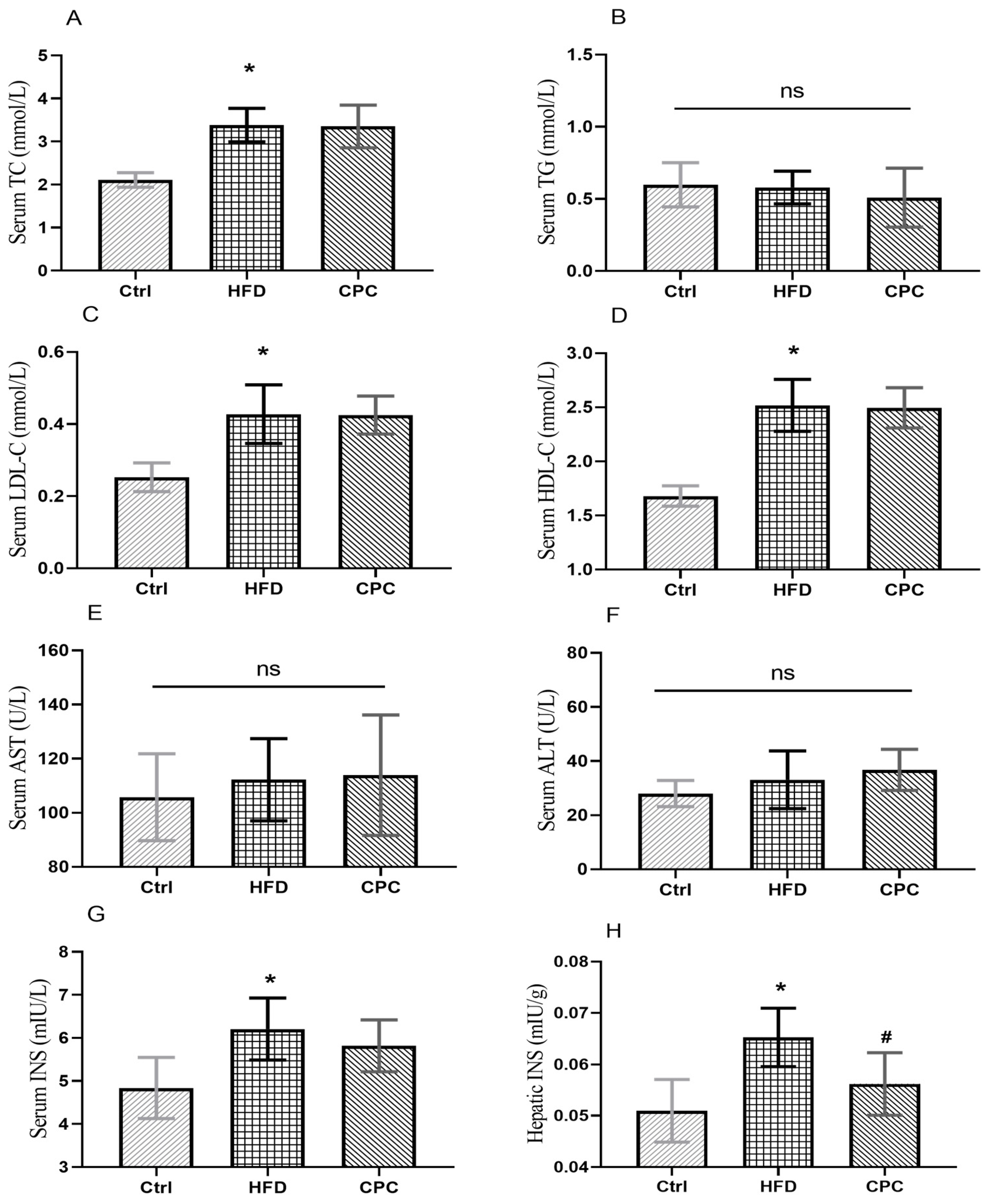
2.4. Biochemical Indicator Measurement
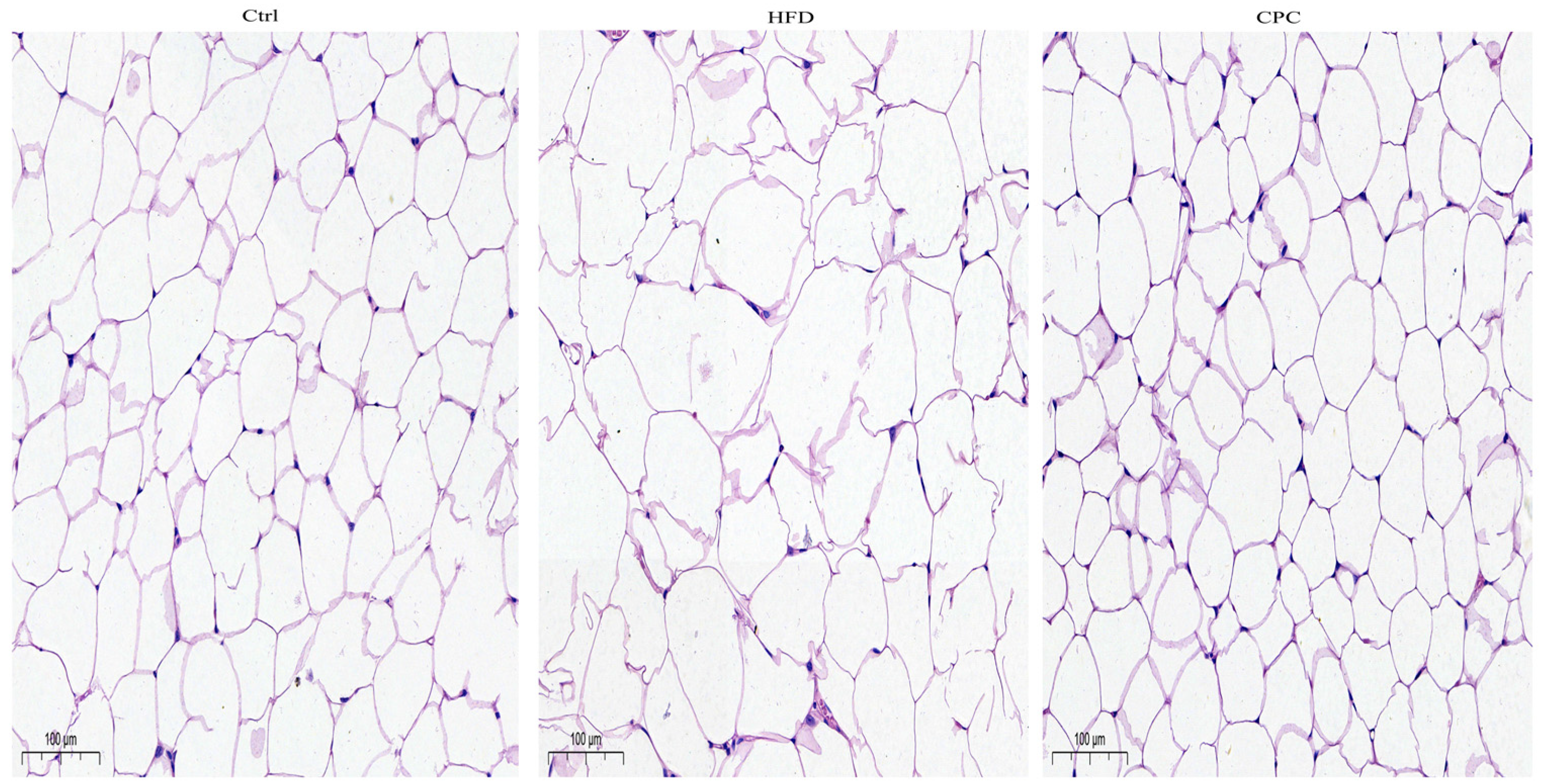
2.5. Histopathological Examination
2.6. Gut Microbiota Analysis
2.7. Untargeted Fecal Metabolomic Analysis
2.8. Data Analysis
3. Results
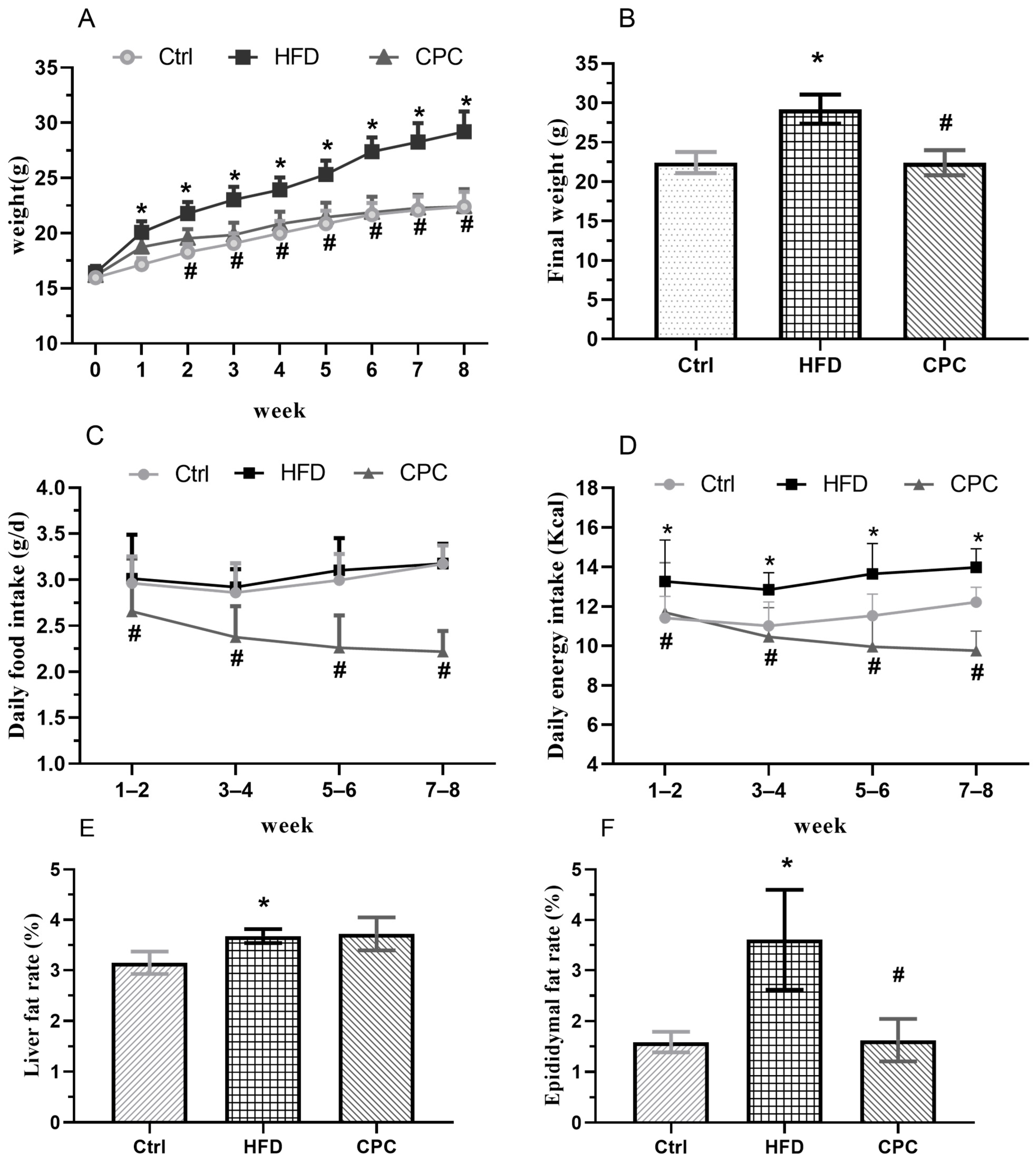
3.1. Impact of the CPC Extract on Body Weight, Food Intake, and Energy Intake
3.2. Impact of the CPC Extract on Epididymal Fat Tissue Mass and Serum Lipid Levels
3.3. Impact of the CPC Extract on Chronic Inflammation
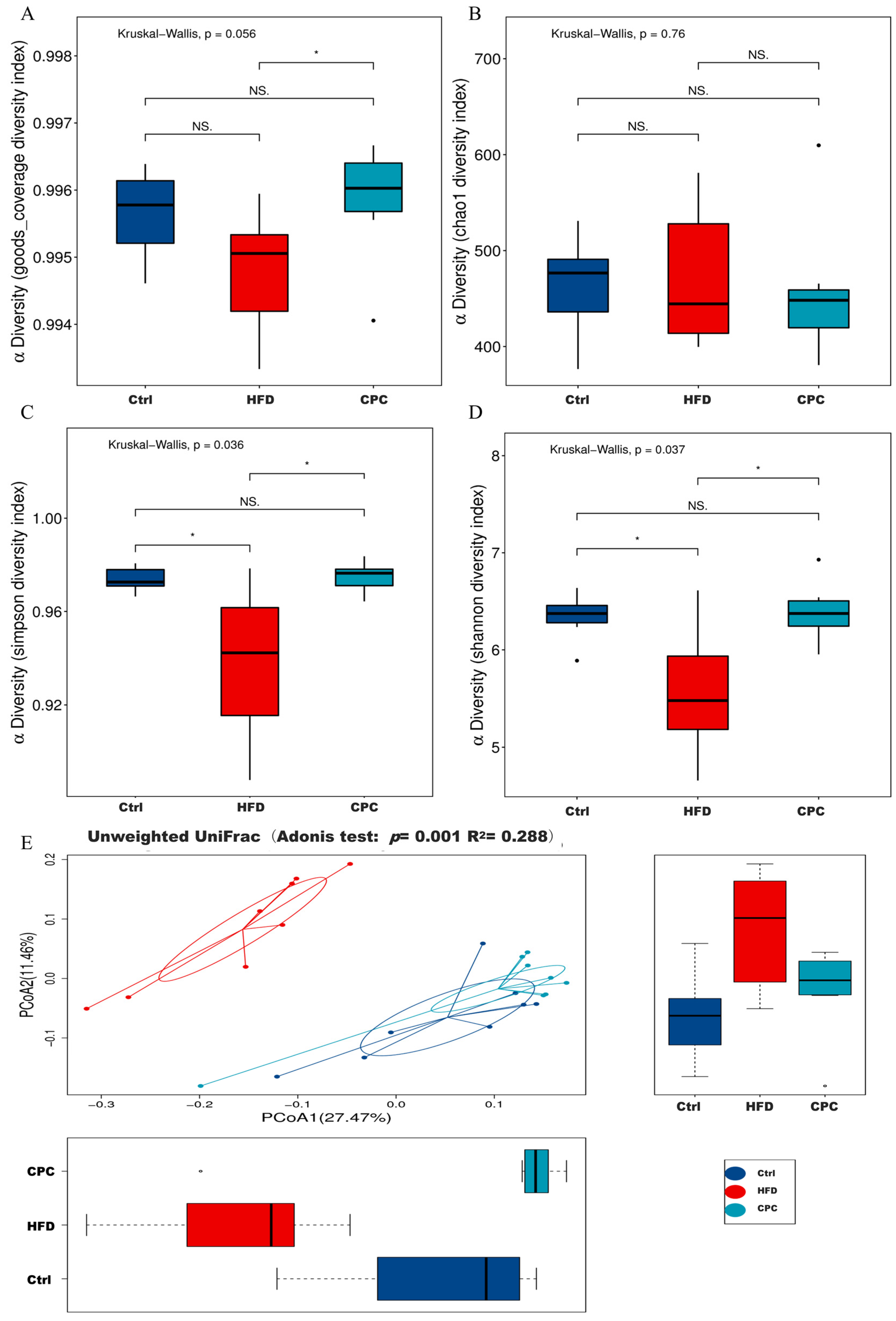
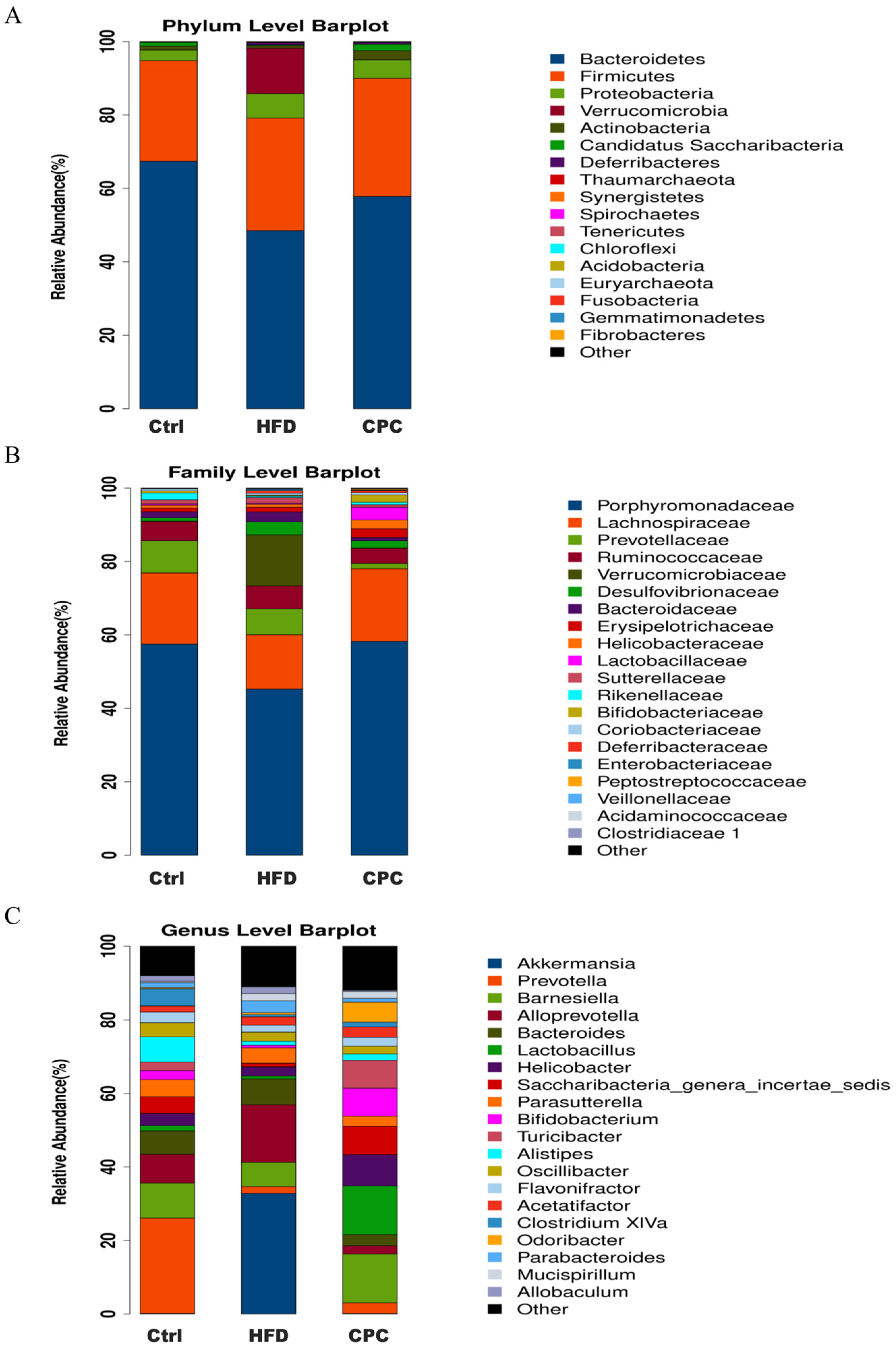
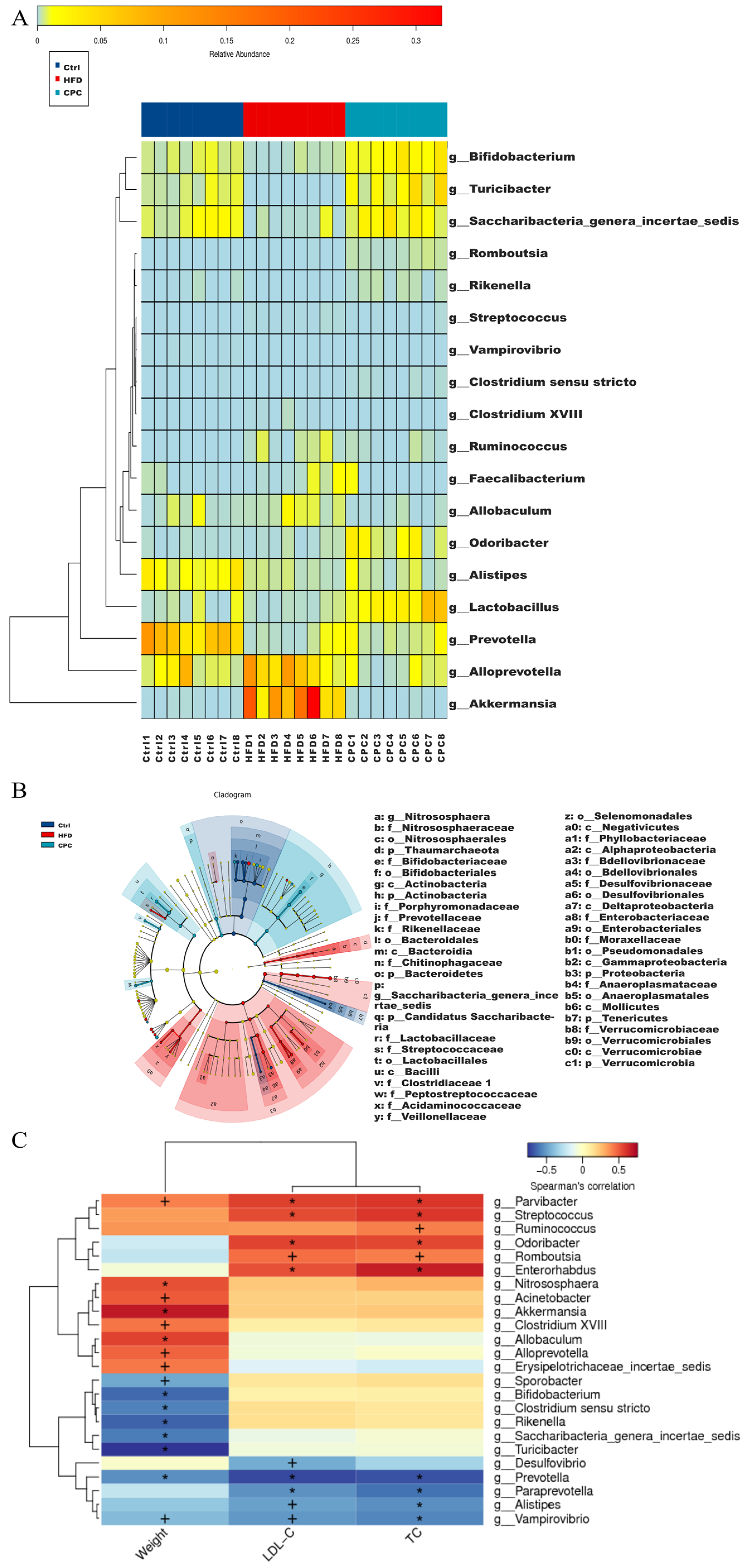
3.4. Impact of the CPC Extract on Diversity and Composition of GM
3.5. Impact of the CPC Extract on Fecal Metabolites
3.6. KEGG Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Madry, E. High-fat, western-style diet, systemic inflammation, and gut microbiota: A narrative review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol. Med. 2015, 21, 702–714. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef]
