Dietary Vitamin E Isoforms Intake: Development of a New Tool to Assess Tocopherols and Tocotrienols Intake in Adults
Abstract
:1. Introduction
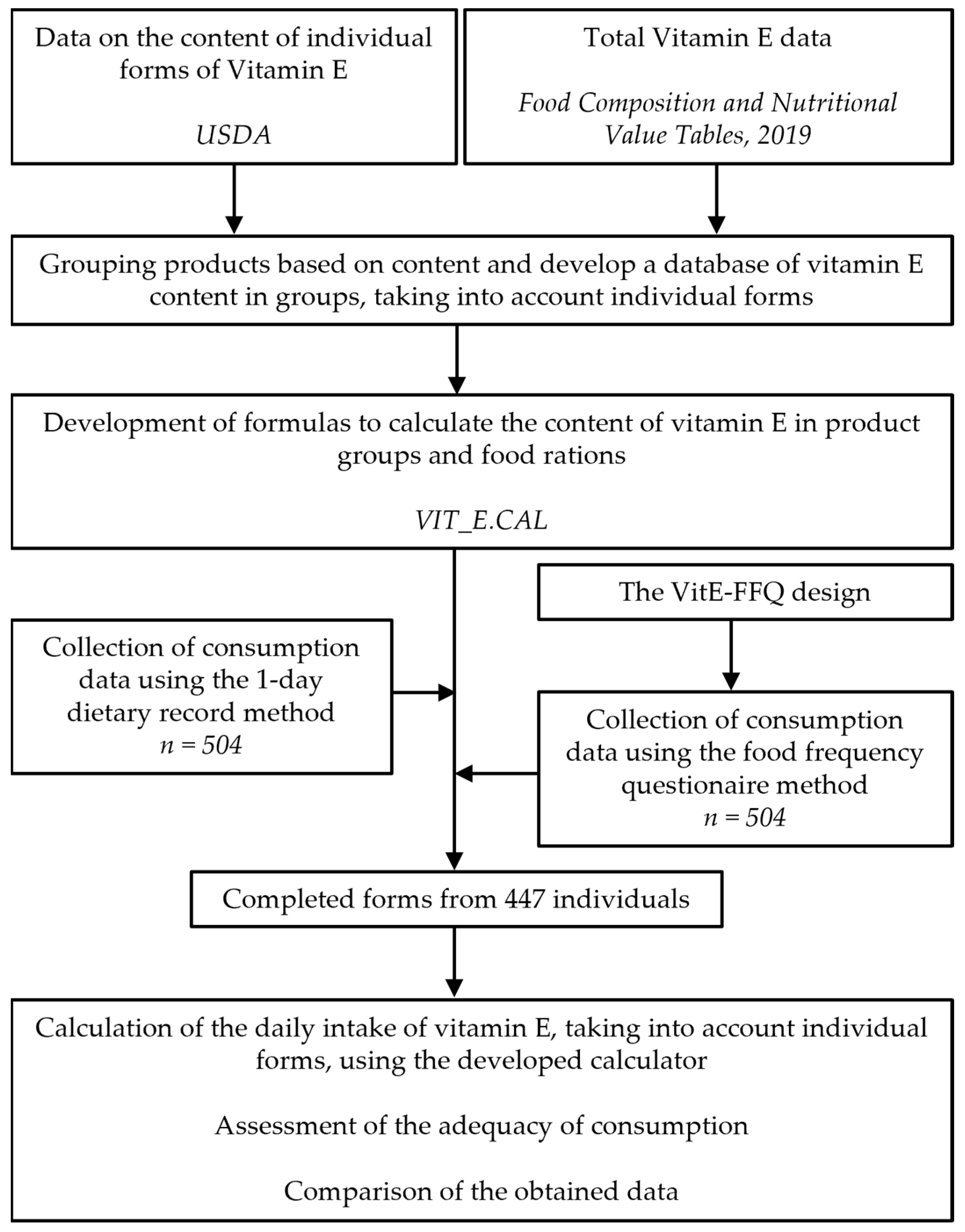
2. Materials and Methods
2.1. Dietary and Socidemographic Data
2.2. The VitE-FFQ Design
2.3. Development of the Tool Vit_E.CAL
2.4. Assessment of Adequecy of Vitamin E and α-Tocopherol Intake
2.5. Statistical Analysis
- -
- Calculation of differences between the intake levels of tocopherols and tocotrienols obtained from the two methods: the normality of the distribution of results was analyzed using the Shapiro–Wilk test, and then the Mann–Whitney U test was applied.
- -
- Analysis of the correlations between results: the normality of distribution of the results was analyzed using the Shapiro–Wilk test, and, afterwards, Spearman’s rank correlation was applied for nonparametric distribution.
- -
- Analysis of the Bland–Altman plots in the assessment of agreement (VitE-FFQ vs. 1-day dietary record); the results were interpreted using the Bland–Altman index, whereas the limits of agreement values (LOA) were calculated as the sum of the mean absolute difference in the consumption of individual forms of tocopherols and tocotrienols, measured by the two methods, and the ± standard deviation of the absolute difference of the assessment compounds intake recorded by the two methods and magnified by 1.96; the Bland–Altman index (%) was calculated as a percentage of persons beyond the limits of agreement (LOA). Good reproducibility of the measurement was proved by a minimum of 95% difference within the ±2 SD limits, which corresponds to the Bland–Altman index amounting to no more than 5%.
