Dietary Fibre Intake in Chile: 13 Years after the Last National Report
Abstract
:1. Introduction
2. Materials and Methods
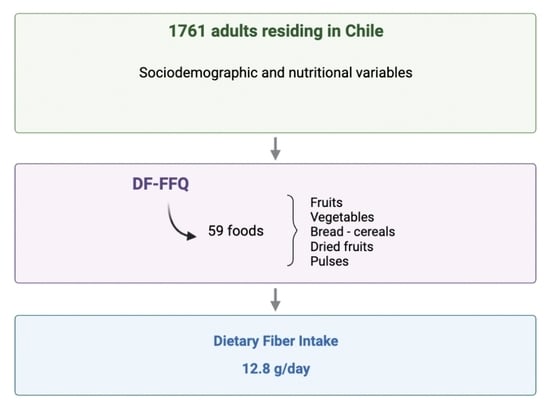
2.1. Study Design and Population
2.2. Determination of Dietary Fiber Intake
2.3. Ethical Aspects
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics, Nutritional Status and Dietary Fiber Intake
3.2. Main Food Sources Contributing to Dietary Fiber
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary Fibre in Europe: Current State of Knowledge on Definitions, Sources, Recommendations, Intakes and Relationships to Health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Stribling, P.; Ibrahim, F. Dietary Fibre Definition Revisited—The Case of Low Molecular Weight Carbohydrates. Clin. Nutr. ESPEN 2023, 55, 340–356. [Google Scholar] [CrossRef] [PubMed]
- European Comission. Dietary Fiber Dietary Fiber. Available online: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/fibre (accessed on 24 July 2023).
- Xue, Y.; Cui, L.; Qi, J.; Ojo, O.; Du, X.; Liu, Y.; Wang, X. The Effect of Dietary Fiber (Oat Bran) Supplement on Blood Pressure in Patients with Essential Hypertension: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2458–2470. [Google Scholar] [CrossRef]
- Ionită-Mîndrican, C.-B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacs, S.M.; Morosan, E.; Dumitrescu, D.-E.; Rosca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2061. [Google Scholar] [CrossRef] [PubMed]
- Vilcanqui-Perez, F.; Vilchez-Perales, C. Dietary Fiber: New Definitions, Functional Properties and Health Benefits. Review. Arch. Latinoam. Nutr. 2017, 67, 146–156. [Google Scholar]
- Soliman, G. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Saghafian, F.; Hajishafiee, M.; Rouhani, P.; Saneei, P. Dietary Fiber Intake, Depression, and Anxiety: A Systematic Review and Meta-Analysis of Epidemiologic Studies. Nutr. Neurosci. 2023, 26, 108–126. [Google Scholar] [CrossRef]
- Miki, T.; Eguchi, M.; Kurotani, K.; Kochi, T.; Kuwahara, K.; Ito, R.; Kimura, Y.; Tsuruoka, H.; Akter, S.; Kashino, I.; et al. Dietary Fiber Intake and Depressive Symptoms in Japanese Employees: The Furukawa Nutrition and Health Study. Nutrition 2016, 32, 584–589. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Song, X.; Li, Z.; Zhang, D. Exploration of the Association between Dietary Fiber Intake and Depressive Symptoms in Adults. Nutrition 2018, 54, 48–53. [Google Scholar] [CrossRef]
- Milajerdi, A.; Ebrahimi-Daryani, N.; Dieleman, L.A.; Larijani, B.; Esmaillzadeh, A. Association of Dietary Fiber, Fruit, and Vegetable Consumption with Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 735–743. [Google Scholar] [CrossRef]
- Dong, W.; Yang, Z. Association of Dietary Fiber Intake with Myocardial Infarction and Stroke Events in US Adults: A Cross-Sectional Study of NHANES 2011–2018. Front. Nutr. 2022, 21, 936926. [Google Scholar] [CrossRef]
- Evans, C. Dietary Fibre and Cardiovascular Health: A Review of Current Evidence and Policy. Proc. Nutr. Soc. 2020, 79, 61–67. [Google Scholar] [CrossRef]
- Thompson, S.V.; Hannon, B.A.; An, R.; Holscher, H.D. Effects of Isolated Soluble Fiber Supplementation on Body Weight, Glycemia, and Insulinemia in Adults with Overweight and Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2017, 106, 1514–1528. [Google Scholar] [CrossRef]