- Deng, X.; Huang, G.; Tu, Q.; Zhou, H.; Li, Y.; Shi, H.; Wu, X.; Ren, H.; Huang, K.; He, X.; et al. Evolution analysis of flavor-active compounds during artificial fermentation of Pu-erh tea. Food Chem. 2021, 357, 129783. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, P.L.; Chen, X.L.; Gong, M.J.; Zhang, L.; Qiu, X.H.; Zhang, J.; Huang, Z.H.; Xu, W. The impact of citrus-tea cofermentation process on chemical composition and contents of Pu-erh tea: An integrated metabolomics study. Front. Nutr. 2021, 8, 737539. [Google Scholar] [CrossRef]
- Ding, L.; Ren, S.; Song, Y.; Zang, C.; Liu, Y.; Guo, H.; Yang, W.; Guan, H.; Liu, J. Modulation of gut microbiota and fecal metabolites by corn silk among high-fat diet-induced hypercholesterolemia mice. Front. Nutr. 2022, 9, 935612. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Zhang, N.; Fu, Y.; Zhang, Z.; Li, Y.; Wang, W.; Li, Y.; Shen, P.; Cao, Y. Ripened Pu-erh tea extract protects mice from obesity by modulating gut microbiota composition. J. Agric. Food Chem. 2019, 67, 6978–6994. [Google Scholar] [CrossRef]
- Yue, S.; Peng, C.; Zhao, D.; Xia, X.; Tan, C.; Wang, Q.; Gong, J. Theabrownin isolated from Pu-erh tea regulates Bacteroidetes to improve metabolic syndrome of rats induced by high-fat, high-sugar and high-salt diet. J. Sci. Food Agric. 