3. Results
3.1. Characteristics of the Participants
3.2. Intake of Tocopherols, Tocotrienols, and α-tocopherol Equivalents
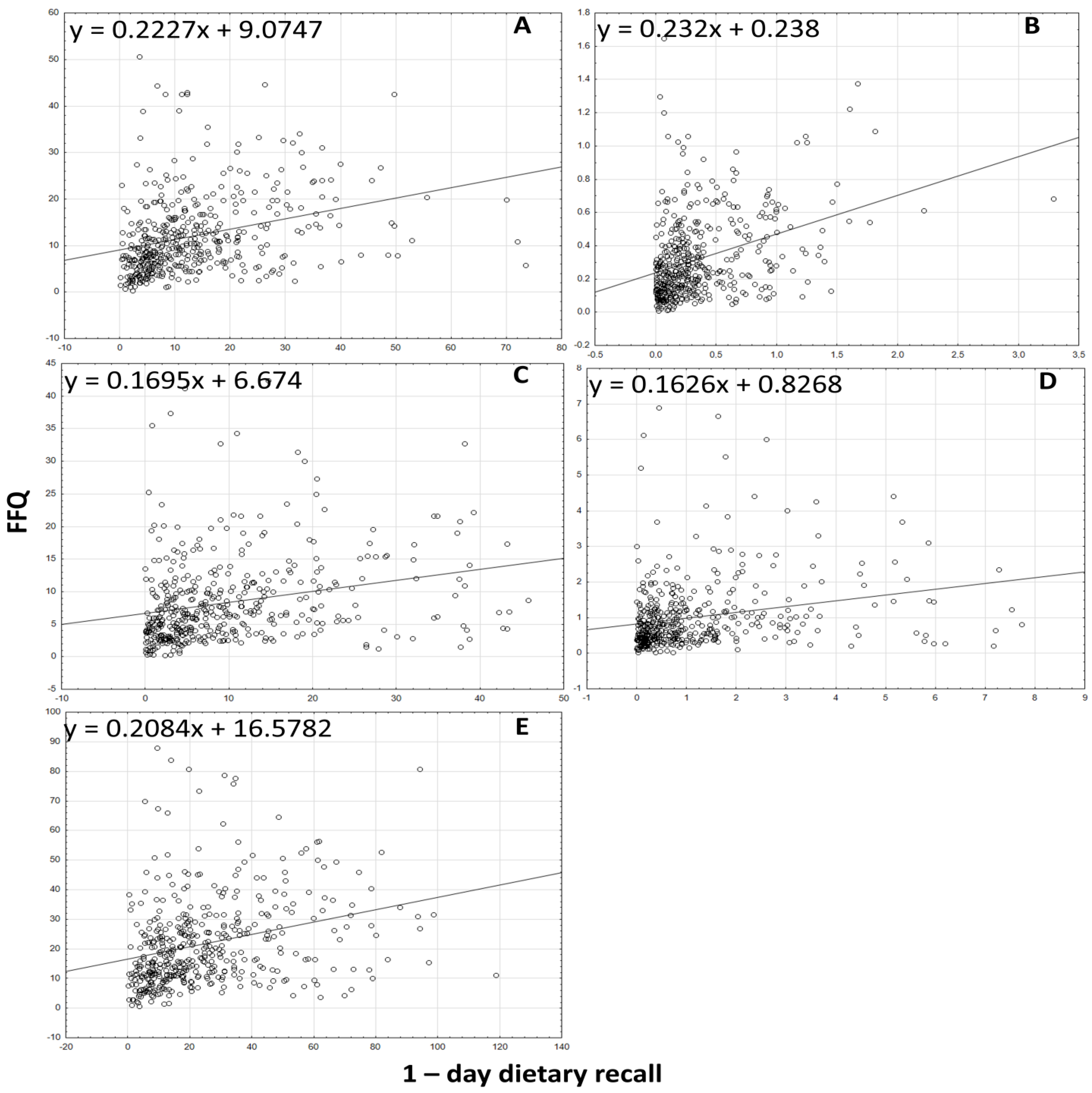
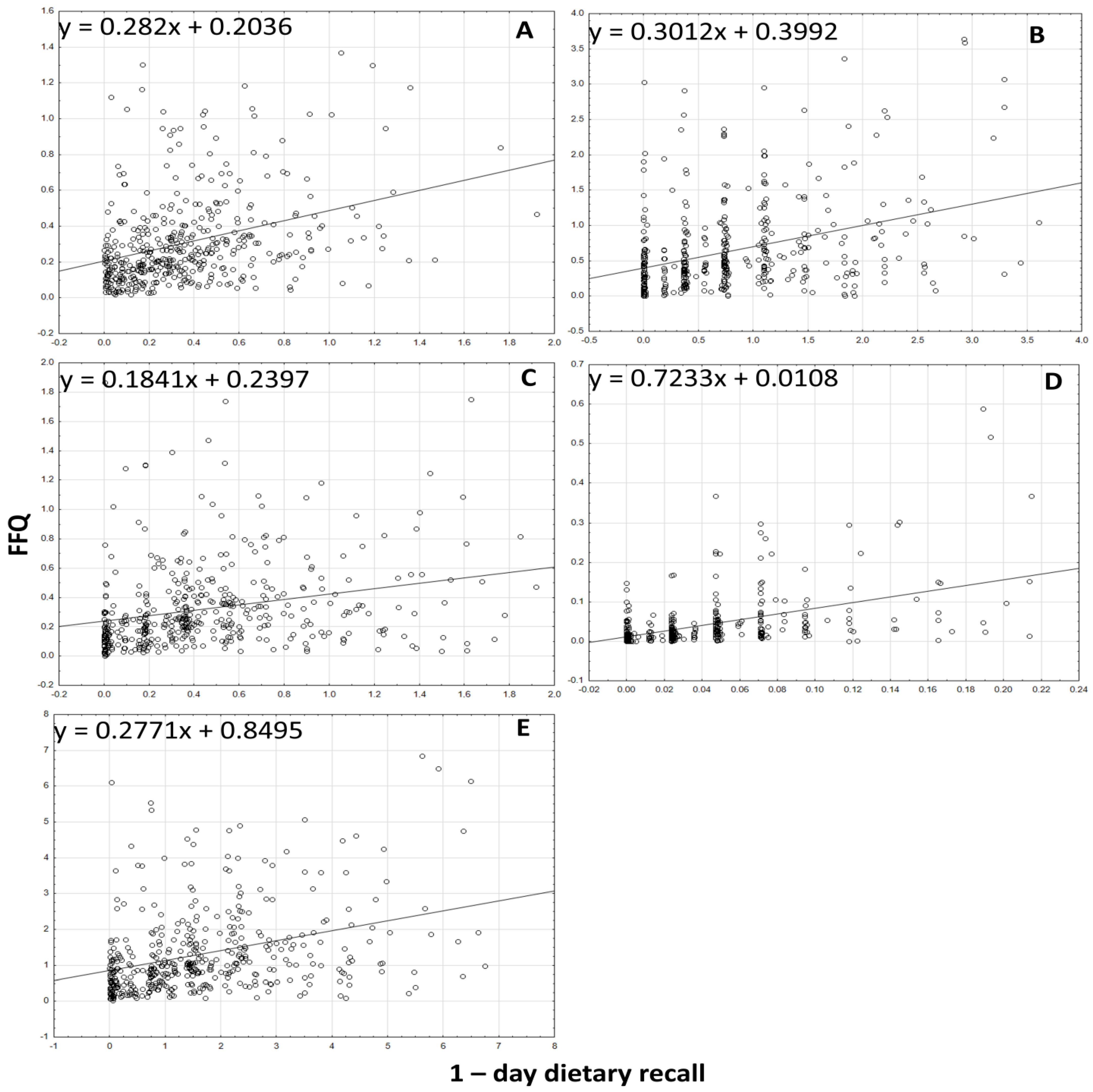
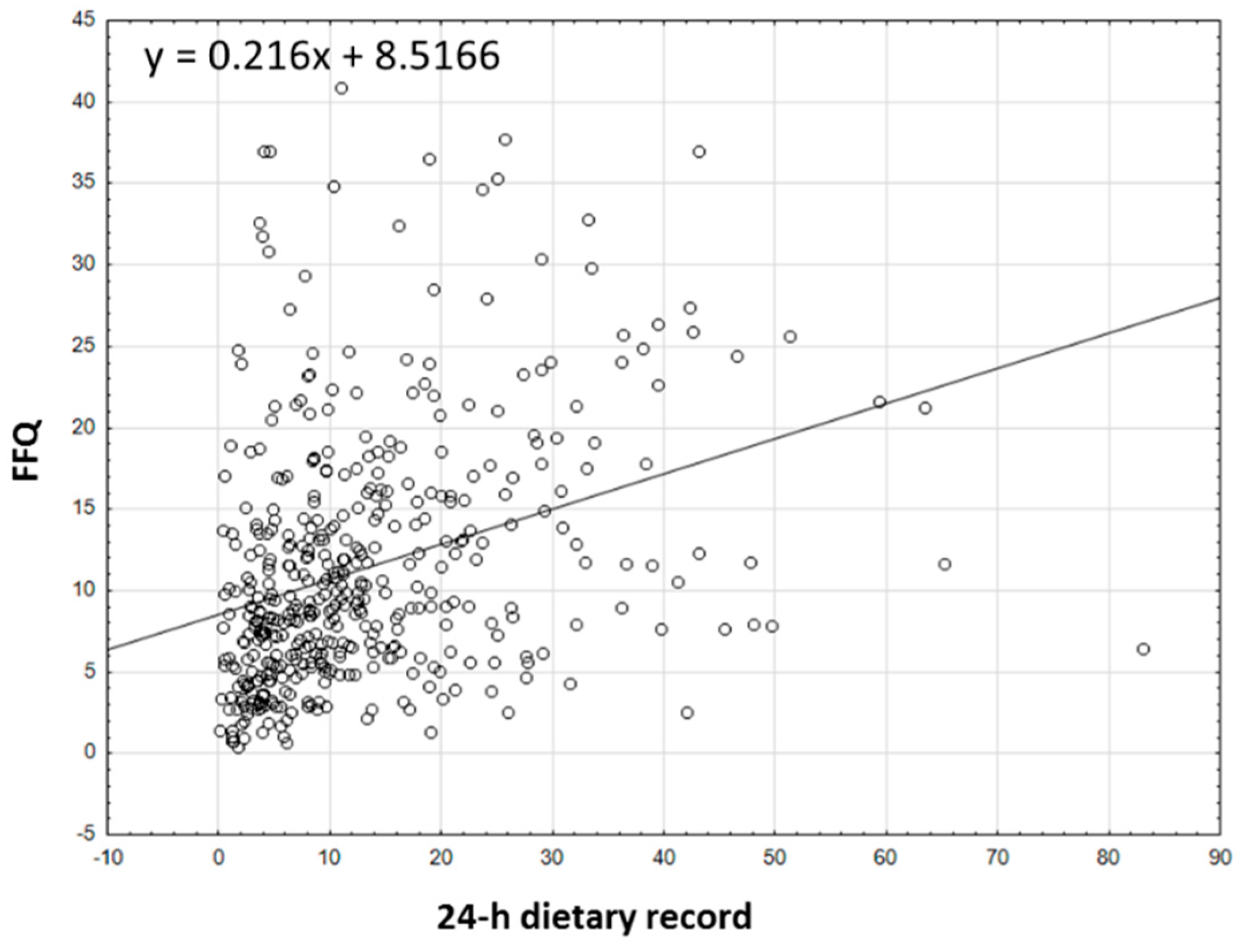
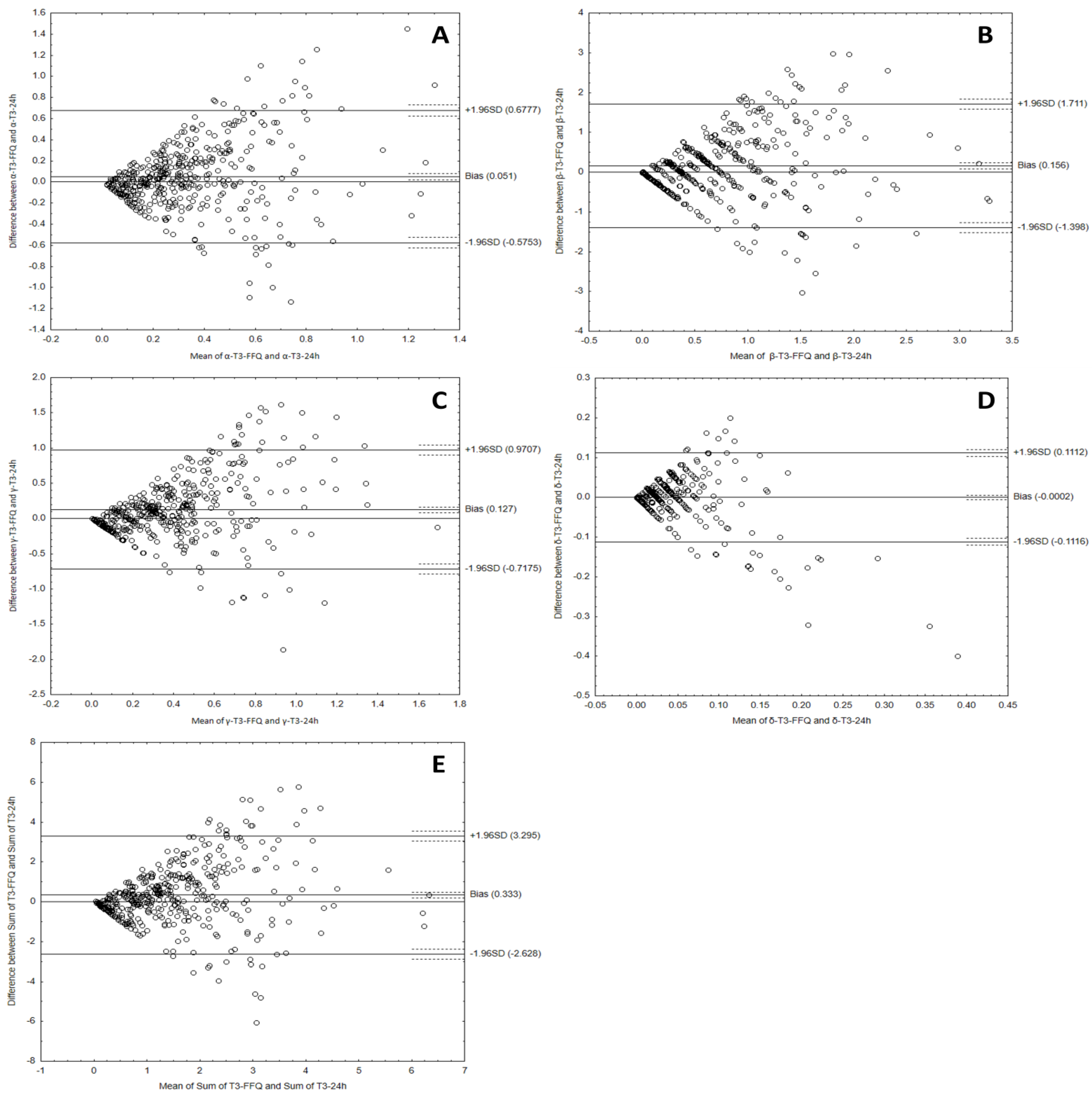
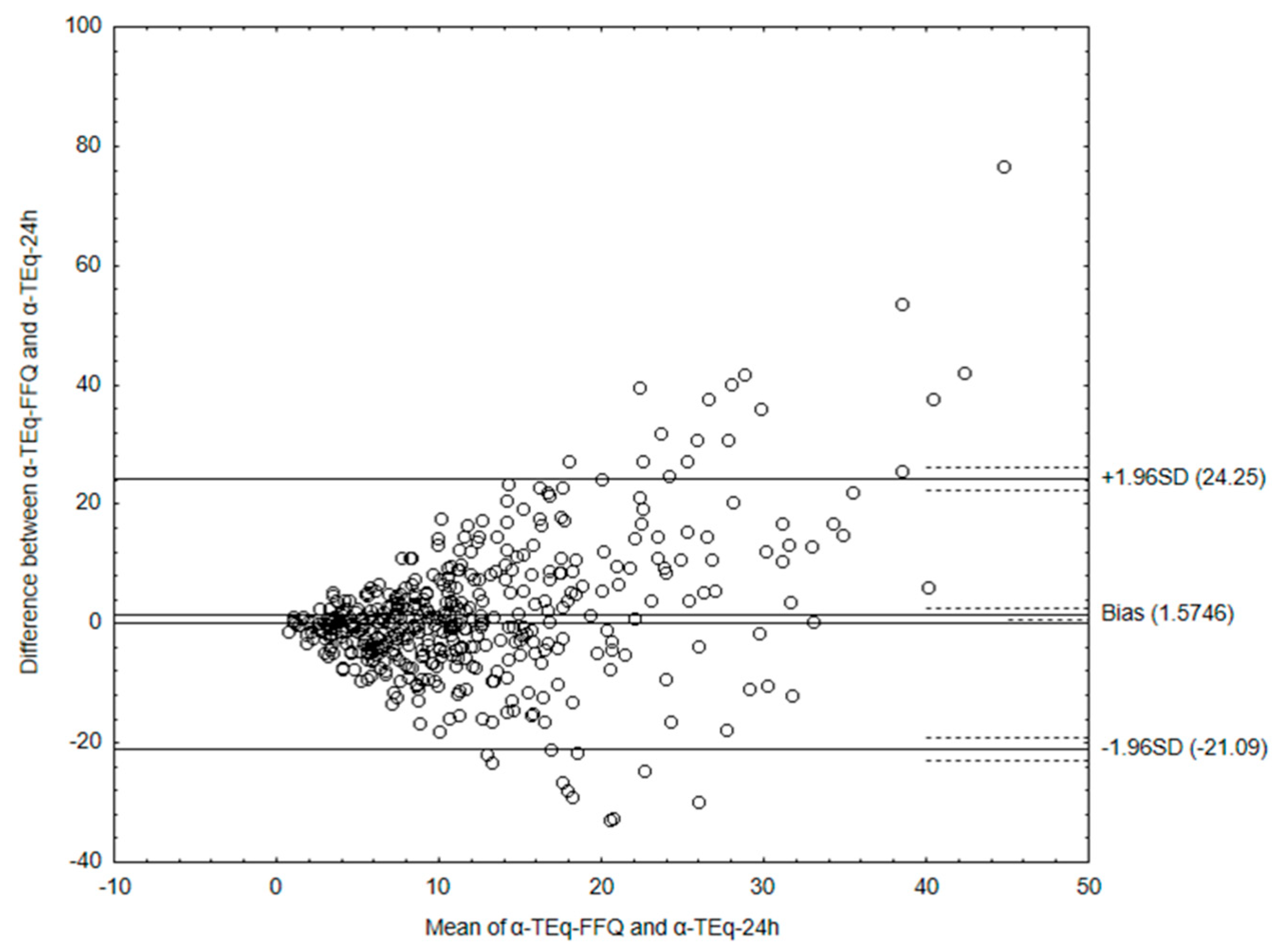
3.3. Analyzing the Agreement between Dietary Data Obtained by FFQ and Dietary Record Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khallouki, F.; Owen, R.W.; Akdad, M.; El Bouhali, B.; Silvente-Poirot, S.; Poirot, M. Vitamin E: An overview. In Molecular Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–66. ISBN 9780128119075. [Google Scholar]
- Malik, A.; Eggersdorfer, M.; Trilok-Kumar, G. Vitamin E status in healthy population in Asia: A review of current literature. Int. J. Vitam. Nutr. Res. 2021, 91, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Ciarcià, G.; Bianchi, S.; Tomasello, B.; Acquaviva, R.; Malfa, G.A.; Naletova, I.; La Mantia, A.; Di Giacomo, C. Vitamin E and Non-Communicable Diseases: A Review. Biomedicines 2022, 10, 2473. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Kono, N. α-Tocopherol transfer protein (α-TTP). Free Radic. Biol. Med. 2021, 176, 162–175. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA PANEL (EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA J. 2015, 13, 4149. [Google Scholar] [CrossRef]
