The Relationship between Diet and the Occurrence of Depressive Symptoms in a Community Example with High Rates of Social Deprivation: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.2.1. Dietary Assessment
2.2.2. Depressive Symptoms
2.2.3. Anthropometric Measurements
2.2.4. Other Socioeconomic and Health Variables
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of Participants
3.2. Dietary Assessment in the Study Group and the Relationship of Dietary Assessment to Selected Socioeconomic and Health Variables
3.3. Occurrence of Depressive Symptoms in the Study Group
3.4. Relationship between Dietary Assessment and Risk of Depressive Symptoms in the Study Population and Gender Strata
4. Discussion
The Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2013 Risk Factors Collaborators; Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental, and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar]
- World Health Organization. Global Status Report on Non-Communicable Diseases. Available online: http://www.who.int/nmh/publications/ncd-status-report-2014/en/ (accessed on 10 June 2023).
- Rummo, P.E.; Meyer, K.A.; Boone-Heinonen, J.; Jacobs, D.R.; Kiefe, C.I., Jr.; Lewis, C.E.; Steffen, L.M.; Gordon-Larsen, P. The neighbourhood availability of convenience stores and diet quality: Findings from 20 years of follow-up in the coronary artery risk development in young adults study. Am. J. Public Health 2015, 105, e65–e73. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.; Lamb, K.E.; Costa, C.; Cutumisu, N.; Ellaway, A.; Kamphuis, C.B.; Mentz, G.; Pearce, J.; Santana, P.; Santos, R.; et al. Neighbourhood socioeconsocio-economictage and fruit and vegetable consumption: A seven countries comparison. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 68. [Google Scholar] [CrossRef]
- Kivimäki, M.; Batty, G.D.; Pentti, J.; Shipley, M.J.; Sipilä, P.N.; Nyberg, S.T.; Suominen, S.B.; Oksanen, T.; Stenholm, S.; Virtanen, M.; et al. The association between socioeconsocio-economicnd the development of mental and physical health conditions in adulthood: A multi-cohort study. Lancet Public Health 2020, 5, e140–e149. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Olstad, D.L.; Leech, R.M.; Ball, K.; Meertens, B.; Potter, J.; Cleanthous, X.; Reynolds, R.; McNaughton, S.A. SocioeconSocio-economices in diet quality and nutrient intakes among Australian adults: Findings from a nationally representative cross-sectional study. Nutrients 2017, 9, 1092. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Lai, W.; Long, E.; Zhang, X.; Li, W.; Zhu, Y.; Chen, C.; Zhong, X.; Liu, Z.; et al. Prevalence of depression and depressive symptoms among outpatients: A systematic review and meta-analysis. BMJ Open 2017, 7, e017173. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. Available online: https://apps.who.int/iris/handle/10665/254610 (accessed on 10 June 2023).
- Hyde, J.S.; Mezulis, A.H.; Abramson, L.Y. The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol. Rev. 2008, 115, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Latorre, A.; Pérez Algorta, G.; Navarro-Guzmán, C.; Serrano-Ripoll, M.J.; Oliván-Blázquez, B. Effectiveness of a lifestyle modification programme in the treatment of depression symptoms in primary care. Front. Med. 2022, 9, 954644. [Google Scholar] [CrossRef]
- Nakamura, M.; Miura, A.; Nagahata, T.; Shibata, Y.; Okada, E.; Ojima, T. Low zinc, copper, and manganese intake is associated with depression and anxiety symptoms in the Japanese working population: Findings from the eating habit and well-being study. Nutrients 2019, 11, 847. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef]
- Nanri, A.; Kimura, Y.; Matsushita, Y.; Ohta, M.; Sato, M.; Mishima, N.; Sasaki, S.; Mizoue, T. Dietary patterns and depressive symptoms among Japanese men and women. Eur. J. Clin. Nutr. 2010, 64, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Mykletun, A.; Berk, M.; Bjelland, I.; Tell, G.S. The association between habitual diet quality and the common mental disorders in community-dwelling adults: The Hordaland Health study. Psychosom. Med. 2011, 73, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.N.; Brunner, E.J.; Ferrie, J.E.; Marmot, M.G.; Kivimaki, M.; Singh-Manoux, A. Dietary pattern and depressive symptoms in middle age. Br. J. Psychiatry. 2009, 195, 408–413. [Google Scholar] [CrossRef]