- Xie, Y.; Gou, L.; Peng, M.; Zheng, J.; Chen, L. Effects of Soluble Fiber Supplementation on Glycemic Control in Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2021, 40, 1800–1810. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary Fibre and Whole Grains in Diabetes Management: Systematic Review and Meta-Analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Juhász, A.E.; Greff, D.; Teutsch, B.; Gede, N.; Hegyi, P.; Horváth, E.M.; Deák, P.Á.; Nyirády, P.; Ács, N.; Juhász, R. Galactomannans Are the Most Effective Soluble Dietary Fibers in Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Am. J. Clin. Nut. 2023, 117, 266–277. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Dietary Fibre Intake and Mortality from Cardiovascular Disease and All Cancers: A Meta-Analysis of Prospective Cohort Studies. Arch. Cardiovasc. Dis. 2016, 109, 39–54. [Google Scholar] [CrossRef]
- Farvid, M.S.; Spence, N.D.; Holmes, M.D.; Barnett, J.B. Fiber Consumption and Breast Cancer Incidence: A Systematic Review and Meta-Analysis of Prospective Studies. Cancer 2020, 126, 3061–3075. [Google Scholar] [CrossRef]
- Xu, K.; Sun, Q.; Shi, Z.; Zou, Y.; Jiang, X.; Wang, Y.; Chong, F.; Song, C. A Dose-Response Meta-Analysis of Dietary Fiber Intake and Breast Cancer Risk. Asia Pac. J. Public Health 2022, 34, 331–337. [Google Scholar] [CrossRef]
- Arayici, M.E.; Mert-Ozupek, N.; Yalcin, F.; Basbinar, Y.; Ellidokuz, H. Soluble and Insoluble Dietary Fiber Consumption and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 2412–2425. [Google Scholar] [CrossRef]
- Nucci, D.; Santangelo, O.E.; Provenzano, S.; Fatigoni, C.; Nardi, M.; Ferrara, P.; Gianfredi, V. Dietary Fiber Intake and Risk of Pancreatic Cancer: Systematic Review and Meta-Analysis of Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 11556. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.W.; Wesselius, A.; Mehrkanoon, S.; Brinkman, M.; van den Brandt, P.; White, E.; Weiderpass, E.; Calvez-Kelm, F.L.; Gunter, M.; Huybrechts, I.; et al. Grain and Dietary Fiber Intake and Bladder Cancer Risk: A Pooled Analysis of Prospective Cohort Studies. Am. J. Clin. Nut. 2020, 112, 1252–1266. [Google Scholar]
- Reynolds, A.N.; Akerman, A.; Kumar, S.; Pham, H.T.D.; Coffey, S.; Mann, J. Dietary Fibre in Hypertension and Cardiovascular Disease Management: Systematic Review and Meta-Analyses. BCM Med. 2022, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.; Wang, Y.-Y.; Wang, L.-L.; Ojo, O.; Feng, Q.-Q.; Jiang, X.-S.; Wang, X.-H. Effect of Dietary Fiber on Gut Barrier Function, Gut Microbiota, Short-Chain Fatty Acids, Inflammation, and Clinical Outcomes in Critically Ill Patients: A Systematic Review and Meta-Analysis. J. Parenter. Enter. Nutr. 2022, 46, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Feng, P.; Xu, Y.; Hou, Y.-Y.; Ojo, O.; Wang, X.-H. The Role of Dietary Fiber Supplementation in Regulating Uremic Toxins in Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2021, 31, 438–447. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Carbohydrates and Dietary Fibre. EFSA J. 2010, 8, 1462. [Google Scholar]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate Quality and Human Health: A Series of Systematic Reviews and Meta-Analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- USDA Food Surveys Research Group: Beltsville, MD. What We Eat in America. U.S. Department of Agriculture. 2017–2018. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1718/Table_1_NIN_GEN_17.pdf (accessed on 6 August 2023).
