The Protective Role of Dietary Polyphenols in Urolithiasis: Insights into Antioxidant Effects and Mechanisms of Action
Abstract
:1. Introduction
2. The Role of Oxidative Stress in Urolithiasis
2.1. Sources of Reactive Oxygen Species in Kidney
2.2. Clinical and Experimental Studies of Oxidative Stress in Urolithiasis
2.3. Antioxidants for Treatment
3. Dietary Polyphenols and Their Biological Significance
3.1. Classification and Sources of Dietary Polyphenols
3.2. Antioxidant Mechanisms of Dietary Polyphenols
4. Antioxidant Potential of Dietary Polyphenols in Urolithiasis: In Vitro and In Vivo Studies
4.1. Flavonoids Compounds
4.2. Non-Flavonoids Compounds
4.3. Plant Sources
5. Antioxidant Potential of Dietary Polyphenols in Urolithiasis: Clinical Investigation
6. Current Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.G. Kidney stones. Nat. Rev. Dis. Primers 2016, 2, 16008. [Google Scholar] [CrossRef]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef] [PubMed]
- Scales, C.D., Jr.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Prevalence of kidney stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, M.R.; Haley, W.E.; Mara, K.C.; Enders, F.T.; Vrtiska, T.J.; Pais, V.M.; Jacobsen, S.J.; McCollough, C.H.; Lieske, J.C.; Rule, A.D. Symptomatic and Radiographic Manifestations of Kidney Stone Recurrence and Their Prediction by Risk Factors: A Prospective Cohort Study. J. Am. Soc. Nephrol. 2019, 30, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, J.A.; Maalouf, N.M.; Pearle, M.S.; Lotan, Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur. Urol. 2014, 66, 724–729. [Google Scholar] [CrossRef]
- Wignall, G.R.; Canales, B.K.; Denstedt, J.D.; Monga, M. Minimally invasive approaches to upper urinary tract urolithiasis. Urol. Clin. North Am. 2008, 35, 441–454, viii. [Google Scholar] [CrossRef]
- Singh, P.; Enders, F.T.; Vaughan, L.E.; Bergstralh, E.J.; Knoedler, J.J.; Krambeck, A.E.; Lieske, J.C.; Rule, A.D. Stone Composition Among First-Time Symptomatic Kidney Stone Formers in the Community. Mayo Clin. Proc. 2015, 90, 1356–1365. [Google Scholar] [CrossRef]
- Finlayson, B. Physicochemical aspects of urolithiasis. Kidney Int. 1978, 13, 344–360. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Frick, K.K.; Nehrke, K. Genetic hypercalciuric stone-forming rats. Curr. Opin. Nephrol. Hypertens 2006, 15, 403–418. [Google Scholar] [CrossRef]
- Khan, S.R.; Canales, B.K. Ultrastructural investigation of crystal deposits in Npt2a knockout mice: Are they similar to human Randall’s plaques? J. Urol. 2011, 186, 1107–1113. [Google Scholar] [CrossRef]
- Mandel, N. Crystal-membrane interaction in kidney stone disease. J. Am. Soc. Nephrol. 1994, 5, S37–S45. [Google Scholar] [CrossRef]
- Khan, S.R.; Byer, K.J.; Thamilselvan, S.; Hackett, R.L.; McCormack, W.T.; Benson, N.A.; Vaughn, K.L.; Erdos, G.W. Crystal-cell interaction and apoptosis in oxalate-associated injury of renal epithelial cells. J. Am. Soc. Nephrol. 1999, 10 (Suppl. S14), S457–S463. [Google Scholar]
- Daudon, M.; Bazin, D.; Letavernier, E. Randall’s plaque as the origin of calcium oxalate kidney stones. Urolithiasis 2015, 43 (Suppl. S1), 5–11. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Canales, B.K.; Dominguez-Gutierrez, P.R. Randall’s plaque and calcium oxalate stone formation: Role for immunity and inflammation. Nat. Rev. Nephrol. 2021, 17, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl. Androl. Urol. 2014, 3, 256–276. [Google Scholar]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food. Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Khan, S.R. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: Evidence from clinical and experimental investigations. J. Urol. 2013, 189, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Roy, Z.; Bansal, R.; Siddiqui, L.; Chaudhary, N. Understanding the Role of Free Radicals and Antioxidant Enzymes in Human Diseases. Curr. Pharm. Biotechnol. 2023, 24, 1265–1276. [Google Scholar] [PubMed]
- Manea, A. NADPH oxidase-derived reactive oxygen species: Involvement in vascular physiology and pathology. Cell Tissue Res. 2010, 342, 325–339. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yi, F.X.; Spurrier, J.L.; Bobrowitz, C.A.; Zou, A.P. Production of superoxide through NADH oxidase in thick ascending limb of Henle’s loop in rat kidney. Am. J. Physiol. Renal. Physiol. 2002, 282, F1111–F1119. [Google Scholar] [CrossRef]
