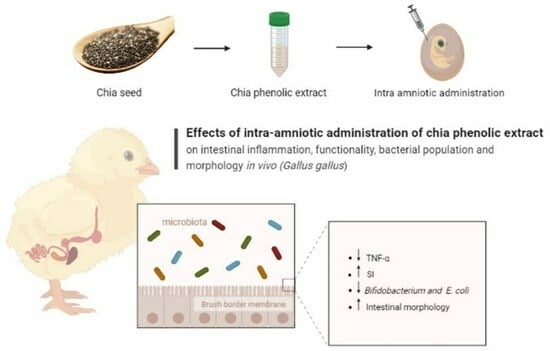
Chia Phenolic Extract Appear to Improve Small Intestinal Functionality, Morphology, Bacterial Populations, and Inflammation Biomarkers In Vivo (Gallus gallus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Material
2.1.1. Chia Phenolic Extract
2.1.2. Determination of Total Phenolics
2.1.3. Antioxidant Capacity
2.2. Intra-Amniotic Administration
2.3. Extraction of the Total RNA from the Duodenum Samples
2.4. Real-Time Polymerase Chain Reaction (RT-PCR) and Prime Design
2.5. Real-Time qPCR Design
2.6. Collection of Microbial Samples and Intestinal Contents DNA Extraction
2.7. Primer’s Design and PCR Amplification of Bacterial 16S rDNA
2.8. Intestinal Morphology
2.9. Statistical Analysis
3. Results
3.1. Chia Phenolic Extract Characterization
3.2. Body Weight
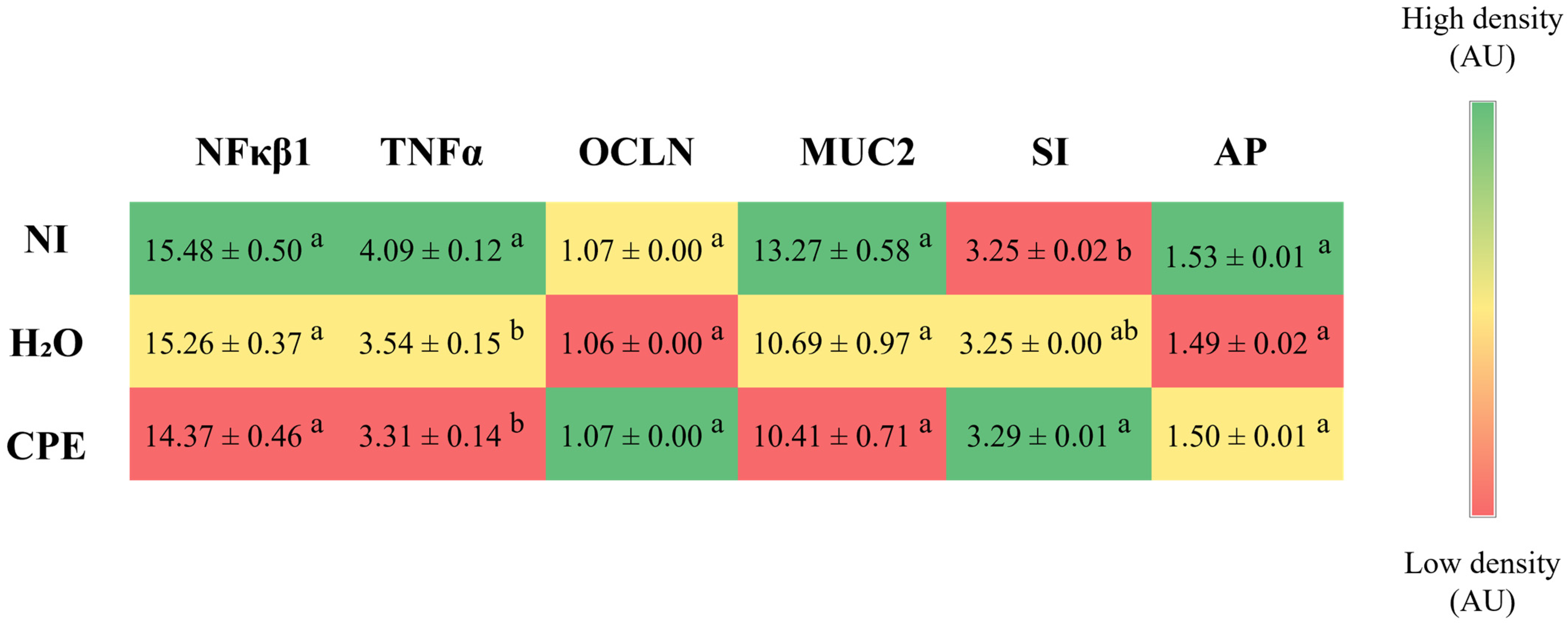
3.3. Effect of Chia Phenolic Extract on Duodenal Gene Expression
3.4. Effect of Chia Phenolic Extract on the Bacterial Population on Cecum Content
3.5. Effect of Chia Phenolic Extract on Morphological Parameters in Duodenum
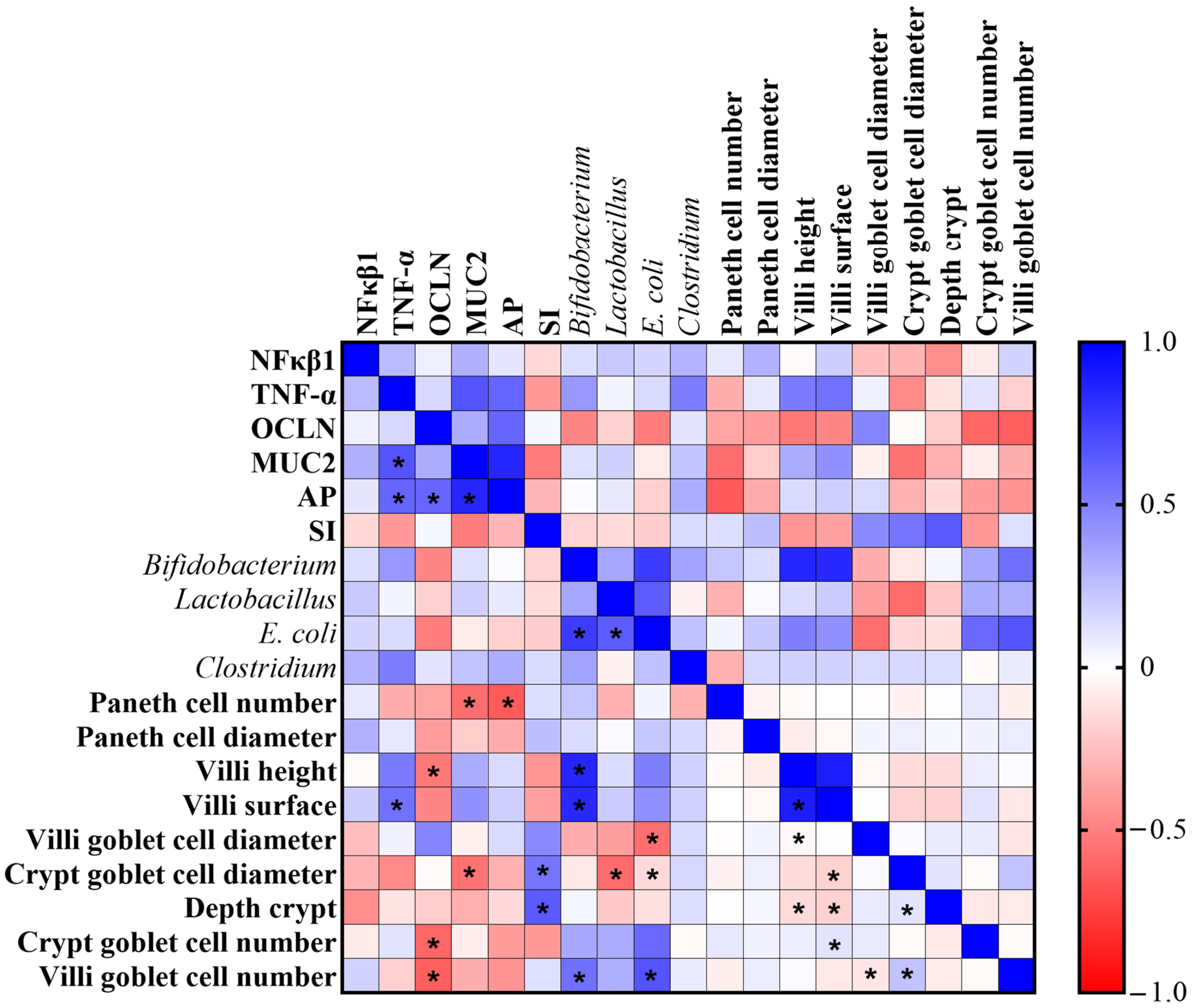
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsumura, Y.; Kitabatake, M.; Kayano, S.I.; Ito, T. Dietary Phenolic Compounds: Their Health Benefits and Association with the Gut Microbiota. Antioxidants 2023, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gómez, V.; González-Barrio, R.; Periago, M.J. Interaction between Dietary Fibre and Bioactive Compounds in Plant By-Products: Impact on Bioaccessibility and Bioavailability. Antioxidants 2023, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, K.; Lin, Y.; Liu, Y. Protective Effects and Molecular Mechanisms of Tea Polyphenols on Cardiovascular Diseases. Front. Nutr. 2023, 10, 1202378. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic Compounds and Related Enzymes as Determinants of Quality in Fruits and Vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Villa-Rodriguez, J.A.; Montiel-Herrera, M.; Pacheco-Ordaz, R.; Roopchand, D.E.; Venema, K.; González-Aguilar, G.A. Phenolic Compounds Promote Diversity of Gut Microbiota and Maintain Colonic Health. Dig. Dis. Sci. 2021, 66, 3270–3289. [Google Scholar] [CrossRef]