- USDA Food Surveys Research Group: Beltsville, MD. What We Eat in America. U.S. Department of Agriculture. 2015–2016. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1516/Table_1_NIN_GEN_15.pdf (accessed on 13 August 2023).
- Guzmán, C.; Espinoza, J.; Fuentealba, F. Pilot Study to Estimate Dietary Fiber Intake in Adults Residing in Chile. Nutrients 2023, 15, 900. [Google Scholar] [CrossRef]
- Gattás, V. Guide to the Nutritional Composition of Natural Foods, Industry and Typical Chilean Preparations, 2nd ed.; University of Chile: Santiago, Chile, 2011. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- International Ethical Guidelines for Health-Related Research Involving Humans, 4th ed.; Council for International Organizations of Medical Sciences (CIOMS): Geneva, Switzerland, 2016.
- National Institute of Statistics of Chile (NIS). Annual Environment Report. 2021. Available online: http://www.ine.gob.cl/ (accessed on 6 December 2022).
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The Joint WHO/FAO Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases: Process, Product and Policy Implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfei, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- McRae, M. The Benefits of Dietary Fiber Intake on Reducing the Risk of Cancer: An Umbrella Review of Meta-Analyses. J. Chiropr. Med. 2018, 17, 90–96. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council of the European Union. Regulation No 1924/2006 of the European Parliament and of the Council on Nutrition and Health Claims on Food; European Parliament: Strasbourg, France, 2006. [Google Scholar]
- National Ministry of Health. Food Sanitary Regulations. No 977/96. Available online: http://www.isl.gob.cl/ (accessed on 6 December 2022).
- Sardinha, A.; Canella, D.; Martins, A.; Claro, R.; Levy, R. Dietary Sources of Fiber Intake in Brazil. Appetite 2014, 79, 134–138. [Google Scholar] [CrossRef]
- Passos, M.D.C.F.; Takemoto, M.L.S.; Guedes, L.S. Patterns of Fiber Intake among Brazilian Adults: Perceptions from an Online Nationwide Survey. Arq. Gastroenterol. 2020, 57, 144–149. [Google Scholar] [CrossRef]
- National Ministry of Health. National Nutrition and Health Survey. Results Document 2007. Available online: http//www.msal.gob.ar/ (accessed on 6 December 2022).
- Lopez-Salido, S.; Llanes-Canedo, C.; Housni, F.; Gonzalez-Flores, J. Knowledge Analysis of Dietary Fiber, Body Mass Index and Socioeconomic Level in the Mexican Population. Interciencia 2020, 45, 469–474. [Google Scholar]
- Rippin, H.; Hutchinson, J.; Jewell, J.; Breda, J.; Cade, J. Adult Nutrient Intakes from Current National Dietary Surveys of European Populations. Nutrients 2017, 9, 1288. [Google Scholar] [CrossRef]
- Ahmed, M.; Prannet, A.; Labbe, M. Nutrient Intakes of Canadian Adults: Results from the Canadian Community Health Survey (CCHS)-2015 Public Use Microdata File. Am. J. Clin. Nutr. 2021, 114, 1131–1140. [Google Scholar] [CrossRef]
- National Federation of Bakers. Report Bread Consumption in Chile. 2020. Available online: http://www.fechipan.cl/ (accessed on 6 December 2022).