- Geiszt, M.; Kopp, J.B.; Várnai, P.; Leto, T.L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 8010–8014. [Google Scholar] [CrossRef]
- Leto, T.L.; Morand, S.; Hurt, D.; Ueyama, T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid. Redox. Signal 2009, 11, 2607–2619. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef]
- Joshi, S.; Peck, A.B.; Khan, S.R. NADPH oxidase as a therapeutic target for oxalate induced injury in kidneys. Oxid Med. Cell Longev. 2013, 2013, 462361. [Google Scholar] [CrossRef]
- Kawahara, T.; Quinn, M.T.; Lambeth, J.D. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol. Biol. 2007, 7, 109. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 2005, 70, 200–214. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Elazar, Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007, 17, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Baggio, B.; Gambaro, G.; Ossi, E.; Favaro, S.; Borsatti, A. Increased urinary excretion of renal enzymes in idiopathic calcium oxalate nephrolithiasis. J. Urol. 1983, 129, 1161–1162. [Google Scholar] [CrossRef]
- Boonla, C.; Wunsuwan, R.; Tungsanga, K.; Tosukhowong, P. Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol. Res. 2007, 35, 185–191. [Google Scholar] [CrossRef]
- Schwille, P.O.; Manoharan, M.; Schmiedl, A. Is idiopathic recurrent calcium urolithiasis in males a cellular disease? Laboratory findings in plasma, urine and erythrocytes, emphasizing the absence and presence of stones, oxidative and mineral metabolism: An observational study. Clin. Chem. Lab. Med. 2005, 43, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Holoch, P.A.; Tracy, C.R. Antioxidants and self-reported history of kidney stones: The National Health and Nutrition Examination Survey. J. Endourol. 2011, 25, 1903–1908. [Google Scholar] [CrossRef]
- Coe, F.L.; Evan, A.P.; Lingeman, J.E.; Worcester, E.M. Plaque and deposits in nine human stone diseases. Urol. Res. 2010, 38, 239–247. [Google Scholar] [CrossRef]
- Linnes, M.P.; Krambeck, A.E.; Cornell, L.; Williams, J.C., Jr.; Korinek, M.; Bergstralh, E.J.; Li, X.; Rule, A.D.; McCollough, C.M.; Vrtiska, T.J.; et al. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int. 2013, 84, 818–825. [Google Scholar] [CrossRef]
- Khan, S.R.; Rodriguez, D.E.; Gower, L.B.; Monga, M. Association of Randall plaque with collagen fibers and membrane vesicles. J. Urol. 2012, 187, 1094–1100. [Google Scholar] [CrossRef]
- Khan, S.R.; Glenton, P.A.; Byer, K.J. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: Experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int. 2006, 70, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Khan, A.; Glenton, P.A.; Khan, S.R. Effect of NADPH oxidase inhibition on the expression of kidney injury molecule and calcium oxalate crystal deposition in hydroxy-L-proline-induced hyperoxaluria in the male Sprague-Dawley rats. Nephrol. Dial. Transplant 2011, 26, 1785–1796. [Google Scholar] [CrossRef]
- Thamilselvan, S.; Hackett, R.L.; Khan, S.R. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J. Urol. 1997, 157, 1059–1063. [Google Scholar] [CrossRef]
- McKee, M.D.; Nanci, A.; Khan, S.R. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J. Bone Miner Res. 1995, 10, 1913–1929. [Google Scholar] [CrossRef] [PubMed]
- de Water, R.; Noordermeer, C.; van der Kwast, T.H.; Nizze, H.; Boevé, E.R.; Kok, D.J.; Schröder, F.H. Calcium oxalate nephrolithiasis: Effect of renal crystal deposition on the cellular composition of the renal interstitium. Am. J Kidney Dis. 1999, 33, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Khan, A.; Byer, K.J. Temporal changes in the expression of mRNA of NADPH oxidase subunits in renal epithelial cells exposed to oxalate or calcium oxalate crystals. Nephrol. Dial. Transplant 2011, 26, 1778–1785. [Google Scholar] [CrossRef]
- Thamilselvan, V.; Menon, M.; Thamilselvan, S. Selective Rac1 inhibition protects renal tubular epithelial cells from oxalate-induced NADPH oxidase-mediated oxidative cell injury. Urol. Res. 2012, 40, 415–423. [Google Scholar] [CrossRef]
- Thamilselvan, V.; Menon, M.; Thamilselvan, S. Oxalate-induced activation of PKC-alpha and -delta regulates NADPH oxidase-mediated oxidative injury in renal tubular epithelial cells. Am. J. Physiol. Renal. Physiol. 2009, 297, F1399–F1410. [Google Scholar] [CrossRef]