- McMartin, S.E.; Jacka, F.N.; Colman, I. The association between fruit and vegetable consumption and mental health disorders: Evidence from five waves of a national survey of Canadians. Prev. Med. 2013, 56, 225–230. [Google Scholar] [CrossRef]
- Tolkien, K.; Bradburn, S.; Murgatroyd, C. An anti-inflammatory diet as a potential intervention for depressive disorders: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 2045–2052. [Google Scholar] [CrossRef]
- Meller, F.O.; Manosso, L.M.; Schäfer, A.A. The influence of diet quality on depression among adults and elderly: A population-based study. J. Affect. Disord. 2021, 282, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Pereira, J.S.; Rea, K.; Nolan, Y.M.; O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Depression’s unholy trinity: Dysregulated stress, immunity, and the microbiome. Annu. Rev. Psychol. 2020, 71, 49–78. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So, depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Butterworth, P. Does reverse causality explain the relationship between diet and depression? J. Affect. Disord. 2015, 175, 248–250. [Google Scholar] [CrossRef]
- Mills, J.G.; Thomas, S.J.; Larkin, T.A.; Deng, C. Overeating and food addiction in major depressive disorder: Links to peripheral dopamine. Appetite 2020, 148, 104586. [Google Scholar] [CrossRef]
- Paans, N.P.G.; Gibson-Smith, D.; Bot, M.; van Strien, T.; Brouwer, I.A.; Visser, M.; Penninx, B.W.J.H. Depression and eating styles are independently associated with dietary intake. Appetite 2019, 134, 103–110. [Google Scholar] [CrossRef]
- Neally, S.J.; Tamura, K.; Langerman, S.D.; Claudel, S.E.; Farmer, N.; Vijayakumar, N.P.; Curlin, K.; Andrews, M.R.; Ceasar, J.N.; Baumer, Y.; et al. Associations between neighbourhood socioeconsocio-economicion and severity of depression: Data from the National Health and Nutrition Examination Survey, 2011–2014. SSM Popul. Health 2022, 18, 101111. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pérez, I.; Bermúdez-Tamayo, C.; Rodríguez-Barranco, M. Socio-ecoSocio-economiclinked with mental health during the recession: A multi-level analysis. Int. J. Equity Health 2017, 16, 45. [Google Scholar] [CrossRef]
- Giannaros, D. Twenty years after the economic restructuring of Eastern Europe: An economic review. Int. Bus. Econ. Res. J. 2008, 7, 35–38. [Google Scholar] [CrossRef]
- Wojtyniak, B.; Moskalewicz, J.; Stokwiszewski, J.; Rabczenko, D. Gender-specific mortality associated with alcohol consumption in Poland in transition. Addiction 2005, 100, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Leinsalu, M.; Stirbu, I.; Vågerö, D.; Kalediene, R.; Kovács, K.; Wojtyniak, B.; Wróblewska, W.; Mackenbach, J.P.; Kunst, A.E. Educational inequalities in mortality in four Eastern European countries: Divergence in trends during the post-communist transition from 1990 to 2000. Int. J. Epidemiol. 2008, 38, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Golinowska, S.; Sowa, A.; Topór-Mądry, R. Health Status and Health Care Systems in Central & Eastern European Countries: Bulgaria, Estonia, Poland, Slovakia and Hungary; European Network of Economic Policy, Research Report No. 31; European Network of Economic Policy Research Institutes: Brussels, Belgium, 2006. [Google Scholar]
- Central Statistical Office (GUS). Report on Results. National Census of Population and Apartments 2011. Available online: https://stat.gov.pl/cps/rde/xbcr/gus/lud_raport_z_wynikow_NSP2011.pdf (accessed on 19 October 2018).
- Statistical Office in Lublin. Registered Unemployment in Lubelskie Voivodship in 2014. Available online: http://wuplublin.praca.gov.pl/-/1355901-sytuacja-na-rynku-pracy-w-wojewodztwie-lubelskim-luty-2015 (accessed on 19 October 2018).