- Clifford, M.N. Diet-Derived Phenols in Plasma and Tissues and Their Implications for Health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as Modifiers of Gut Microbial Communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Fathi Hafshejani, S.; Lotfi, S.; Rezvannejad, E.; Mortazavi, M.; Riahi-Madvar, A. Correlation between Total Phenolic and Flavonoid Contents and Biological Activities of 12 Ethanolic Extracts of Iranian Propolis. Food Sci. Nutr. 2023, 11, 4308–4325. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. Dietary Polyphenols: Antioxidants or Not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases—Promises, Perspectives, and Pitfalls. Oxid. Med. Cell. Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of Tea Phenolics and Their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; Abd El-Hack, M.E. The Role of Polyphenols in Poultry Nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef]
- Lara, A.R.; Mesa-García, M.D.; Medina, K.A.D.; Quirantes Piné, R.; Casuso, R.A.; Segura Carretero, A.; Huertas, J.R. Assessment of the Phytochemical and Nutrimental Composition of Dark Chia Seed (Salvia Hispánica L.). Foods 2021, 10, 3001. [Google Scholar] [CrossRef]
- Martínez-Cruz, O.; Paredes-López, O. Phytochemical Profile and Nutraceutical Potential of Chia Seeds (Salvia Hispanica L.) by Ultra High Performance Liquid Chromatography. J. Chromatogr. A 2014, 1346, 43–48. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Anunciação, P.C.; da Silva Matyelka, J.C.; Della Lucia, C.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chemical Composition of Brazilian Chia Seeds Grown in Different Places. Food Chem. 2017, 221, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Motyka, S.; Skała, E.; Ekiert, H.; Szopa, A. Health-Promoting Approaches of the Use of Chia Seeds. J. Funct. Foods 2023, 103, 105480. [Google Scholar] [CrossRef]
- Enes, B.N.; Moreira, L.D.P.D.; Toledo, R.C.L.; Moraes, É.A.; de Castro Moreira, M.E.; Hermsdorff, H.H.M.; Noratto, G.; Mertens-Talcott, S.U.; Talcott, S.; Martino, H.S.D.; et al. Effect of Different Fractions of Chia (Salvia Hispanica L.) on Glucose Metabolism, in Vivo and in Vitro. J. Funct. Foods 2020, 71, 157–164. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Kolba, N.; Martino, H.S.D.; Hart, J.; Tako, E. Soluble Extracts from Chia Seed (Salvia Hispanica L.) Affect Brush Border Membrane Functionality, Morphology and Intestinal Bacterial Populations in Vivo (Gallus Gallus). Nutrients 2019, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of Flavonoids on Intestinal Inflammation, Barrier Integrity and Changes in Gut Microbiota during Diet-Induced Obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The Gut Microbiota: A Key Factor in the Therapeutic Effects of (Poly)Phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef]