- Byer, K.; Khan, S.R. Citrate provides protection against oxalate and calcium oxalate crystal induced oxidative damage to renal epithelium. J. Urol. 2005, 173, 640–646. [Google Scholar] [CrossRef]
- Lieske, J.C.; Hammes, M.S.; Hoyer, J.R.; Toback, F.G. Renal cell osteopontin production is stimulated by calcium oxalate monohydrate crystals. Kidney Int. 1997, 51, 679–686. [Google Scholar] [CrossRef]
- Umekawa, T.; Chegini, N.; Khan, S.R. Oxalate ions and calcium oxalate crystals stimulate MCP-1 expression by renal epithelial cells. Kidney Int. 2002, 61, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Asselman, M.; Verhulst, A.; De Broe, M.E.; Verkoelen, C.F. Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J. Am. Soc. Nephrol. 2003, 14, 3155–3166. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Xia, Q.D.; Xu, J.Z.; Liu, C.Q.; Sun, J.X.; Xun, Y.; Wang, S.G. Identification of the pivotal role of SPP1 in kidney stone disease based on multiple bioinformatics analysis. BMC Med. Genomics 2022, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, A.; Asselman, M.; Persy, V.P.; Schepers, M.S.; Helbert, M.F.; Verkoelen, C.F.; De Broe, M.E. Crystal retention capacity of cells in the human nephron: Involvement of CD44 and its ligands hyaluronic acid and osteopontin in the transition of a crystal binding- into a nonadherent epithelium. J. Am. Soc. Nephrol. 2003, 14, 107–115. [Google Scholar] [CrossRef]
- Joshi, S.; Clapp, W.L.; Wang, W.; Khan, S.R. Osteogenic changes in kidneys of hyperoxaluric rats. Biochim. Biophys Acta 2015, 1852, 2000–2012. [Google Scholar] [CrossRef]
- Okada, A.; Yasui, T.; Hamamoto, S.; Hirose, M.; Kubota, Y.; Itoh, Y.; Tozawa, K.; Hayashi, Y.; Kohri, K. Genome-wide analysis of genes related to kidney stone formation and elimination in the calcium oxalate nephrolithiasis model mouse: Detection of stone-preventive factors and involvement of macrophage activity. J. Bone Miner Res. 2009, 24, 908–924. [Google Scholar] [CrossRef]
- Talham, D.R.; Backov, R.; Benitez, I.O.; Sharbaugh, D.M.; Whipps, S.; Khan, S.R. Role of lipids in urinary stones: Studies of calcium oxalate precipitation at phospholipid langmuir monolayers. Langmuir 2006, 22, 2450–2456. [Google Scholar] [CrossRef]
- Huang, H.S.; Chen, J.; Chen, C.F.; Ma, M.C. Vitamin E attenuates crystal formation in rat kidneys: Roles of renal tubular cell death and crystallization inhibitors. Kidney Int. 2006, 70, 699–710. [Google Scholar] [CrossRef]
- Thamilselvan, S.; Menon, M. Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int. 2005, 96, 117–126. [Google Scholar] [CrossRef]
- Jeong, B.C.; Kim, B.S.; Kim, J.I.; Kim, H.H. Effects of green tea on urinary stone formation: An in vivo and in vitro study. J. Endourol. 2006, 20, 356–361. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef]
- Sok, D.E.; Cui, H.S.; Kim, M.R. Isolation and bioactivities of furfuran type lignan compounds from edible plants. Recent Pat. Food Nutr. Agric. 2009, 1, 87–95. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, J.; Zheng, S.; Feng, Q.; Xiao, X. Curcumin, A Polyphenolic Curcuminoid With Its Protective Effects and Molecular Mechanisms in Diabetes and Diabetic Cardiomyopathy. Front. Pharmacol. 2018, 9, 472. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S310–S329. [Google Scholar] [CrossRef]
- La Rosa, G.; Lonardo, M.S.; Cacciapuoti, N.; Muscariello, E.; Guida, B.; Faraonio, R.; Santillo, M.; Damiano, S. Dietary Polyphenols, Microbiome, and Multiple Sclerosis: From Molecular Anti-Inflammatory and Neuroprotective Mechanisms to Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 7247. [Google Scholar] [CrossRef]
- Fanaro, G.B.; Marques, M.R.; Calaza, K.D.C.; Brito, R.; Pessoni, A.M.; Mendonça, H.R.; Lemos, D.E.A.; de Brito Alves, J.L.; de Souza, E.L.; Cavalcanti Neto, M.P. New Insights on Dietary Polyphenols for the Management of Oxidative Stress and Neuroinflammation in Diabetic Retinopathy. Antioxidants 2023, 12, 1237. [Google Scholar] [CrossRef]
- Manach, C.; Donovan, J.L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic. Res. 2004, 38, 771–785. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds against Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Bandy, B. Mechanisms of flavonoid protection against myocardial ischemia-reperfusion injury. J. Mol. Cell Cardiol. 2009, 46, 309–317. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Testai, L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci. 2015, 135, 68–76. [Google Scholar] [CrossRef]