- Gonzalez, N.; Peña, F.; Durán, S. Characterization of Food Intake and Nutrient in Elderly Chilean Adults. Rev. Chil. Nutr. 2016, 43, 347–352. [Google Scholar]
- Oh, H.; Kim, H.; Lee, D.H.; Lee, A.; Giovannucci, E.L.; Kang, S.S.; Keum, N. Different Dietary Fibre Sources and Risks of Colorectal Cancer and Adenoma: A Dose-Response Meta-Analysis of Prospective Studies. Br. J. Nutr. 2019, 122, 605–615. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Kumanyika, S.K.; Lemaitre, R.N.; Olson, J.L.; Burke, G.L.; Siscovick, D.S. Cereal, Fruit, and Vegetable Fiber Intake and the Risk of Cardiovascular Disease in Elderly Individuals. JAMA 2003, 289, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, A.T.; Herrington, D.M.; Mozaffarian, D.; Lichtenstein, A.H. Cereal Fiber and Whole-Grain Intake Are Associated with Reduced Progression of Coronary-Artery Atherosclerosis in Postmenopausal Women with Coronary Artery Disease. Am. Heart J. 2005, 150, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Flint, A.; Pai, J.K.; Forman, J.P.; Hu, F.B.; Willett, W.C.; Rexrode, K.M.; Mukamal, K.J.; Rimm, E.B. Dietary Fiber Intake and Mortality among Survivors of Myocardial Infarction: Prospective Cohort Study OPEN ACCESS. BMJ 2014, 348, g2659. [Google Scholar] [CrossRef] [PubMed]
- McEligot, A.J.; Largent, J.; Ziogas, A.; Peel, D.; Anton-Culver, H. Dietary Fat, Fiber, Vegetable, and Micronutrients Are Associated with Overall Survival in Postmenopausal Women Diagnosed with Breast Cancer. Nutr. Cancer 2006, 55, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Buyken, A.E.; Flood, V.; Empson, M.; Rochtchina, E.; Barclay, A.W.; Brand-Miller, J.; Mitchell, P. Carbohydrate Nutrition and Inflammatory Disease Mortality in Older Adults. Am. J. Clin. Nutr. 2010, 92, 634–643. [Google Scholar] [CrossRef]
- Ames, N.; Blewett, H.; Storsley, J.; Thandapilly, S.J.; Zahradka, P.; Taylor, C. A Double-Blind Randomised Controlled Trial Testing the Effect of a Barley Product Containing Varying Amounts and Types of Fibre on the Postprandial Glucose Response of Healthy Volunteers. Br. J. Nutr. 2015, 113, 1373–1383. [Google Scholar] [CrossRef]
- Weickert, M.O.; Roden, M.; Isken, F.; Hoffmann, D.; Nowotny, P.; Osterhoff, M.; Blaut, M.; Alpert, C.; Gögebakan, Ö.; Bumke-Vogt, C.; et al. Effects of Supplemented Isoenergetic Diets Differing in Cereal Fiber and Protein Content on Insulin Sensitivity in Overweight Humans. Am. J. Clin. Nutr. 2011, 94, 459–471. [Google Scholar] [CrossRef]