- Verediano, T.A.; Agarwal, N.; Martino, H.S.D.; Grancieri, M.; Paes, M.C.D.; Tako, E. Effect of Black Corn Anthocyanin-Rich Extract (Zea Mays L.) on Cecal Microbial Populations In Vivo (Gallus Gallus). Nutrients 2022, 14, 4679. [Google Scholar] [CrossRef]
- Mishima, M.D.V.; Martino, H.S.D.; Kolba, N.; Shah, D.D.; Grancieri, M.; Dos Santos, K.M.O.; Lima, J.P.; Da Silva, B.P.; de Mejia, E.G.; Tako, E. Effects of Intra-Amniotic Administration of the Hydrolyzed Protein of Chia (Salvia Hispanica L.) and Lacticaseibacillus Paracasei on Intestinal Functionality, Morphology, and Bacterial Populations, In Vivo (Gallus Gallus). Nutrients 2023, 15, 1831. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Polyphen. Flavonoids 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Grancieri, M.; Stampini, H.; Martino, D.; Gonzalez, E.; Mejia, D. Digested Total Protein and Protein Fractions from Chia Seed (Salvia Hispanica L.) Had High Scavenging Capacity and Inhibited 5-LOX, COX-1-2, and INOS Enzymes. Food Chem. 2019, 289, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Martino, H.S.D.; Kolba, N.; Tako, E. Yacon (Smallanthus Sonchifolius) Flour Soluble Extract Improve Intestinal Bacterial Populations, Brush Border Membrane Functionality and Morphology in Vivo (Gallus Gallus). Food Res. Int. 2020, 137, 109705. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, L.; Xu, G.; Li, Z.; Appiah, M.O.; Yang, L.; Lu, W. Quercetin Reduces Inflammation and Protects Gut Microbiota in Broilers. Molecules 2022, 27, 3269. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Wang, J.; Zheng, T.; Wu, C.; Cui, M.; Feng, Y.; Ye, H.; Dong, Z.; Dang, Y. Plant Polyphenols Attenuate DSS-Induced Ulcerative Colitis in Mice via Antioxidation, Anti-Inflammation and Microbiota Regulation. Int. J. Mol. Sci. 2023, 24, 10828. [Google Scholar] [CrossRef] [PubMed]
- Bing, X.; Xuelei, L.; Wanwei, D.; Linlang, L.; Keyan, C. EGCG Maintains Th1/Th2 Balance and Mitigates Ulcerative Colitis Induced by Dextran Sulfate Sodium through TLR4/MyD88/NF-ΚB Signaling Pathway in Rats. Can. J. Gastroenterol. Hepatol. 2017, 2017, 3057268. [Google Scholar] [CrossRef]