- Ozgová, S.; Hermánek, J.; Gut, I. Different antioxidant effects of polyphenols on lipid peroxidation and hydroxyl radicals in the NADPH-, Fe-ascorbate- and Fe-microsomal systems. Biochem. Pharmacol. 2003, 66, 1127–1137. [Google Scholar] [CrossRef]
- Azimi, A.; Eidi, A.; Mortazavi, P.; Rohani, A.H. Protective effect of apigenin on ethylene glycol-induced urolithiasis via attenuating oxidative stress and inflammatory parameters in adult male Wistar rats. Life Sci. 2021, 279, 119641. [Google Scholar] [CrossRef]
- Ding, T.; Zhao, T.; Li, Y.; Liu, Z.; Ding, J.; Ji, B.; Wang, Y.; Guo, Z. Vitexin exerts protective effects against calcium oxalate crystal-induced kidney pyroptosis in vivo and in vitro. Phytomedicine 2021, 86, 153562. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Jeong, B.C.; Sung, M.K.; Park, M.Y.; Choi, E.Y.; Kim, B.S.; Kim, H.H.; Kim, J.I. Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J. Urol. 2008, 179, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Guzel, A.; Yunusoglu, S.; Calapoglu, M.; Candan, I.A.; Onaran, I.; Oncu, M.; Ergun, O.; Oksay, T. Protective Effects of Quercetin on Oxidative Stress-Induced Tubular Epithelial Damage in the Experimental Rat Hyperoxaluria Model. Medicina 2021, 57, 566. [Google Scholar] [CrossRef]
- Gamero-Estevez, E.; Andonian, S.; Jean-Claude, B.; Gupta, I.; Ryan, A.K. Temporal Effects of Quercetin on Tight Junction Barrier Properties and Claudin Expression and Localization in MDCK II Cells. Int. J. Mol. Sci. 2019, 20, 4889. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, L.; Li, W.; Li, D.; Li, F. Hyperoside protects human kidney-2 cells against oxidative damage induced by oxalic acid. Mol. Med. Rep. 2018, 18, 486–494. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, Y.F.; Feng, Y.; Peng, B.; Che, J.P.; Liu, M.; Zheng, J.H. Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis 2014, 42, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Sun, X.; Liu, X.; Hutterer, G.; Pummer, K.; Hager, B.; Ye, Z.; Chen, Z. Kaempferol alleviates calcium oxalate crystal-induced renal injury and crystal deposition via regulation of the AR/NOX2 signaling pathway. Phytomedicine 2021, 86, 153555. [Google Scholar] [CrossRef]
- Zhai, W.; Zheng, J.; Yao, X.; Peng, B.; Liu, M.; Huang, J.; Wang, G.; Xu, Y. Catechin prevents the calcium oxalate monohydrate induced renal calcium crystallization in NRK-52E cells and the ethylene glycol induced renal stone formation in rat. BMC Complement Altern. Med. 2013, 13, 228. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Shang, P.; Bao, J.; Lu, J.; Yue, Z. Anti-nephrolithic potential of catechin in melamine-related urolithiasis via the inhibition of ROS, apoptosis, phospho-p38, and osteopontin in male Sprague-Dawley rats. Free Radic Res. 2015, 49, 1249–1258. [Google Scholar] [CrossRef]
- Grases, F.; Prieto, R.M.; Gomila, I.; Sanchis, P.; Costa-Bauzá, A. Phytotherapy and renal stones: The role of antioxidants. A pilot study in Wistar rats. Urol. Res. 2009, 37, 35–40. [Google Scholar] [CrossRef]
- Kanlaya, R.; Singhto, N.; Thongboonkerd, V. EGCG decreases binding of calcium oxalate monohydrate crystals onto renal tubular cells via decreased surface expression of alpha-enolase. J. Biol. Inorg. Chem. 2016, 21, 339–346. [Google Scholar] [CrossRef]
- Fong-Ngern, K.; Vinaiphat, A.; Thongboonkerd, V. Microvillar injury in renal tubular epithelial cells induced by calcium oxalate crystal and the protective role of epigallocatechin-3-gallate. Faseb. J. 2017, 31, 120–131. [Google Scholar] [CrossRef]
- Ye, T.; Yang, X.; Liu, H.; Lv, P.; Lu, H.; Jiang, K.; Peng, E.; Ye, Z.; Chen, Z.; Tang, K. Theaflavin protects against oxalate calcium-induced kidney oxidative stress injury via upregulation of SIRT1. Int. J. Biol. Sci. 2021, 17, 1050–1060. [Google Scholar] [CrossRef]
- Jing, G.H.; Liu, Y.D.; Liu, J.N.; Jin, Y.S.; Yu, S.L.; An, R.H. Puerarin prevents calcium oxalate crystal-induced renal epithelial cell autophagy by activating the SIRT1-mediated signaling pathway. Urolithiasis 2022, 50, 545–556. [Google Scholar] [CrossRef]
- Zhou, D.; Wu, Y.; Yan, H.; Shen, T.; Li, S.; Gong, J.; Li, G.; Mai, H.; Wang, D.; Tan, X. Gallic acid ameliorates calcium oxalate crystal-induced renal injury via upregulation of Nrf2/HO-1 in the mouse model of stone formation. Phytomedicine 2022, 106, 154429. [Google Scholar] [CrossRef]