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of Dietary Fiber on Human Health. Food Sci. Hum. Wellness 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Garcia, I.; Mendez, S.; Aguirre, N.; Sanchez, M.; Matias, D.; Perez, E. Increasing Consumption of Dietary Fiber Complementary to the Treatment of Metabolic Syndrome. Nutr. Hosp. 2018, 35, 582–587. [Google Scholar]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, L.; Walton, J.; Flynn, A. Nutritional challenges for older adults in Europe: Current status and future directions. Proc. Nutr. Soc. 2019, 78, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Galesi-Pacheco, L.F.; Leandro-Merhi, V.A.; Carmo-Azzolin, G.M.C.; Marquez de Oliveira, M.R. Oral condition of institutionalized older people: Influence on anthropometric indicators, food and nutrient intake. Rev. Chil. Nutr. 2021, 48, 9–17. [Google Scholar] [CrossRef]
- Alanís-García, E.; González-Rubio, P.Y.; Delgado-Olivares, L.; Cruz-Cansino, N. del S. Fibra dietética: Historia, definición y efectos en la salud. Educ. Salud Boletín Científico Inst. Cienc. Salud Univ. Autónoma Estado Hidalgo 2021, 9, 187–195. [Google Scholar]
- Sun, W.; Li, S.; Chen, C.; Lu, Z.; Zhang, D. Dietary fiber intake is positively related with cognitive function in US older adults. J. Funct. Foods 2022, 90, 104986. [Google Scholar] [CrossRef]
- Lanuza, F.; Petermann-Rocha, F.; Celis-Morales, C.; Concha-Cisternas, Y.; Nazar, G.; Troncoso-Pantoja, C.; Lassere-Laso, N.; Martínez-Sanguinetti, M.A.; Parra-Soto, S.; Zamora-Ros, R.; et al. A healthy eating score is inversely associated with depression in older adults: Results from the Chilean National Health Survey 2016–2017. Public Health Nutr. 2021, 25, 2864–2875. [Google Scholar] [CrossRef]
- Kao, C.C.; Yang, Z.Y.; Chen, W.L. The association between dietary fiber intake and sarcopenia. J. Funct. Foods 2023, 102, 105437. [Google Scholar] [CrossRef]
- Çıtar Dazıroğlu, M.E.; Tek, N. Evaluation of Dietary Fiber Adequacy According to Malnutrition Status in Elderly People. Focus Med. Sci. 2021, 7, 1–5. [Google Scholar]
- Ronda-Pérez, E.; Campos-Mora, J.; de Juan, A.; Gea, T.; Reid, A.; Caballero, P. Differences in the Prevalence of Fruit and Vegetable Consumption in Spanish Workers. Nutrients 2020, 12, 3848. [Google Scholar] [CrossRef]
- Franco, A.D.S.; Castro, I.R.R.D.; Wolkoff, D.B. Impacto da promoção sobre consumo de frutas e hortaliças em ambiente de trabalho. Rev. Saúde Pública 2013, 47, 29–36. [Google Scholar] [CrossRef]
- Bandoni, D.; Sarno, F.; Jaime, P. Impact of an intervention on the availability and consumption of fruits and vegetables in the workplace. Public Health Nutr. 2011, 14, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, G.; Howlett, G.; Wilson, C. Increasing Employees’ Fruit Consumption through Access and Peer Support at Work. Food Nutr. Sci. 2013, 4, 88–95. [Google Scholar] [CrossRef]