- Quarta, S.; Massaro, M.; Carluccio, M.A.; Calabriso, N.; Bravo, L.; Sarria, B.; García-Conesa, M.T. An Exploratory Critical Review on TNF-α as a Potential Inflammatory Biomarker Responsive to Dietary Intervention with Bioactive Foods and Derived Products. Foods 2022, 11, 2524. [Google Scholar] [CrossRef]
- Lima, M.S.R.; De Lima, V.C.O.; Piuvezam, G.; De Azevedo, K.P.M.; Maciel, B.L.L.; De Araújo Morais, A.H. Mechanisms of Action of Anti-Inflammatory Proteins and Peptides with Anti-TNF-Alpha Activity and Their Effects on the Intestinal Barrier: A Systematic Review. PLoS ONE 2022, 17, e0270749. [Google Scholar] [CrossRef]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour Necrosis Factor Signalling in Health and Disease [Version 1; Referees: 2 Approved]. F1000Research 2019, 8, 111. [Google Scholar] [CrossRef]
- Lu, Y.H.; Hong, Y.; Zhang, T.Y.; Chen, Y.X.; Wei, Z.J.; Gao, C.Y. Rosmarinic Acid Exerts Anti-Inflammatory Effect and Relieves Oxidative Stress via Nrf2 Activation in Carbon Tetrachloride-Induced Liver Damage. Food Nutr. Res. 2022, 66, 1–13. [Google Scholar] [CrossRef]
- Lai, K.M.; Chen, S.Y.; Wang, G.Y.; Shahidi, F.; Yen, G.C. Protective Effect of Rosmarinic Acid-Rich Extract from Trichodesma Khasianum Clarke against Microbiota Dysbiosis in High-Fat Diet-Fed Obese Mice. Food Res. Int. 2023, 164, 112344. [Google Scholar] [CrossRef]
- Zhao, G.R.; Zhang, H.M.; Ye, T.X.; Xiang, Z.J.; Yuan, Y.J.; Guo, Z.X.; Zhao, L. Bin Characterization of the Radical Scavenging and Antioxidant Activities of Danshensu and Salvianolic Acid B. Food Chem. Toxicol. 2008, 46, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Xiong, D.; Li, Y.; Gong, S.; Zhang, L.; Li, B.; Pan, J.; Qian, J.; Qu, H. Inhibition of Nuclear Factor Kappa B as a Mechanism of Danshensu during Toll-like Receptor 2-Triggered Inflammation in Macrophages. Int. Immunopharmacol. 2020, 83, 106419. [Google Scholar] [CrossRef]
- Tang, Z.; Shu, G.; Du, H.; Zheng, Y.; Fu, H.; Zhang, W.; Lv, C.; Xu, F.; Li, H.; Ouyang, P.; et al. Effects of Dietary Ferulic Acid on Intestinal Health and Ileal Microbiota of Tianfu Broilers Challenged with Lipopolysaccharide. Molecules 2023, 28, 1720. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-alves, S.C.; Vendramini-costa, D.B.; Baú, C.; Cazarin, B.; Roberto, M.; Júnior, M.; Pedro, J.; Ferreira, B.; Bento, A.; Alexandre, M.; et al. Characterization of Phenolic Compounds in Chia (Salvia Hispanica L.) Seeds, Fiber Flour and Oil. Food Chem. 2017, 232, 295–305. [Google Scholar] [CrossRef]