- Central Statistical Office (GUS). Beneficiaries of Social Assistance and Family Benefits in 2012. Available online: https://stat.gov.pl/files/gfx/portalinformacyjny/pl/defaultaktualnosci/5487/6/3/5/beneficjenci_pomocy_spolecznej_i_swiadczen_rodzinnych_2012.pdf (accessed on 19 October 2018).
- Program PL 13. Publication of Mortality Rates for Selected Districts. Available online: http://archiwum.zdrowie.gov.pl/aktualnosc-27-2136-Program_PL_13___publikacja_wskaznikow_umieralnosci_dla_wybranych_powiatow.html (accessed on 19 October 2018).
- Gawęcki, J.; Hryniewiecki, L. Human nutrition. In The Basics of Nutrition Science; PWN: Warsaw, Poland, 2006. [Google Scholar]
- Kmiecik, D.; Poślednik, B.; Waszkowiak, K.; Kobus-Cisowska, J.; Jędrusek-Golińska, A. Evaluation of kindergarden menus offered by kindergarden kitchen and catering companies in leszczynski district. Bromat. Chem. Toksykol. 2016, 49, 521–525. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Siu, A.L.; US Preventive Services Task Force (USPSTF); Bibbins-Domingo, K.; Grossman, D.C.; Baumann, L.C.; Davidson, K.W.; Ebell, M.; García, F.A.; Gillman, M.; Herzstein, J.; et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 315, 380–387. [Google Scholar] [CrossRef]
- Ślusarska, B.J.; Nowicki, G.; Piasecka, H.; Zarzycka, D.; Mazur, A.; Saran, T.; Bednarek, A. Validation of the Polish language version of the Patient Health Questionnaire-9 in a population of adults aged 35–64. Ann. Agric. Environ. Med. 2019, 26, 420–424. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Physical Status: The Use and Interpretation of Anthropometry; Report of a WHO Expert Committee; WHO Technical Report Series 854; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Różańska, D.; Czekajło, A.; Zatońska, K.; Szuba, A.; Regulska-Ilow, B. Association between dietary glycaemic load and selected demographic, socio-ecosocio-economicstyle factors in a group of adult Poles in Lower Silesia—Results of the PURE Poland Study. Ann. Agric. Environ. Med. 2020, 27, 49–55. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D.; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE). Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Haase, A.M.; Steptoe, A.; Nillapun, M.; Jonwutiwes, K.; Bellisle, F. Gender differences in food choice: The contribution of health beliefs and dieting. Ann. Behav. Med. 2004, 27, 107–116. [Google Scholar] [CrossRef]
- Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Guessous, I. Socio-demographic and lifestyle determinants of dietary patterns in French-speaking Switzerland, 2009–2012. BMC Public Health 2018, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, A.Đ. Healthy eating habits among the population of Serbia: Gender and age differences. J. Health Popul. Nutr. 2015, 33, 76–84. [Google Scholar] [PubMed]
- Kałucka, S.; Kaleta, D.; Makowiec-Dabrowska, T. Prevalence of dietary behaviour and determinants of quality of diet among beneficiaries of government welfare assistance in Poland. Int. J. Environ. Res. Public Health 2019, 16, 501. [Google Scholar] [CrossRef]
- de Abreu, D.; Guessous, I.; Vaucher, J.; Preisig, M.; Waeber, G.; Vollenweider, P.; Marques-Vidal, P. Low compliance with dietary recommendations for food intake among adults. Clin. Nutr. 2013, 32, 783–788. [Google Scholar] [CrossRef]
- Drewnowski, A. The cost of US foods as related to their nutritive value. Am. J. Clin. Nutr. 2010, 92, 1181–1188. [Google Scholar] [CrossRef]
- Backholer, K.; Spencer, E.; Gearon, E.; Magliano, D.J.; McNaughton, S.A.; Shaw, J.E.; Peeters, A. The association between socio-ecosocio-economic and diet quality in Australian adults. Public Health Nutr. 2016, 19, 477–485. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Endo, S.; Yoshimura, I. A new whole-mouth gustatory test procedure. II. Effects of aging, gender and smoking. Acta Otolaryngol. Suppl. 2002, 546, 49–59. [Google Scholar] [CrossRef]