2022, 102, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, C.C. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Lecube, A.; Lopez-Cano, C. Obesity, a diet-induced inflammatory disease. Nutrients 2019, 11, 2284. [Google Scholar] [CrossRef] [PubMed]
- Greiner, T.; Backhed, F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metab. 2011, 22, 117–123. [Google Scholar] [CrossRef]
- Asai, A.; Chou, P.M.; Bu, H.F.; Wang, X.; Rao, M.S.; Jiang, A.; DiDonato, C.J.; Tan, X.D. Dissociation of hepatic insulin resistance from susceptibility of nonalcoholic fatty liver disease induced by a high-fat and high-carbohydrate diet in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G496–G504. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Just, S.; Mondot, S.; Ecker, J.; Wegner, K.; Rath, E.; Gau, L.; Streidl, T.; Hery-Arnaud, G.; Schmidt, S.; Lesker, T.R.; et al. The gut microbiota drives the impact of bile acids and fat source in diet on mouse metabolism. Microbiome 2018, 6, 134. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone disease, obesity and the Firmicutes/Bacteroidetes ratio as a possible biomarker of gut dysbiosis. J. Pers. Med. 2020, 11, 13. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Song, C.; Li, L.; Wang, T.; Hu, J.; Zhu, L.; Yue, T. Lactobacillus alleviated obesity induced by high-fat diet in mice. J. Food Sci. 2021, 86, 5439–5451. [Google Scholar] [CrossRef] [PubMed]
- Greetham, H.L.; Gibson, G.R.; Giffard, C.; Hippe, H.; Merkhoffer, B.; Steiner, U.; Falsen, E.; Collins, M.D. Allobaculum stercoricanis gen. nov., sp. nov., isolated from canine feces. Anaerobe 2004, 10, 301–307. [Google Scholar] [CrossRef]
- Zheng, Z.; Lyu, W.; Ren, Y.; Li, X.; Zhao, S.; Yang, H.; Xiao, Y. Allobaculum involves in the modulation of intestinal ANGPTLT4 expression in mice treated by high-fat diet. Front. Nutr. 2021, 8, 690138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Raychaudhuri, S.; Page, R.; Shahinozzaman, M.; Obanda, D.N. Metagenomic insights into the effects of Urtica dioica vegetable on the gut microbiota of C57BL/6J obese mice, particularly the composition of Clostridia. J. Nutr. Biochem. 2021, 91, 108594. [Google Scholar] [CrossRef]