- Péter, S.; Eggersdorfer, M.; Weber, P.; Péter, S.; Eggersdorfer, M.; Weber, P. Vitamin E Intake and Serum Levels in the General Population: A Global Perspective. In Vitamin E in Human Health; Humana: Cham, Switzerland, 2019; pp. 175–188. [Google Scholar] [CrossRef]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef]
- Vitamin E-Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 11 January 2023).
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Vitamin E. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000; pp. 186–283. ISBN 0-309-59719-6. [Google Scholar]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2014; ISBN 9789289326704. [Google Scholar]
- Dietary Reference Values for Vitamins and Minerals. Vitamin E. ANSES-French Agency for Food, Environmental and Occupational Health & Safety. Available online: https://www.anses.fr/fr/content/les-références-nutritionnelles-en-vitamines-et-minéraux (accessed on 24 April 2023).
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i Ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warszawa, Poland, 2020; ISBN 9788365870285. (In Polish) [Google Scholar]
- Godoy-Parejo, C.; Deng, C.; Zhang, Y.; Liu, W.; Chen, G. Roles of vitamins in stem cells. Cell. Mol. Life Sci. 2020, 77, 1771–1791. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Mohd Mutalip, S.; Ab-Rahim, S.; Rajikin, M. Vitamin E as an Antioxidant in Female Reproductive Health. Antioxidants 2018, 7, 22. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E and metabolites—Regulation of cancer cell death and underlying mechanisms. IUBMB Life 2019, 71, 495–506. [Google Scholar] [CrossRef]
- Stonehouse, W.; Brinkworth, G.D.; Thompson, C.H.; Abeywardena, M.Y. Short term effects of palm-tocotrienol and palm-carotenes on vascular function and cardiovascular disease risk: A randomised controlled trial. Atherosclerosis 2016, 254, 205–214. [Google Scholar] [CrossRef]
- Wagner, K.H.; Kamal-Eldin, A.; Elmadfa, I. Gamma-tocopherol-An underestimated vitamin? Ann. Nutr. Metab. 2004, 48, 169–188. [Google Scholar] [CrossRef]
- Abdullah, A.; Atia, A.; Salem Alrawaiq, N.; Kamil Md Yusof, M.; Fadzli Rusli, M. Palm Oil Tocotrienols in Cancer Chemoprevention and Treatment. In Elaeis Guineensis; IntechOpen: London, UK, 2022; ISBN 978-1-83962-762-0. [Google Scholar]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Vitamin E as a potential interventional treatment for metabolic syndrome: Evidence from animal and human studies. Front. Pharmacol. 