- Akhlaghi, M. The Role of Dietary Fibers in Regulating Appetite, an Overview of Mechanisms and Weight Consequences. Crit. Rev. Food Sci. Nutr. 2022, 4, 12. [Google Scholar] [CrossRef]
- Vitaglione, P.; Lumaga, R.; Stanzione, A.; Scalfi, L.; Fogliano, V. Beta-Glucan-Enriched Bread Reduces Energy Intake and Modifies Plasma Ghrelin and Peptide YY Concentrations in the Short Term. Appetite 2009, 53, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.; O’Neil, C.; Greenway, F. Dietary Fiber and Satiety: The Effects of Oats on Satiety. Nutr. Rev. 2016, 74, 131–147. [Google Scholar] [CrossRef]




| Kerrypnx | Total Population | Men | Women | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Age | ||||||
| 777 | 44.1 | 307 | 51.2 | 465 | 40.3 |
| 880 | 50.0 | 264 | 44.0 | 615 | 53.2 |
| 104 | 5.9 | 29 | 4.8 | 75 | 6.5 |
| Macrozone | ||||||
| 54 | 3.1 | 20 | 3.3 | 34 | 2.9 |
| 780 | 44.3 | 200 | 33.3 | 577 | 50.0 |
| 249 | 14.1 | 62 | 10.3 | 186 | 16.1 |
| 678 | 38.5 | 318 | 53.0 | 358 | 31.0 |
| Residence | ||||||
| 1630 | 92.6 | 560 | 93.3 | 1065 | 92.2 |
| 131 | 7.4 | 40 | 6.7 | 90 | 7.8 |
| Occupation | ||||||
| 816 | 46.3 | 283 | 47.2 | 533 | 46.1 |
| 190 | 10.8 | 76 | 12.7 | 113 | 9.8 |
| 614 | 34.9 | 213 | 35.5 | 396 | 34.3 |
| 49 | 2.8 | 20 | 3.3 | 29 | 2.5 |
| 51 | 2.9 | 0 | 0 | 51 | 4.4 |
| 41 | 2.3 | 8 | 1.3 | 33 | 2.9 |
| BMI Category | ||||||
| 32 | 1.8 | 6 | 1.0 | 26 | 2.3 |
| 787 | 44.7 | 220 | 36.7 | 563 | 48.7 |
| 613 | 34.8 | 246 | 41.0 | 365 | 31.6 |
| 329 | 18.7 | 128 | 21.3 | 201 | 17.4 |
| Total Population | Men | Women | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p Value | |
| Age | |||||||
| 13.3 | 7.4 | 13.4 | 8.1 | 13.3 | 6.9 | 0.591 |
| 12.3 | 6.9 | 12.2 | 7.1 | 12.4 | 6.8 | 0.789 |
| 13.5 | 6.0 | 13.9 | 7.2 | 13.3 | 5.5 | 0.848 |
| p value | 0.005 * | 0.248 | 0.020 * | ||||
| Macrozone | |||||||
| 13.6 | 6.3 | 11.9 | 5.6 | 14.6 | 6.6 | 0.128 |
| 12.9 | 7.0 | 13.8 | 7.9 | 12.7 | 6.7 | 0.407 |
| 12.5 | 6.9 | 13.8 | 8.9 | 12.0 | 6.1 | 0.214 |
| 12.7 | 7.3 | 12.2 | 7.3 | 13.1 | 7.3 | 0.073 |
| p value | 0.429 | 0.073 | 0.096 | ||||
| Residence | |||||||
| 12.8 | 7.2 | 12.9 | 7.7 | 12.8 | 6.9 | 0.277 |
| 12.7 | 5.7 | 13.1 | 6.0 | 12.5 | 5.6 | 0.700 |
| p value | 0.529 | 0.403 | 0.810 | ||||
| Occupation | |||||||
| 12.1 | 6.5 | 11.9 | 6.5 | 12.2 | 6.5 | 0.554 |
| 13.2 | 7.2 | 12.6 | 7.7 | 13.7 | 6.9 | 0.291 |
| 13.5 | 7.7 | 13.9 | 8.8 | 13.3 | 7.0 | 0.767 |
| 13.9 | 8.2 | 14.4 | 6.6 | 13.7 | 9.3 | 0.416 |
| 12.8 | 6.0 | 0 | 0.0 | 12.8 | 6.0 | - |
| 13.4 | 7.1 | 16.3 | 8.1 | 12.7 | 6.7 | 0.250 |
| p value | 0.024 * | 0.092 | 0.130 | ||||
| BMI Category | |||||||