- Kemis, J.H.; Linke, V.; Barrett, K.L.; Boehm, F.J.; Traeger, L.L.; Keller, M.P.; Rabaglia, M.E.; Schueler, K.L.; Stapleton, D.S.; Gatti, D.M.; et al. Genetic determinants of gut microbiota composition and bile acid profiles in mice. PLoS Genet. 2019, 15, e1008073. [Google Scholar] [CrossRef]
- Johnson, A.M.; Costanzo, A.; Gareau, M.G.; Armando, A.M.; Quehenberger, O.; Jameson, J.M.; Olefsky, J.M. High fat diet causes depletion of intestinal eosinophils associated with intestinal permeability. PLoS ONE 2015, 10, e0122195. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- He, W.S.; Li, L.; Rui, J.; Li, J.; Sun, Y.; Cui, D.; Xu, B. Tomato seed oil attenuates hyperlipidemia and modulates gut microbiota in C57BL/6J mice. Food Funct. 2020, 11, 4275–4290. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, K.H.; Nakamura, Y.; Kawakami, S.; Shimada, K.; Hayakawa, T.; Onoue, H.; Fukushima, M. Dietary L-cysteine improves the antioxidative potential and lipid metabolism in rats fed a normal diet. Biosci. Biotechnol. Biochem. 2013, 77, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Y.; Gao, N.; Lan, J.; Dou, X.; Li, J.; Shan, A. L-Threonine upregulates the expression of beta-defensins by activating the NF-kappaB signaling pathway and suppressing SIRT1 expression in porcine intestinal epithelial cells. Food Funct. 