- Hoseinynejad, K.; Mard, S.A.; Mansouri, Z.; Lamoochi, Z.; Kazemzadeh, R. Efficacy of chlorogenic acid against ethylene glycol-induced renal stone model: The role of NFKB-RUNX2-AP1-OSTERIX signaling pathway. Tissue Cell 2022, 79, 101960. [Google Scholar] [CrossRef]
- Yasir, F.; Wahab, A.T.; Choudhary, M.I. Protective effect of dietary polyphenol caffeic acid on ethylene glycol-induced kidney stones in rats. Urolithiasis 2018, 46, 157–166. [Google Scholar] [CrossRef]
- Nile, S.H.; Keum, Y.S.; Nile, A.S.; Kwon, Y.D.; Kim, D.H. Potential cow milk xanthine oxidase inhibitory and antioxidant activity of selected phenolic acid derivatives. J. Biochem. Mol. Toxicol. 2018, 32, e22005. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, H.J.; Sohn, E.J.; Ko, H.S.; Shim, B.S.; Ahn, K.S.; Kim, S.H. Anti-nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacol. Rep. 2013, 65, 970–979. [Google Scholar] [CrossRef]
- Oksay, T.; Yunusoğlu, S.; Calapoğlu, M.; Aydın Candan, I.; Onaran, İ.; Ergün, O.; Özorak, A. Protective impact of resveratrol in experimental rat model of hyperoxaluria. Int. Urol. Nephrol. 2017, 49, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xun, Y.; Zhang, J.; Hu, H.; Qin, B.; Wang, T.; Wang, S.; Li, C.; Lu, Y. Resveratrol Attenuates Oxalate-Induced Renal Oxidative Injury and Calcium Oxalate Crystal Deposition by Regulating TFEB-Induced Autophagy Pathway. Front Cell Dev. Biol. 2021, 9, 638759. [Google Scholar] [CrossRef]
- Ghodasara, J.; Pawar, A.; Deshmukh, C.; Kuchekar, B. Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacognosy Res. 2010, 2, 388–392. [Google Scholar]
- Li, Y.; Zhang, J.; Liu, H.; Yuan, J.; Yin, Y.; Wang, T.; Cheng, B.; Sun, S.; Guo, Z. Curcumin ameliorates glyoxylate-induced calcium oxalate deposition and renal injuries in mice. Phytomedicine 2019, 61, 152861. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, S.J.; Park, M.N.; Linnes, M.; Han, H.J.; Kim, J.H.; Lieske, J.C.; Kim, S.H. Gallotannin suppresses calcium oxalate crystal binding and oxalate-induced oxidative stress in renal epithelial cells. Biol. Pharm Bull. 2012, 35, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Saha, A.; Fujiki, H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011, 102, 317–323. [Google Scholar] [CrossRef]
- Maeda, K.; Kuzuya, M.; Cheng, X.W.; Asai, T.; Kanda, S.; Tamaya-Mori, N.; Sasaki, T.; Shibata, T.; Iguchi, A. Green tea catechins inhibit the cultured smooth muscle cell invasion through the basement barrier. Atherosclerosis 2003, 166, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Yasui, T.; Okada, A.; Tozawa, K.; Hayashi, Y.; Kohri, K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol 2005, 173, 271–275. [Google Scholar] [CrossRef]
- Li, Z.; Chang, L.; Ren, X.; Hu, Y.; Chen, Z. Modulation of Rat Kidney Stone Crystallization and the Relative Oxidative Stress Pathway by Green Tea Polyphenol. ACS Omega 2021, 6, 1725–1731. [Google Scholar] [CrossRef]
- Ghalayini, I.F.; Al-Ghazo, M.A.; Harfeil, M.N. Prophylaxis and therapeutic effects of raspberry (Rubus idaeus) on renal stone formation in Balb/c mice. Int. Braz J. Urol. 2011, 37, 259–266; discussion 267. [Google Scholar] [CrossRef]
- Tugcu, V.; Kemahli, E.; Ozbek, E.; Arinci, Y.V.; Uhri, M.; Erturkuner, P.; Metin, G.; Seckin, I.; Karaca, C.; Ipekoglu, N.; et al. Protective effect of a potent antioxidant, pomegranate juice, in the kidney of rats with nephrolithiasis induced by ethylene glycol. J. Endourol. 2008, 22, 2723–2731. [Google Scholar] [CrossRef]
- Ilbey, Y.O.; Ozbek, E.; Simsek, A.; Cekmen, M.; Somay, A.; Tasci, A.I. Effects of pomegranate juice on hyperoxaluria-induced oxidative stress in the rat kidneys. Ren. Fail 2009, 31, 522–531. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, A.; Dong, J.; Zheng, S.; Yu, H.; Wang, X.; Jiang, H.; Yang, L. Screening out key compounds of Glechomae Herba for antiurolithic activity and quality control based on spectrum-effect relationships coupled with UPLC-QDA. Biomed. Pharmacother. 2022, 149, 112829. [Google Scholar] [CrossRef]
- Li, J.; Wen, Q.; Feng, Y.; Zhang, J.; Luo, Y.; Tan, T. Characterization of the multiple chemical components of Glechomae Herba using ultra high performance liquid chromatography coupled to quadrupole-time-of-flight tandem mass spectrometry with diagnostic ion filtering strategy. J. Sep. Sci. 2019, 42, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, M.; Ergene, B.; Süntar, I.; Ozbilgin, S.; Saltan Çitoğlu, G.; Demirel, M.A.; Keleş, H.; Altun, L.; Küpeli Akkol, E. Preclinical Evaluation of Antiurolithiatic Activity of Viburnum opulus L. on Sodium Oxalate-Induced Urolithiasis Rat Model. Evid. Based Complement Alternat Med. 2014, 2014, 578103. [Google Scholar] [CrossRef]