| 13.9 | 6.8 | 11.5 | 6.1 | 14.5 | 6.9 | 0.412 |
| 13.6 | 7.4 | 14.5 | 8.2 | 13.3 | 7.1 | 0.300 |
| 12.1 | 7.0 | 11.9 | 7.7 | 12.2 | 6.6 | 0.188 |
| 12.2 | 6.1 | 12.0 | 5.9 | 12.3 | 6.3 | 0.676 |
| p value | <0.001 * | 0.001 * | 0.050 | ||||
| Fruits | Vegetables | Bread | Cereals | Dried Fruits | Pulses | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI | |
| Age (years) | ||||||||||||||||||
| 1.51 | 1.49 | 1.40–1.61 | 1.83 | 1.58 | 1.71–1.94 | 4.51 | 3.19 | 4.28–4.73 | 2.77 | 3.20 | 2.54–3.00 | 1.14 | 1.89 | 1.01–1.27 | 1.55 | 1.90 | 1.42–1.69 |
| 1.49 | 1.46 | 1.49–1.58 | 1.83 | 1.58 | 1.72–1.93 | 4.26 | 2.91 | 4.06–4.45 | 1.89 | 2.41 | 1.73–2.05 | 1.31 | 1.98 | 1.18–1.44 | 1.52 | 2.29 | 1.37–1.68 |
| 2.15 | 1.62 | 1.83–2.46 | 2.10 | 1.59 | 1.79–2.41 | 4.56 | 3.03 | 3.97–5.15 | 1.59 | 1.89 | 1.22–1.96 | 1.63 | 2.01 | 1.24–2.02 | 1.41 | 1.17 | 1.18–1.64 |
| p value | <0.001 * | 0.025 * | 0.422 | <0.001 * | <0.001 * | 0.523 | ||||||||||||
| Macrozone | ||||||||||||||||||
| 1.74 | 1.54 | 1.32–2.16 | 2.15 | 1.77 | 1.66–2.63 | 4.20 | 2.69 | 3.46–4.93 | 2.50 | 2.72 | 1.75–3.24 | 1.60 | 2.26 | 0.99–2.22 | 1.39 | 1.56 | 0.96–1.82 |
| 1.56 | 1.53 | 1.45–1.67 | 1.80 | 1.55 | 1.69–1.91 | 4.52 | 3.01 | 4.31–4.73 | 2.28 | 2.85 | 2.08–2.48 | 1.22 | 1.96 | 1.09–1.36 | 1.57 | 1.87 | 1.44–1.70 |
| 1.35 | 1.36 | 1.18–1.52 | 1.73 | 1.59 | 1.53–1.93 | 4.45 | 3.27 | 4.04–4.86 | 2.26 | 2.87 | 1.90–2.62 | 1.21 | 1.91 | 0.97–1.44 | 1.45 | 1.57 | 1.25–1.64 |
| 1.55 | 1.49 | 1.44–1.66 | 1.91 | 1.61 | 1.79–2.03 | 4.22 | 3.03 | 3.99–4.45 | 2.22 | 2.73 | 2.01–2.42 | 1.28 | 1.91 | 1.13–1.42 | 1.54 | 2.4 | 1.34–1.71 |
| p value | 0.156 | 0.116 | 0.120 | 0.717 | 0.701 | 0.013 * | ||||||||||||
| Sex | ||||||||||||||||||
| 1.61 | 1.50 | 1.52–1.69 | 1.93 | 1.59 | 1.84–2.02 | 4.23 | 2.90 | 4.06–4.40 | 2.22 | 2.65 | 2.07–2.37 | 1.22 | 1.76 | 1.12–1.33 | 1.55 | 2.18 | 1.42–1.68 |
| 1.40 | 1.47 | 1.28–1.52 | 1.67 | 1.56 | 1.55–1.80 | 4.69 | 3.29 | 4.43–4.69 | 2.32 | 3.08 | 2.07–2.57 | 1.30 | 2.25 | 1.12–1.48 | 1.48 | 1.84 | 1.33–1.62 |
| p value | 0.001 * | <0.001 * | 0.021 * | 0.537 | 0.299 | 0.158 | ||||||||||||
| Residence | ||||||||||||||||||
| 1.55 | 1.50 | 1.47–1.62 | 1.83 | 1.59 | 1.76–1.91 | 4.36 | 3.05 | 4.21–4.51 | 2.28 | 2.83 | 2.14–2.42 | 1.25 | 1.95 | 1.16–1.35 | 1.54 | 2.13 | 1.43–1.64 |
| 1.37 | 1.32 | 1.14–1.60 | 1.94 | 1.48 | 1.68–2.19 | 4.65 | 3.06 | 4.12–5.18 | 2.00 | 2.47 | 1.58–2.43 | 1.25 | 1.89 | 0.92–1.57 | 1.45 | 1.16 | 1.24–1.65 |
| p value | 0.435 | 0.161 | 0.219 | 0.451 | 0.994 | 0.302 | ||||||||||||
| Occupation | ||||||||||||||||||
| 1.46 | 1.43 | 1.36–1.56 | 1.76 | 1.52 | 1.65–1.86 | 4.23 | 2.77 | 4.04–4.42 | 1.93 | 2.35 | 1.77–2.09 | 1.23 | 1.77 | 1.11–1.35 | 1.48 | 2.20 | 1.33–1.63 |