- Senftleber, N.K.; Ramne, S.; Moltke, I.; Jørgensen, M.E.; Albrechtsen, A.; Hansen, T.; Andersen, M.K. Genetic Loss of Sucrase-Isomaltase Function: Mechanisms, Implications, and Future Perspectives. Appl. Clin. Genet. 2023, 16, 31–39. [Google Scholar] [CrossRef]
- Simsek, M.; Quezada-Calvillo, R.; Nichols, B.L.; Hamaker, B.R. Phenolic Compounds Increase the Transcription of Mouse Intestinal Maltase-Glucoamylase and Sucrase-Isomaltase. Food Funct. 2017, 8, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Chang, Y.; Li, Y.; Zhou, Y.; Qin, J.; Sun, Z.; Li, H. Caffeic Acid Phenethyl Ester (Propolis Extract) Ameliorates Insulin Resistance by Inhibiting JNK and NF-ΚB Inflammatory Pathways in Diabetic Mice and HepG2 Cell Models. J. Agric. Food Chem. 2017, 65, 9041–9053. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Naimi, M.; Murphy, B.; Hudlicky, T.; Tsiani, E. Rosmarinic Acid, a Rosemary Extract Polyphenol, Increases Skeletal Muscle Cell Glucose Uptake and Activates AMPK. Molecules 2017, 22, 1669. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Y. Polyphenol Supplementation Benefits Human Health via Gut Microbiota: A Systematic Review via Meta-Analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Lasik-Kurdyś, M.; Gumienna, M.; Górna, B.; Adzahan, N.M. Influence of Green Tea Added to Cherry Wine on Phenolic Content, Antioxidant Activity and Alpha-Glucosidase Inhibition during an In Vitro Gastrointestinal Digestion. Foods 2022, 11, 3298. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Al-Quwaie, D.A.; Ashkan, M.F.; Alharbi, M.; Alanazi, H.; Binothman, N.; Aljadani, M.; Majrashi, K.A.; Huwaikem, M.; et al. Extraction, Characterization of Polyphenols from Certain Medicinal Plants and Evaluation of Their Antioxidant, Antitumor, Antidiabetic, Antimicrobial Properties, and Potential Use in Human Nutrition. Front. Nutr. 2023, 10, 1125106. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red Wine Polyphenols Modulate Fecal Microbiota and Reduce Markers of the Metabolic Syndrome in Obese Patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Deng, X.; Jian, S.; Zhang, M.; Wen, C.; Xin, Z.; Zhang, L.; Tong, A.; Ye, S.; Liao, P.; et al. Gallic Acid Alleviates Gut Dysfunction and Boosts Immune and Antioxidant Activities in Puppies Under Environmental Stress Based on Microbiome–Metabolomics Analysis. Front. Immunol. 2022, 12, 813890. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on Gut Microbiota Composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, S.; Huang, W.; Li, K.; Wu, M.; Liu, W.; Han, J. Chlorogenic Acid Improves Intestinal Morphology by Enhancing Intestinal Stem-Cell Activity. J. Sci. Food Agric. 2023, 103, 3287–3294. [Google Scholar] [CrossRef]