2017, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Mo, H.; Wang, S.; Zu, Y.; Elfakhani, M.; Rios, S.R.; Chyu, M.C.; Yang, R.S.; Shen, C.L. Potential roles of vitamin E in age-related changes in skeletal muscle health. Nutr. Res. 2018, 49, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Galmés, S.; Serra, F.; Palou, A. Vitamin E Metabolic Effects and Genetic Variants: A Challenge for Precision Nutrition in Obesity and Associated Disturbances. Nutrients 2018, 10, 1919. [Google Scholar] [CrossRef]
- Idamokoro, E.M.; Falowo, A.B.; Oyeagu, C.E.; Afolayan, A.J. Multifunctional activity of vitamin E in animal and animal products: A review. Anim. Sci. J. 2020, 91, e13352. [Google Scholar] [CrossRef]
- Shahidi, F.; De Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.I.; Adnan, M.; Rahman, M.M. Natural Sources of Tocotrienols: A Note on Absorption. J. Silico Vitr. Pharmacol. 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album Fotografii Produktów i Potraw. Available online: https://scholar.google.com/scholar_lookup?title=Atlas+of+Portion+Sizes+of+Food+Products+and+Dishes&author=Szponar,+L.&author=Wolnicka,+K.&author=Rychlik,+E.&publication_year=2000 (accessed on 1 April 2018).