| 1.66 | 1.62 | 1.43–1.89 | 2.15 | 1.73 | 1.91–2.40 | 4.48 | 3.32 | 4.01–4.96 | 1.98 | 2.50 | 1.62–2.34 | 1.47 | 2.23 | 1.15–1.79 | 1.46 | 1.61 | 1.23–1.69 |
| 1.53 | 1.51 | 1.41–1.65 | 1.80 | 1.57 | 1.68–1.93 | 4.48 | 3.28 | 4.22–4.74 | 2.88 | 3.35 | 2.61–3.15 | 1.17 | 1.96 | 1.01–1.32 | 1.63 | 2.20 | 1.46–1.80 |
| 1.43 | 1.42 | 1.03–1.84 | 2.06 | 1.76 | 1.56–2.57 | 5.06 | 3.33 | 4.11–6.02 | 2.50 | 3.30 | 1.55–3.45 | 1.26 | 2.60 | 0.51–2.01 | 1.64 | 1.10 | 1.32–1.96 |
| 1.91 | 1.63 | 1.45–2.37 | 2.19 | 1.91 | 1.65–2.73 | 4.56 | 2.75 | 3.79–5.33 | 1.14 | 1.74 | 0.65–1.63 | 1.47 | 2.36 | 0.80–2.13 | 1.54 | 1.21 | 1.20–1.88 |
| 2.04 | 1.50 | 1.56–2.51 | 1.95 | 1.52 | 1.47–2.43 | 4.51 | 3.37 | 3.45–5.58 | 1.92 | 2.09 | 1.26–2.58 | 1.69 | 2.12 | 1.02–2.36 | 1.28 | 0.95 | 0.98–1.58 |
| p value | 0.021 * | 0.021 * | 0.669 | <0.001 * | 0.012 * | 0.110 | ||||||||||||
| BMI Category | ||||||||||||||||||
| 1.66 | 1.54 | 1.11–2.21 | 2.17 | 1.85 | 1.51–2.84 | 4.69 | 4.01 | 3.24–6.14 | 2.59 | 2.38 | 1.73–3.45 | 1.02 | 1.55 | 0.45–1.58 | 1.79 | 1.60 | 1.21–2.37 |
| 1.72 | 1.58 | 1.62–1.84 | 1.94 | 1.61 | 1.83–2.05 | 4.13 | 2.98 | 3.92–4.34 | 2.65 | 2.97 | 2.44–2.86 | 1.48 | 2.14 | 1.33–1.63 | 1.65 | 2.29 | 1.49–1.81 |
| 1.41 | 1.37 | 1.29–1.52 | 1.73 | 1.50 | 1.61–1.85 | 4.27 | 2.87 | 4.05–4.50 | 2.05 | 2.77 | 1.83–2.27 | 1.13 | 1.81 | 0.99–1.28 | 1.50 | 2.09 | 1.33–1.66 |
| 1.31 | 1.42 | 1.15–1.46 | 1.79 | 1.63 | 1.61–1.97 | 5.17 | 3.29 | 4.81–5.52 | 1.66 | 2.31 | 1.41–1.91 | 0.95 | 1.65 | 0.77–1.13 | 1.30 | 1.40 | 1.15–1.45 |
| p value | <0.001 * | 0.053 | <0.001 * | <0.001 * | <0.001 * | 0.011 * | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Pincheira, C.; Espinoza, J.; Durán-Agüero, S.; Obregón, A.M.; Fuentealba, F. Dietary Fibre Intake in Chile: 13 Years after the Last National Report. Nutrients 2023, 15, 3671. https://doi.org/10.3390/nu15173671
Guzmán-Pincheira C, Espinoza J, Durán-Agüero S, Obregón AM, Fuentealba F. Dietary Fibre Intake in Chile: 13 Years after the Last National Report. Nutrients. 2023; 15(17):3671. https://doi.org/10.3390/nu15173671
Chicago/Turabian StyleGuzmán-Pincheira, Carla, Jonathan Espinoza, Samuel Durán-Agüero, Ana María Obregón, and Fabiola Fuentealba. 2023. "Dietary Fibre Intake in Chile: 13 Years after the Last National Report" Nutrients 15, no. 17: 3671. https://doi.org/10.3390/nu15173671
APA StyleGuzmán-Pincheira, C., Espinoza, J., Durán-Agüero, S., Obregón, A. M., & Fuentealba, F. (2023). Dietary Fibre Intake in Chile: 13 Years after the Last National Report. Nutrients, 15(17), 3671. https://doi.org/10.3390/nu15173671