- Chao, Y.; Gao, S.; Li, N.; Zhao, H.; Qian, Y.; Zha, H.; Chen, W.; Dong, X. Lipidomics Reveals the Therapeutic Effects of EtOAc Extract of Orthosiphon stamineus Benth. on Nephrolithiasis. Front. Pharmacol. 2020, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jin, J.; Li, X.; Zhao, Z.; Zhang, L.; Wang, Q.; Li, J.; Zhang, Q.; Xiang, S. Total flavonoids of Desmodium styracifolium attenuates the formation of hydroxy-L-proline-induced calcium oxalate urolithiasis in rats. Urolithiasis 2018, 46, 231–241. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wu, J.S.; Chang, Y.F.; Sun, Z.J.; Chang, C.J.; Lu, F.H.; Yang, Y.C. Increased amount and duration of tea consumption may be associated with decreased risk of renal stone disease. World J. Urol. 2019, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Cai, H.; Xiang, Y.B.; Li, H.; Lipworth, L.; Miller, N.L.; Zheng, W.; Shu, X.O.; Hsi, R.S. Green tea intake and risk of incident kidney stones: Prospective cohort studies in middle-aged and elderly Chinese individuals. Int. J. Urol. 2019, 26, 241–246. [Google Scholar] [CrossRef]
- Zhuo, D.; Li, M.; Cheng, L.; Zhang, J.; Huang, H.; Yao, Y. A Study of Diet and Lifestyle and the Risk of Urolithiasis in 1519 Patients in Southern China. Med. Sci. Monit. 2019, 25, 4217–4224. [Google Scholar] [CrossRef]
- Wu, Z.B.; Jiang, T.; Lin, G.B.; Wang, Y.X.; Zhou, Y.; Chen, Z.Q.; Xu, Y.M.; Ye, H.B.; Chen, B.J.; Bao, X.Z.; et al. Tea Consumption is Associated with Increased Risk of Kidney Stones in Northern Chinese: A Cross-sectional Study. Biomed Environ. Sci. 2017, 30, 922–926. [Google Scholar]
- Rode, J.; Bazin, D.; Dessombz, A.; Benzerara, Y.; Letavernier, E.; Tabibzadeh, N.; Hoznek, A.; Tligui, M.; Traxer, O.; Daudon, M.; et al. Daily Green Tea Infusions in Hypercalciuric Renal Stone Patients: No Evidence for Increased Stone Risk Factors or Oxalate-Dependent Stones. Nutrients 2019, 11, 256. [Google Scholar] [CrossRef]
- Rodgers, A.; Mokoena, M.; Durbach, I.; Lazarus, J.; de Jager, S.; Ackermann, H.; Breytenbach, I.; Okada, A.; Usami, M.; Hirose, Y.; et al. Do teas rich in antioxidants reduce the physicochemical and peroxidative risk factors for calcium oxalate nephrolithiasis in humans? Pilot studies with Rooibos herbal tea and Japanese green tea. Urolithiasis 2016, 44, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Tracy, C.R.; Henning, J.R.; Newton, M.R.; Aviram, M.; Bridget Zimmerman, M. Oxidative stress and nephrolithiasis: A comparative pilot study evaluating the effect of pomegranate extract on stone risk factors and elevated oxidative stress levels of recurrent stone formers and controls. Urolithiasis 2014, 42, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Prasongwatana, V.; Woottisin, S.; Sriboonlue, P.; Kukongviriyapan, V. Uricosuric effect of Roselle (Hibiscus sabdariffa) in normal and renal-stone former subjects. J. Ethnopharmacol. 2008, 117, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.G.; Behura, S.K.; Kumar, R. Litholytic property of Kulattha (Dolichous biflorus) vs potassium citrate in renal calculus disease: A comparative study. J. Assoc. Physicians India 2010, 58, 286–289. [Google Scholar]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact 2020, 328, 109211. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Eleazu, C.; Eleazu, K.; Kalu, W. Management of Benign Prostatic Hyperplasia: Could Dietary Polyphenols Be an Alternative to Existing Therapies? Front. Pharmacol. 2017, 8, 234. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Mah, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Serafini, M.; Testa, M.F.; Villaño, D.; Pecorari, M.; van Wieren, K.; Azzini, E.; Brambilla, A.; Maiani, G. Antioxidant activity of blueberry fruit is impaired by association with milk. Free Radic. Biol. Med. 2009, 46, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Kirkpatrick, N.J.; Polagruto, J.A.; Ensunsa, J.L.; Schmitz, H.H.; Keen, C.L. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003, 73, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediators Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.O.; Ofosu, F.K.; Kim, N.H.; Kilonzi, S.M.; Oh, D.H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Mignet, N.; Seguin, J.; Chabot, G.G. Bioavailability of polyphenol liposomes: A challenge ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef]