- Szewczyk, K.; Daniluk, P.; Górnicka, M. Assessment of Tocotrienols Intake in Adults—A Pilot Study. Biol. Life Sci. Forum 2022, 12, 16. [Google Scholar] [CrossRef]
- Przygoda, B.; Kunachowicz, H.; Nadolna, I.; Iwanow, K. Food Composition Tables, 7th ed.; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2019. [Google Scholar]
- FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/ (accessed on 24 April 2023).
- Polito, A.; Intorre, F.; Andriollo-Sanchez, M.; Azzini, E.; Raguzzini, A.; Meunier, N.; Ducros, V.; O’Connor, J.M.; Coudray, C.; Roussel, A.M.; et al. Estimation of intake and status of vitamin A, vitamin E and folate in older European adults: The ZENITH. Eur. J. Clin. Nutr. 2005, 59, S42–S47. [Google Scholar] [CrossRef]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar]
- Dietrich, M.; Jacques, P.F.; Traber, M.G.; Cross, C.E.; Hu, Y.; Block, G. Does γ-Tocopherol Play a Role in the Primary Prevention of Heart Disease and Cancer? A Review. J. Am. Coll. Nutr. 2013, 25, 292–299. [Google Scholar] [CrossRef]
- Sookwong, P.; Nakagawa, K.; Yamaguchi, Y.; Miyazawa, T.; Kato, S.; Kimura, F.; Miyazawa, T. Tocotrienol Distribution in Foods: Estimation of Daily Tocotrienol Intake of Japanese Population. J. Agric. Food Chem. 2010, 58, 3350–3355. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Kim, K.N.; Lee, J.S.; Yoon, M.O.; Lee, H.S. Dietary intake and major source foods of vitamin E among Koreans: Findings of the Korea National Health and Nutrition Examination Survey 2016–2019. Nutr Res Pract. 2022, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Bronkowska, M.; Biernat, J.; Sadowska, B.; Człowieka, Z.Ż. Ocena zawartości witamin w racjach pokarmowych kobiet w okresie okołomenopauzalnym. Bromatol. I Chem. Toksykol. 2009, 1, 18–23. [Google Scholar]
- Górnicka, M.; Pindral, J.; Hamułka, J.; Warzyniak, A. Ocena spożycia witamin antyoksydacyjnych przez studentów SGGW w Warszawie. Rocz. Państwowego Zakładu Hig. 2011, 62, 397–402. [Google Scholar]
- Górna, I.; Drabowicz, E.; Przysławski, J. Ocena zawartości wybranych witamin i składników mineralnych w całodziennych racjach pokarmowych grupy kobiet z zaburzeniami płodności. Bromat. Chem. Toksykol. 2012, 3, 1060–1064. [Google Scholar]
- Gorzela, J.; Gorzelańczyk, J.; Frąckiewicz, J. Spożycie witamin C, E wśród kobiet uprawiających fitness. In Współczesne Kierunki Działań Prozdrowotnych; WSliZ: Warszawa, Poland, 2015; pp. 169–186. [Google Scholar]
- Dao, M.C.; Subar, A.F.; Warthon-Medina, M.; Cade, J.E.; Burrows, T.; Golley, R.K.; Forouhi, N.G.; Pearce, M.; Holmes, B.A. Dietary Assessment Toolkits: An Overview. Public Health Nutr. 2019, 22, 404. [Google Scholar] [CrossRef]
- Poslusna, K.; Ruprich, J.; De Vries, J.H.M.; Jakubikova, M.; Van’t Veer, P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br. J. Nutr. 2009, 101 (Suppl. 2), S73–S85. [Google Scholar] [CrossRef]
- Schoeller, D.A.; Thomas, D.; Archer, E.; Heymsfield, S.B.; Blair, S.N.; Goran, M.I.; Hill, J.O.; Atkinson, R.L.; Corkey, B.E.; Foreyt, J.; et al. Self-report–based estimates of energy intake offer an inadequate basis for scientific conclusions. Am. J. Clin. Nutr. 2013, 97, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Kipnis, V.; Troiano, R.P.; Midthune, D.; Schoeller, D.A.; Bingham, S.; Sharbaugh, C.O.; Trabulsi, J.; Runswick, S.; Ballard-Barbash, R.; et al. Using Intake Biomarkers to Evaluate the Extent of Dietary Misreporting in a Large Sample of Adults: The OPEN Study. Am. J. Epidemiol. 2003, 158, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Goris, A.H.C.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Undereating and underrecording of habitual food intake in obese men: Selective underreporting of fat intake. Am. J. Clin. Nutr. 2000, 71, 130–134. [Google Scholar] [CrossRef]
- Sauvageot, N.; Alkerwi, A.; Albert, A.; Guillaume, M. Use of food frequency questionnaire to assess relationships between dietary habits and cardiovascular risk factors in NESCAV study: Validation with biomarkers. Nutr. J. 2013, 12, 143. [Google Scholar] [CrossRef]
- Kowalkowska, J.; Wadolowska, L.; Czarnocinska, J.; Czlapka-Matyasik, M.; Galinski, G.; Jezewska-Zychowicz, M.; Bronkowska, M.; Dlugosz, A.; Loboda, D.; Wyka, J. Reproducibility of a Questionnaire for Dietary Habits, Lifestyle and Nutrition Knowledge Assessment (KomPAN) in Polish Adolescents and Adults. Nutrients 2018, 10, 1845. [Google Scholar] [CrossRef]