- Agarwal, N.; Shukla, V.; Kolba, N.; Jackson, C.; Cheng, J.; Padilla-Zakour, O.I.; Tako, E. Comparing the Effects of Concord Grape (Vitis Labrusca L.) Puree, Juice, and Pomace on Intestinal Morphology, Functionality, and Bacterial Populations In Vivo (Gallus Gallus). Nutrients 2022, 14, 3539. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Zhu, L.; Ma, S.; Luo, Y.; Liang, H.; Liu, Q.; Chen, J.; Guli, S.; Chen, X. Orchestration of MUC2—The Key Regulatory Target of Gut Barrier and Homeostasis: A Review. Int. J. Biol. Macromol. 2023, 236, 123862. [Google Scholar] [CrossRef]
- Mishima, M.D.V.; Da Silva, B.P.; Gomes, M.J.C.; Toledo, R.C.L.; Mantovani, H.C.; de São José, V.P.B.; Costa, N.M.B.; Tako, E.; Martino, H.S.D. Effect of Chia (Salvia Hispanica L.) Associated with High-Fat Diet on the Intestinal Health of Wistar Rats. Nutrients 2022, 14, 4924. [Google Scholar] [CrossRef]
- Mishima, M.D.V.; Da Silva, B.P.; Gomes, M.J.C.; Toledo, R.C.L.; Pereira, C.E.R.; Costa, N.M.B.; Martino, H.S.D. Effect of Chia Flour Associated with High Fat Diet on Intestinal Health in Female Ovariectomized Wistar Rats. Eur. J. Nutr. 2022, 62, 905–919. [Google Scholar] [CrossRef]
- de Morais, V.N.; Gomes, M.J.C.; Grancieri, M.; Moreira, L.D.P.D.; Toledo, R.C.L.; Costa, N.M.B.; da Silva, B.P.; Martino, H.S.D. Chia (Salvia Hispanica L.) Flour Modulates the Intestinal Microbiota in Wistar Rats Fed a High-Fat and High-Fructose Diet. Food Res. Int. 2023, 172, 113095. [Google Scholar] [CrossRef]
- Chen, X.; Pan, S.; Li, F.; Xu, X.; Xing, H. Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity. Biomolecules 2022, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary Factors Affecting Polyphenol Bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]



| Gene | Oligonucleotides (5′-3′) | |
|---|---|---|
| Forward Primer (5′-3′) | Reverse Primer (5′-3′) | |
| BBM functionality | ||
| AP | CGTCAGCCAGTTTGACTATGTA | CTCTCAAAGAAGCTGAGGATGG |
| SI | CCAGCAATGCCAGCATATTG | CGGTTTCTCCTTACCACTTCTT |
| 18S rRNA | GCAAGACGAACTAAAGCGAAAG | TCGGAACTACGACGGTATCT |