2021, 12, 5821–5836. [Google Scholar] [CrossRef]
- Skupsky, J.; Sabui, S.; Hwang, M.; Nakasaki, M.; Cahalan, M.D.; Said, H.M. Biotin supplementation ameliorates murine colitis by preventing NF-kappaB activation. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 557–567. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acids as metabolic regulators and nutrient sensors. Annu. Rev. Nutr. 2019, 39, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid metabolism in liver pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Barone, J.J.; Roberts, H.R. Caffeine consumption. Food Chem. Toxicol. 1996, 34, 119–129. [Google Scholar] [CrossRef]
- Summers, R.M.; Louie, T.M.; Yu, C.L.; Subramanian, M. Characterization of a broad-specificity non-haem iron N-demethylase from Pseudomonas putida CBB5 capable of utilizing several purine alkaloids as sole carbon and nitrogen source. Microbiol. (Read.) 2011, 157, 583–592. [Google Scholar] [CrossRef]
- Nakabayashi, H.; Hashimoto, T.; Ashida, H.; Nishiumi, S.; Kanazawa, K. Inhibitory effects of caffeine and its metabolites on intracellular lipid accumulation in murine 3T3-L1 adipocytes. Biofactors 2008, 34, 293–302. [Google Scholar] [CrossRef]
- Ahmed, W.H.; Boulet, N.; Briot, A.; Ryan, B.J.; Kinsella, G.K.; O’Sullivan, J.; Les, F.; Mercader-Barcelo, J.; Henehan, G.T.M.; Carpene, C. Novel facet of an old dietary molecule? direct influence of caffeine on glucose and biogenic amine handling by human adipocytes. Molecules 2021, 26, 3831. [Google Scholar] [CrossRef]


| Composition | Content (per 1000 g) | |
|---|---|---|
| High-Fat Diet | Control Diet | |
| Casein | 233.06 | 189.58 |
| Cystine | 3.50 | 2.84 |
| Corn starch | 84.83 | 298.59 |
| Maltose dextrin | 116.53 | 33.18 |
| Sucrose | 201.36 | 331.77 |
| Cellulose | 58.26 | 47.40 |
| Soybean oil | 29.13 | 23.70 |
| Lard | 206.84 | 18.96 |
| Mineral mixture M1002 | 11.65 | 9.48 |
| Dicalcium phosphate | 15.15 | 12.32 |
| Calcium carbonate | 6.41 | 5.21 |
| Potassium citrate | 19.23 | 15.64 |
| Vitamin mixture V1001 | 11.56 | 9.48 |
| Hydrocholine Tartaric acid | 2.33 | 1.90 |