- Welch, A.A. Dietary intake measurement: Methodology. Encycl. Hum. Nutr. 2013, 2–4, 65–73. [Google Scholar] [CrossRef]
- Lee, C.W.; Chen, H.J.; Xie, G.R.; Shih, C.K. Reproducibility of A Non-Quantitative Food Frequency Questionnaire (62-Item FFQ-6) and PCA-Driven Dietary Pattern Identification in 13–21-Year-Old Females. Nutrients 2019, 11, 2183. [Google Scholar] [CrossRef]
- Lai, J.S.; Attia, J.; Mcevoy, M.; Hure, A.J.; Au, A.J.H. Biochemical Validation of the Older Australian’s Food Frequency Questionnaire Using Carotenoids and Vitamin E. Nutrients 2014, 6, 4906–4917. [Google Scholar] [CrossRef]
- Malekahmadi, M.; Naeini, A.A.; Shab-Bidar, S.; Feizi, A.; Djazayery, A. Development, validity, and reliability of a food frequency questionnaire for antioxidants in elderly Iranian people. J. Res. Med. Sci. 2016, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Houston, Z.L.; McNaughton, S.A.; Kruger, R. Development and evaluation of a food frequency questionnaire to assess nutrient intakes of adult women in New Zealand. Nutr. Diet. 2020, 77, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zalaket, J.; Matta, J.; Hanna-Wakim, L. Development, validity, and reproducibility of a semiquantitative food frequency questionnaire for the assessment of antioxidant vitamins intake in Lebanon. Nutrition 2019, 58, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mumu, S.J.; Merom, D.; Ali, L.; Fahey, P.P.; Hossain, I.; Rahman, A.K.M.F.; Allman-Farinelli, M. Validation of a food frequency questionnaire as a tool for assessing dietary intake in cardiovascular disease research and surveillance in Bangladesh. Nutr. J. 2020, 19, 42. [Google Scholar] [CrossRef]
- Kowalkowska, J.; Wadolowska, L. The 72-Item Semi-Quantitative Food Frequency Questionnaire (72-Item SQ-FFQ) for Polish Young Adults: Reproducibility and Relative Validity. Nutrients 2022, 14, 2696. [Google Scholar] [CrossRef] [PubMed]

| Food Product | Serving Size | Household Measures |
|---|---|---|
| Vegetables | ||
| Broccoli/brussels sprouts/kale/asparagus/leeks | 100 g | 2/3 cup; 2 handfuls of leaves |
| Carrots/parsley root | 100 g | 2/3 cup |
| Green peas/tomatoes | 100 g | 2/3 cup |
| Beetroot/beet greens/pumpkin/red peppers/turnip | 100 g | 2/3 cup; 2 handfuls of leaves |
| Lettuce/romaine lettuce/spinach/chicory/rucola | 100 g | 2/3 cup; 2 handfuls |
| Corn | 20 g | 1 tablespoon |
| Carrot juice/multivegetable juices | 200 mL | 1 cup |
| Other vegetables | 100 g | 2/3 cup; 2 handfuls of leaves |
| Fruit and Fruit Products | ||
| Kiwifruit | 100 g | 2/3 cup |
| Raspberries/blackberries/blueberries | 100 g | 2/3 cup |
| Avocado | 70 g | 1/2 medium piece |
| Other fruit | 100 g | 2/3 cup |
| Legumes and Legume Products | ||
| Peas/lentils—dry seeds | 15 g | 1 tablespoon |
| Hummus | 10 g | 1 teaspoon |
| Nuts and Oilseeds | ||
| Pumpkin seeds | 30 g | 2 tablespoons |
| Linseeds | 30 g | 2 tablespoons |
| Pistachios/pecans | 30 g | 2 tablespoons |
| Almonds/sunflower seeds | 30 g | 2 tablespoons |
| Almond drink | 200 mL | 1 cup |
| Hazelnuts | 30 g | 2 tablespoons |
| Peanuts | 30 g | 2 tablespoons |
| Pine nuts | 30 g | 2 tablespoons |
| Other nuts, e.g., walnuts, cashews | 30 g | 2 tablespoons |
| Fats | ||
| Canola oil | 10 g | 1 tablespoon |
| Sunflower oil | 10 g | 1 tablespoon |
| Olive oil | 10 g | 1 tablespoon |
| Grape-seed oil | 10 g | 1 tablespoon |
| Peanut oil | 10 g | 1 tablespoon |
| Wheat germ oil | 10 g | 1 tablespoon |
| Soybean oil | 10 g | 1 tablespoon |
| Coconut oil | 10 g | 1 tablespoon |
| Margarine | 10 g | 1 tablespoon |
| Mayonnaise | 10 g | 1 tablespoon |
| Pesto | 10 g | 1 teaspoon |
| Eggs | 50 g | 1 piece |
| Cereals | ||
| Wholemeal bread, whole wheat pasta/brown rice/cornflakes | bread 35 g or 75 g other | 1 slice or cup of dry product |
| Fish and Fish Products | ||
| Fresh fish, e.g., salmon, trout, mackerel, herring | 100 g | 1 piece |
| Canned fish in oil, e.g., mackerel, tuna, herring, sardines | 150 g | 1 medium can |
| Other fish, e.g., canned fish in water/in tomatoes, flounder, carp, fish fingers | 150 g | 1 medium can |
| Snacks and Others | ||
| Dark chocolate | 5 g | 1 cube |
| Chips/crackers/nachos | 10 g | 1 handful |
| NIPH–NIH–NRI | NIH | EFSA | ||||
|---|---|---|---|---|---|---|