- Ning, P.; Lü, S.; Bai, X.; Wu, X.; Gao, C.; Wen, N.; Liu, M. High encapsulation and localized delivery of curcumin from an injectable hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 121–129. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Bøhn, S.K.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Effects of tea and coffee on cardiovascular disease risk. Food Funct. 2012, 3, 575–591. [Google Scholar] [CrossRef]
- Babu, V.R.; Thakur, M.S.; Patra, S. Effect of physicochemical parameters on enzymatic biodecaffeination during tea fermentation. Appl. Biochem. Biotechnol. 2012, 166, 112–126. [Google Scholar] [CrossRef]
- Sansone, R.; Ottaviani, J.I.; Rodriguez-Mateos, A.; Heinen, Y.; Noske, D.; Spencer, J.P.; Crozier, A.; Merx, M.W.; Kelm, M.; Schroeter, H.; et al. Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: Randomized, double-masked controlled studies. Am. J. Clin. Nutr. 2017, 105, 352–360. [Google Scholar] [CrossRef]
- Nakagawa, K.; Nakayama, K.; Nakamura, M.; Sookwong, P.; Tsuduki, T.; Niino, H.; Kimura, F.; Miyazawa, T. Effects of co-administration of tea epigallocatechin-3-gallate (EGCG) and caffeine on absorption and metabolism of EGCG in humans. Biosci. Biotechnol. Biochem. 2009, 73, 2014–2017. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Calniquer, G.; Khanin, M.; Ovadia, H.; Linnewiel-Hermoni, K.; Stepensky, D.; Trachtenberg, A.; Sedlov, T.; Braverman, O.; Levy, J.; Sharoni, Y. Combined Effects of Carotenoids and Polyphenols in Balancing the Response of Skin Cells to UV Irradiation. Molecules 2021, 26, 1931. [Google Scholar] [CrossRef]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J. Nutr. 2009, 139, 120–127. [Google Scholar] [CrossRef]
- Ma, S.; Kim, C.; Neilson, A.P.; Griffin, L.E.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Comparison of Common Analytical Methods for the Quantification of Total Polyphenols and Flavanols in Fruit Juices and Ciders. J. Food Sci. 2019, 84, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Abd El Mohsen, M.M.; Minihane, A.M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. Int. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W.; Lee, C.Y. Phenolic compounds and their changes in apples during maturation and cold storage. J. Agric. Food Chem. 1990, 38, 945–948. [Google Scholar] [CrossRef]
- Peng, Y.H.; Sweet, D.H.; Lin, S.P.; Yu, C.P.; Lee Chao, P.D.; Hou, Y.C. Green tea inhibited the elimination of nephro-cardiovascular toxins and deteriorated the renal function in rats with renal failure. Sci. Rep. 2015, 5, 16226. [Google Scholar] [CrossRef]
- Murakami, A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Hirose, M.; Takahashi, S.; Ogawa, K.; Shirai, T.; Ito, N. Forestomach and kidney carcinogenicity of caffeic acid in F344 rats and C57BL/6N x C3H/HeN F1 mice. Cancer Res. 1991, 51, 5655–5660. [Google Scholar]
- Zhu, B.T.; Liehr, J.G. Inhibition of catechol O-methyltransferase-catalyzed O-methylation of 2- and 4-hydroxyestradiol by quercetin. Possible role in estradiol-induced tumorigenesis. J. Biol. Chem. 1996, 271, 1357–1363. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Yang, C.S. Possible controversy over dietary polyphenols: Benefits vs risks. Chem. Res. Toxicol. 2007, 20, 583–585. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]


| Dietary Polyphenols Groups | Dietary Polyphenols Subgroups | Example | Dietary Source |
|---|---|---|---|
| Flavonoids | Flavones | Luteolin, Apigenin, Chrysin, Vitexin | Parsley, Celery, Thyme, Capsicum pepper |
| Flavonols | Quercetin, Hyperoside, Kaempferol, Myricetin, Galangin, Fisetin | Red cabbage, Onion, Leek, Curly pale, Cherry, Tomato, Broccoli, Blueberry, Apricot, Apple, Black Grape, Green and black tea, Beans, Red wine | |
| Flavanones | Hesperetin, Naringenin, Eriodictyol, Diosmin, Isosakuranetin | Orange, Grapefruit, Lemon juice | |
| Flavanols | (Epi)Catechin, (Epi)Gallocatechin, Epigallocatechin gallate, Theaflavin | Green and black tea, Cocoa, Chocolates, Apricots, Beans, Grapes, Berries, Apples, Red wine | |
| Isoflavones | Genistein, Genistin, Daidzein, Daidzin, Biochanin A, Puerarin, Formononetin | Soybeans, Soy foods, Legumes | |