| Edible red dye | 0.058 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Guan, H.; Yang, W.; Guo, H.; Zang, C.; Liu, Y.; Ren, S.; Liu, J. Modulatory Effects of Co-Fermented Pu-erh Tea with Aqueous Corn Silk Extract on Gut Microbes and Fecal Metabolites in Mice Fed High-Fat Diet. Nutrients 2023, 15, 3642. https://doi.org/10.3390/nu15163642
Ding L, Guan H, Yang W, Guo H, Zang C, Liu Y, Ren S, Liu J. Modulatory Effects of Co-Fermented Pu-erh Tea with Aqueous Corn Silk Extract on Gut Microbes and Fecal Metabolites in Mice Fed High-Fat Diet. Nutrients. 2023; 15(16):3642. https://doi.org/10.3390/nu15163642
Chicago/Turabian StyleDing, Lin, Hong Guan, Wenqing Yang, Hao Guo, Chuangang Zang, Yuchao Liu, Shan Ren, and Jicheng Liu. 2023. "Modulatory Effects of Co-Fermented Pu-erh Tea with Aqueous Corn Silk Extract on Gut Microbes and Fecal Metabolites in Mice Fed High-Fat Diet" Nutrients 15, no. 16: 3642. https://doi.org/10.3390/nu15163642
APA StyleDing, L., Guan, H., Yang, W., Guo, H., Zang, C., Liu, Y., Ren, S., & Liu, J. (2023). Modulatory Effects of Co-Fermented Pu-erh Tea with Aqueous Corn Silk Extract on Gut Microbes and Fecal Metabolites in Mice Fed High-Fat Diet. Nutrients, 15(16), 3642. https://doi.org/10.3390/nu15163642