| Vitamin E | α-T Eq [mg/d] | α-T [mg/d] | α-T [mg/d] | |||
| DRIs | AI | UL | RDA | UL | AI | UL |
| Men | 10 | 300 | 15 | 1000 | 13 | 300 |
| Women | 8 | 11 | ||||
| Variables | Total n = 447 | Women n = 327 (73%) | Men n = 120 (27%) |
|---|---|---|---|
| Age group, % | |||
| 18–25 years | 60 | 62 | 52 |
| 26–40 years | 29 | 27 | 32 |
| 41–60 years | 10 | 9 | 15 |
| >60 years | 1 | 2 | 1 |
| Education, % | |||
| Primary and vocational | 3 | 18 | 5 |
| Secondary | 24 | 7 | 25 |
| University | 52 | 53 | 48 |
| While studying | 21 | 22 | 22 |
| Place of living, % | |||
| Village | 21 | 20 | 25 |
| City < 100,000 inhab. | 21 | 21 | 22 |
| City > 100,000 inhab. | 48 | 50 | 41 |
| City > 500,000 inhab. | 10 | 9 | 12 |
| Anthropometrics (mean ± SD) | |||
| Height, cm | 171 ± 9.0 | 166.2 ± 6.0 | 181.1 ± 6.4 |
| Body weight, kg | 70.3 ± 33.2 | 65.1 ± 36.0 | 84.6 ± 16.9 |
| BMI | 24.1 ± 11.3 | 23.5 ± 12.8 | 25.8 ± 5.2 |
| Vitamin E Isoforms | Methods | p * | |||||
|---|---|---|---|---|---|---|---|
| VitE-FFQ (mg/day) | 1-Day Dietary Record (mg/day) | ||||||
| Mean ± SD | Median | Min.–Max. | Mean ± SD | Median | Min.–Max. | ||
| α-T | 12.0 ± 8.5 | 9.7 | 0.4–50.6 | 13.3 ± 11.7 | 10.1 | 0.2–73.5 | NS |
| β-T | 0.3 ± 0.2 | 0.2 | 0.1–1.6 | 0.3 ± 0.4 | 0.2 | 0.1–3.3 | NS |
| γ-T | 8.3 ± 6.6 | 6.5 | 0.2–42.3 | 9.8 ± 9.8 | 6.3 | 0.1–45.7 | NS |
| δ-T | 1.1 ± 0.9 | 0.7 | 0.1–6.9 | 1.1 ± 1.4 | 0.6 | 0.1–7.7 | NS |
| Sum of Ts | 21.7 ± 15.1 | 17.7 | 0.7–87.9 | 24.6 ± 20.5 | 18.3 | 0.2–118.7 | NS |
| α-T3 | 0.3 ± 0.2 | 0.2 | 0.1–1.4 | 0.4 ± 0.3 | 0.3 | 0.1–1.9 | NS |
| β-T3 | 0.6 ± 0.6 | 0.4 | 0.1–3.6 | 0.8 ± 0.7 | 0.7 | 0.0–3.6 | NS |
| γ-T3 | 0.3 ± 0.3 | 0.2 | 0.1–1.9 | 0.5 ± 0.4 | 0.4 | 0.0–1.9 | NS |
| δ-T3 | 0.1 ± 0.1 | 0.1 | 0.0–0.6 | 0.1 ± 0.1 | 0.1 | 0.0–0.2 | NS |
| Sum of T3s | 1.3 ± 1.2 | 1.0 | 0.1–6.8 | 1.6 ± 1.4 | 1.4 | 0.1–6.7 | NS |
| α-T Eq | 11.3 ± 7.6 | 9.2 | 0.4–40.9 | 12.8 ± 11.6 | 9.5 | 0.1–83.0 | NS |
| DRIs | Percentage (%) of Individuals According to Method | ||
|---|---|---|---|
| FFQ | 1-D | ||
| n = 447 | n = 447 | ||
| α-T; RDA (NIH) | Adequate intake | 42 | 45 |
| Inadequate intake | 58 | 55 | |
| α-T equivalents; AI (NIPH–NIH–NRI) | Adequate intake | 57 | 55 |
| Inadequate intake | 43 | 45 | |
| α-T equivalents; AI (EFSA) | Adequate intake | 40 | 47 |
| Inadequate intake | 60 | 53 | |
| α-T equivalents; UL | Excessive intake | 0 | 0 |
| Vitamin E Isoforms | Mean Absolute Difference (mg) | Lower LOA | Upper LOA | Number of Individuals beyond the LOA | Bland–Altman Index (%) |
|---|---|---|---|---|---|
| α-tocopherol | 1.29 | −22.54 | 25.13 | 422 out of 447 | 5.6 |
| β-tocopherol | 0.034 | −0.704 | 0.772 | 413 out of 447 | 7.6 |
| γ-tocopherol | 1.46 | −18.77 | 21.69 | 416 out of 447 | 6.9 |
| δ-tocopherol | 0.141 | −2.845 | 3.126 | 420 out of 447 | 6.04 |
| Sum of tocopherols | 2.93 | −39.77 | 45.63 | 415 out of 447 | 7.2 |
| Vitamin E Isoforms | Mean Absolute Difference (mg) | Lower LOA | Upper LOA | Number of Individuals beyond the LOA | Bland–Altman Index (%) |
|---|---|---|---|---|---|
| α-tocotrienol | 0.051 | −0.5753 | 0.6777 | 415 out of 447 | 7.2% |
| β-tocotrienol | 0.156 | −1.398 | 1.711 | 412 out of 447 | 7.8% |
| γ-tocotrienol | 0.127 | 0.7175 | 0.9707 | 415 out of 447 | 7.2% |
| δ-tocotrienol | 0.0002 | −0.1116 | 0.1112 | 417 out of 447 | 6.7% |
| Sum of tocotrienols | 0.333 | −2.628 | 3.295 | 415 out of 447 | 7.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk, K.; Górnicka, M. Dietary Vitamin E Isoforms Intake: Development of a New Tool to Assess Tocopherols and Tocotrienols Intake in Adults. Nutrients 2023, 15, 3759. https://doi.org/10.3390/nu15173759
Szewczyk K, Górnicka M. Dietary Vitamin E Isoforms Intake: Development of a New Tool to Assess Tocopherols and Tocotrienols Intake in Adults. Nutrients. 2023; 15(17):3759. https://doi.org/10.3390/nu15173759
Chicago/Turabian StyleSzewczyk, Kacper, and Magdalena Górnicka. 2023. "Dietary Vitamin E Isoforms Intake: Development of a New Tool to Assess Tocopherols and Tocotrienols Intake in Adults" Nutrients 15, no. 17: 3759. https://doi.org/10.3390/nu15173759
APA StyleSzewczyk, K., & Górnicka, M. (2023). Dietary Vitamin E Isoforms Intake: Development of a New Tool to Assess Tocopherols and Tocotrienols Intake in Adults. Nutrients, 15(17), 3759. https://doi.org/10.3390/nu15173759