| Anthocyanins | Cyanidin, Delphinidin, Pelargonidin, Peonidin, Petunidin, Malvidin | Red, blue, and purple berries, Red and purple grapes, Red wine, Cherry, Rhubarb | |
| Phenolic acids | Hydroxybenzoic acid | Gallic acid, Protocatechuic acid | Blackberry, Raspberry, Strawberry, Black currant |
| Hydroxycinnamic acid | Chlorogenic acid, Ferulic acid, Caffeic acid, Coumaric acid | Blueberry, Kiwi, Cherry, Plum, Apple, Pear, Peach, Chicory, Artichoke, Potato, Coffee | |
| Stilbenes | - | Resveratrol | Grapes, Red wine, Pomegranate, Groundnut |
| Lignans | - | Secoisolariciresinol | Linseed, Lentils, Garlic, Asparagus, Carrots, Pears, Prunes |
| Polyphenols Compounds | Experimental Model | Mechanisms of Action | References |
|---|---|---|---|
| Apigenin | Wistar rats drink water containing 0.75% ethylene glycol and 1% ammonium chloride. | Inhibition of the TGF-β pathway | [81] |
| Vitexin | C57BL/6 mice with intraperitoneal injection of 100 mg/kg/d glyoxylate. HK-2 cells treated with COM. THP-1 cells treated with COM. | Inhibition of pyroptosis, apoptosis, epithelial–mesenchymal transition, and macrophage infiltration | [82] |
| Quercetin | SD rats fed chow containing 3% sodium oxalate. MDCK cells treated with sodium oxalate. | Inhibition of lipid peroxidation, Activation of SOD and CAT activities | [83] |
| Quercetin | Wistar rats drink water containing 1% ethylene glycol. | Inhibition of the p38-MAPK pathway | [84] |
| Hyperoside | HK-2 cells treated with oxalate. | Activation of the Nrf2/HO-1/NQO1 pathway | [86] |
| Quercetin+Hyperoside | SD rats drink water containing 0.5% ethylene glycol. | - | [87] |
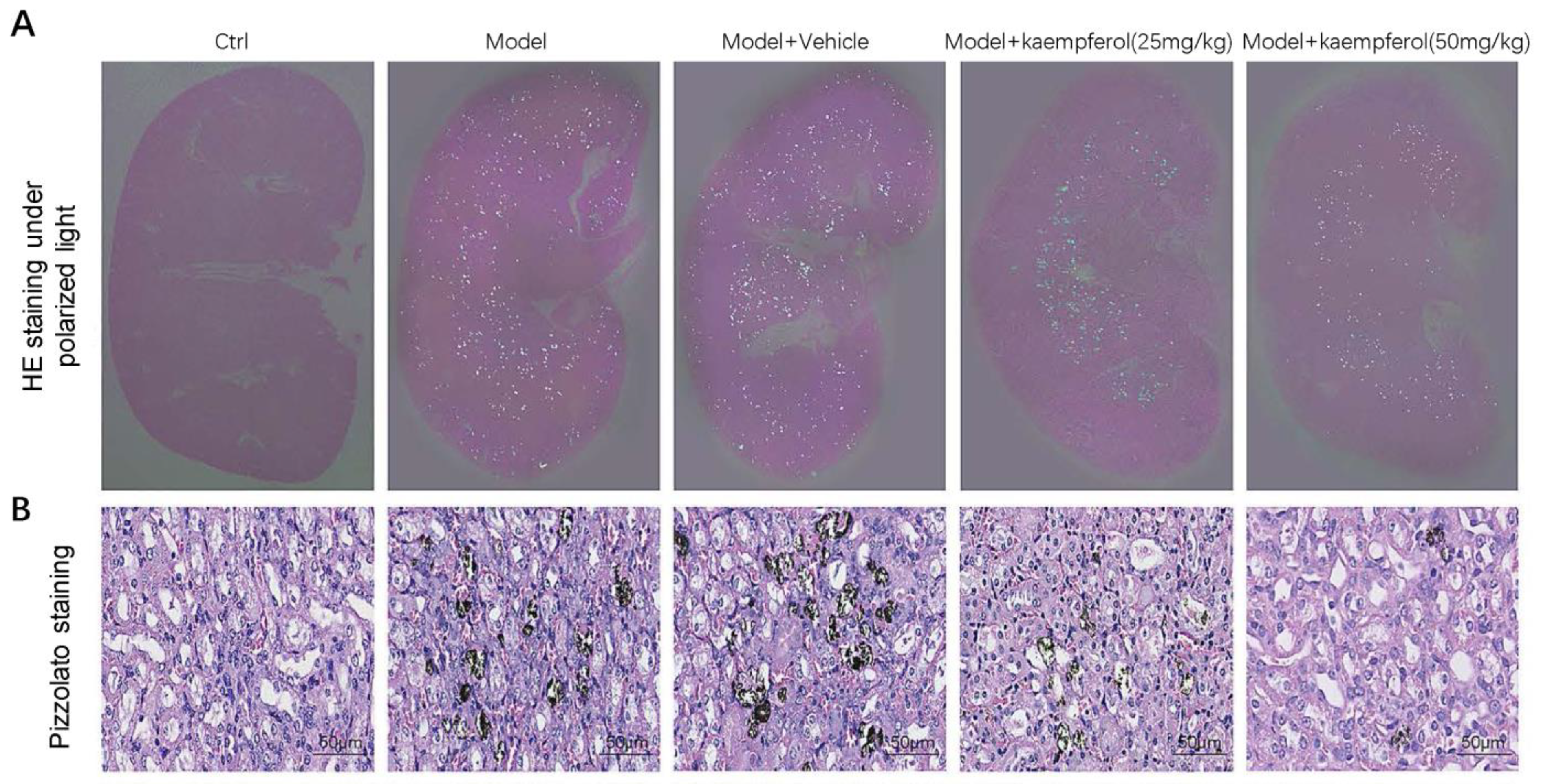
| Kaempferol | C57BL/6 mice with intraperitoneal injection of 100 mg/kg/d glyoxylate. HK-2 cells treated with COM. | Inhibition of the AR/NOX2 pathway | [88] |
| Catechin | Wistar rats drink water containing 5% ethylene glycol. NRK-52E cells treated with COM. | Inhibition of the changes in mitochondrial membrane potential, Inhibition of lipid peroxidation, Inhibition of apoptosis | [89] |
| Epigallocatechin gallate | SD rats fed chow containing 3% sodium oxalate. NRK-52E cells treated with oxalate. | - | [61] |
| Epigallocatechin gallate | MDCK cells treated with COM. | Inhibition of oxidized protein expression | [93] |
| Theaflavin | SD rats drink water containing 0.8% ethylene glycol and 0.8% ammonium chloride. HK-2 cells treated with COM | Regulation of the miR-128/SIRT1 axis | [94] |
| Puerarin | C57BL/6 mice with intraperitoneal injection of 100 mg/kg/d glyoxylate. HK-2 Cells treated with COM. | Activation of the SIRT1/AKT/p38 pathway | [95] |
| Gallic acid | C57BL/6 mice with intraperitoneal injection of 75 mg/kg/d glyoxylate. HK-2 cells treated with COM. | Activation of the Nrf2/HO-1 pathway | [96] |
| Chlorogenic acid | SD rats drink water containing 1% ethylene glycol. | Inhibition of the NF-κB/Runx2/AP-1/Osterix pathway | [97] |
| Resveratrol | SD rats drink water containing 0.8% ethylene glycol and 1% ammonium chloride. Human primary renal epithelial cells treated with oxalate. | Inhibition of NADPH oxidase subunits (p22phox and p47phox), MCP-1, OPN, TGF-1, TGFR-I/II and hyaluronan expression | [100] |
| Resveratrol | SD rats with intraperitoneal injection of 100 mg/kg/day glyoxylate. NRK-52E cells treated with oxalate. | Activation of a TFEB-induced autophagy | [102] |
| Curcumin | C57BL/6 mice with intraperitoneal injection of 100 mg/kg/d glyoxylate. | Activation of the Nrf2/HO-1/NQO1 pathway | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-Y.; Qin, B.-L. The Protective Role of Dietary Polyphenols in Urolithiasis: Insights into Antioxidant Effects and Mechanisms of Action. Nutrients 2023, 15, 3753. https://doi.org/10.3390/nu15173753
Hong S-Y, Qin B-L. The Protective Role of Dietary Polyphenols in Urolithiasis: Insights into Antioxidant Effects and Mechanisms of Action. Nutrients. 2023; 15(17):3753. https://doi.org/10.3390/nu15173753
Chicago/Turabian StyleHong, Sen-Yuan, and Bao-Long Qin. 2023. "The Protective Role of Dietary Polyphenols in Urolithiasis: Insights into Antioxidant Effects and Mechanisms of Action" Nutrients 15, no. 17: 3753. https://doi.org/10.3390/nu15173753
APA StyleHong, S.-Y., & Qin, B.-L. (2023). The Protective Role of Dietary Polyphenols in Urolithiasis: Insights into Antioxidant Effects and Mechanisms of Action. Nutrients, 15(17), 3753. https://doi.org/10.3390/nu15173753