| Inflammation | ||
| TNF-α | GACAGCCTATGCCAACAAGTA | TTACAGGAAGGGCAACTCATC |
| NF-κβ1 | CACAGCTGGAGGGAAGTAAAT | TTGAGTAAGGAAGTGAGGTTGAG |
| Intestinal barrier | ||
| MUC2 | CCTGCTGCAAGGAAGTAGAA | GGAAGATCAGAGTGGTGCATAG |
| OCLN | GTCTGTGGGTTCCTCATCGT | GTTCTTCACCCACTCCTCCA |
| Variable | Mean ± SD |
|---|---|
| Total phenolic compounds (mg of GAE/g of sample) | 405.70 ± 17.58 |
| Antiradical activity (µmol of Trolox equivalent/g of sample) | 3.06 ± 0.05 |
| NI | H2O | CPE | |
|---|---|---|---|
| Villi goblet cell diameter (µM) | 2.46 ± 0.06 a | 2.20 ± 0.05 a | 2.64 ± 0.08 a |
| Crypt goblet cell diameter (µM) | 2.92 ± 0.05 b | 3.13 ± 0.05 a | 2.96 ± 0.06 ab |
| Villi goblet cell number | 24.68 ± 0.74 b | 38.38 ± 0.91 a | 23.78 ± 0.60 b |
| Acidic | 15.28 ± 0.71 b | 26.71 ± 1.12 a | 11.73 ± 0.65 c |
| Neutral | 0.79 ± 0.13 a | 0.10 ± 0.04 c | 0.50 ± 0.11 b |
| Mixed | 8.68 ± 0.57 b | 11.57 ± 0.66 a | 11.55 ± 0.46 a |
| Crypt goblet cell number | 12.67 ± 0.55 a | 10.95 ± 0.62 b | 9.33 ± 0.35 b |
| Acidic | 8.53 ± 0.43 a | 7.88 ± 0.51 ab | 6.99 ± 0.28 b |
| Neutral | 0.41 ± 0.06 a | 0.50 ± 0.07 a | 0.10 ± 0.03 b |
| Mixed | 3.73 ± 0.27 a | 2.58 ± 0.21 b | 2.25 ± 0.16 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishima, M.D.V.; Martino, H.S.D.; Kolba, N.; Agarwal, N.; Jackson, C.; da Silva, B.P.; Grancieri, M.; de Assis, A.; São José, V.P.B.d.; Tako, E. Chia Phenolic Extract Appear to Improve Small Intestinal Functionality, Morphology, Bacterial Populations, and Inflammation Biomarkers In Vivo (Gallus gallus). Nutrients 2023, 15, 3643. https://doi.org/10.3390/nu15163643
Mishima MDV, Martino HSD, Kolba N, Agarwal N, Jackson C, da Silva BP, Grancieri M, de Assis A, São José VPBd, Tako E. Chia Phenolic Extract Appear to Improve Small Intestinal Functionality, Morphology, Bacterial Populations, and Inflammation Biomarkers In Vivo (Gallus gallus). Nutrients. 2023; 15(16):3643. https://doi.org/10.3390/nu15163643
Chicago/Turabian StyleMishima, Marcella Duarte Villas, Hércia Stampini Duarte Martino, Nikolai Kolba, Nikita Agarwal, Cydney Jackson, Bárbara Pereira da Silva, Mariana Grancieri, Andressa de Assis, Vinícius Parzanini Brilhante de São José, and Elad Tako. 2023. "Chia Phenolic Extract Appear to Improve Small Intestinal Functionality, Morphology, Bacterial Populations, and Inflammation Biomarkers In Vivo (Gallus gallus)" Nutrients 15, no. 16: 3643. https://doi.org/10.3390/nu15163643