- Vennemann, M.M.; Hummel, T.; Berger, K. The association between smoking and smell and taste impairment in the general population. J. Neurol. 2008, 255, 1121–1126. [Google Scholar] [CrossRef]
- Albar, S.A.; Alwan, N.A.; Evans, C.E.; Cade, J.E. Is there an association between food portion size and BMI among British adolescents? Br. J. Nutr. 2014, 112, 841–851. [Google Scholar] [CrossRef]
- Levis, B.; Yan, X.W.; He, C.; Sun, Y.; Benedetti, A.; Thombs, B.D. Comparison of depression prevalence estimates in meta-analyses based on screening tools and rating scales versus diagnostic interviews: A meta-research review. BMC Med. 2019, 17, 65. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Oliveira Bierhals, I.; Gonçalves Soares, A.L.; Hellwig, N.; Tomasi, E.; Formoso Assunção, M.C.; Gonçalves, H. Interrelationship between diet quality and depressive symptoms in elderly. J. Nutr. Health Aging 2018, 22, 387–392. [Google Scholar] [CrossRef]
- Ljungberg, T.; Bondza, E.; Lethin, C. Evidence of the importance of dietary habits regarding depressive symptoms and depression. Int. J. Environ. Res. Public Health 2020, 17, 1616. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Smith, D.; Bot, M.; Brouwer, I.A.; Visser, M.; Giltay, E.J.; Penninx, B.W.J.H. Association of food groups with depression and anxiety disorders. Eur. J. Nutr. 2020, 59, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Grases, G.; Colom, M.A.; Sanchis, P.; Grases, F. Possible relation between consumption of different food groups and depression. BMC Psychol. 2019, 7, 14. [Google Scholar] [CrossRef]
- Ju, S.Y.; Park, Y.K. Low fruit and vegetable intake is associated with depression among Korean adults in data from the 2014 Korea National Health and Nutrition Examination Survey. J. Health Popul. Nutr. 2019, 38, 39. [Google Scholar] [CrossRef]
- Vermeulen, E.; Stronks, K.; Snijder, M.B.; Schene, A.H.; Lok, A.; de Vries, J.H.; Visser, M.; Brouwer, I.A.; Nicolaou, M. A combined high-sugar and high-saturated-fat dietary pattern is associated with more depressive symptoms in a multi-ethnic population: The HELIUS (Healthy Life in an Urban Setting) study. Public Health Nutr. 2017, 20, 2374–2382. [Google Scholar] [CrossRef]
- Kocot, J.; Dziemidok, P.; Kiełczykowska, M.; Hordyjewska, A.; Szcześniak, G.; Musik, I. Adipokine profile in patients with type 2 diabetes depends on degree of obesity. Med. Sci. Monit. 2017, 23, 4995–5004. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gower, B.A.; Shelton, R.C.; Wu, X. Gender-specific relationship between obesity and major depression. Front. Endocrinol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Valles, A.; Inoue, W.; Rummel, C.; Luheshi, G.N. Obesity, adipokines and neuroinflammation. Neuropharmacology 2015, 6, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Huang, T.Y.; Garza, J.C.; Chua, S.C.; Lu, X.Y. Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int. J. Neuropsychopharmacol. 2013, 16, 857–867. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Cooney, L.G.; Dokras, A. Depression and anxiety in polycystic ovary syndrome: Etiology and treatment. Curr. Psychiatry Rep. 2017, 19, 83. [Google Scholar] [CrossRef]
- Buvat, J.; Maggi, M.; Guay, A.; Torres, L.O. Testosterone deficiency in men: Systematic review and standard operating procedures for diagnosis and treatment. J. Sex Med. 2013, 10, 245–284. [Google Scholar] [CrossRef]
- Albert, P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Public Opinion Research Centre. Excessive Food Consumption. Available online: https://www.cbos.pl/EN/publications/public_opinion.php (accessed on 10 June 2023).
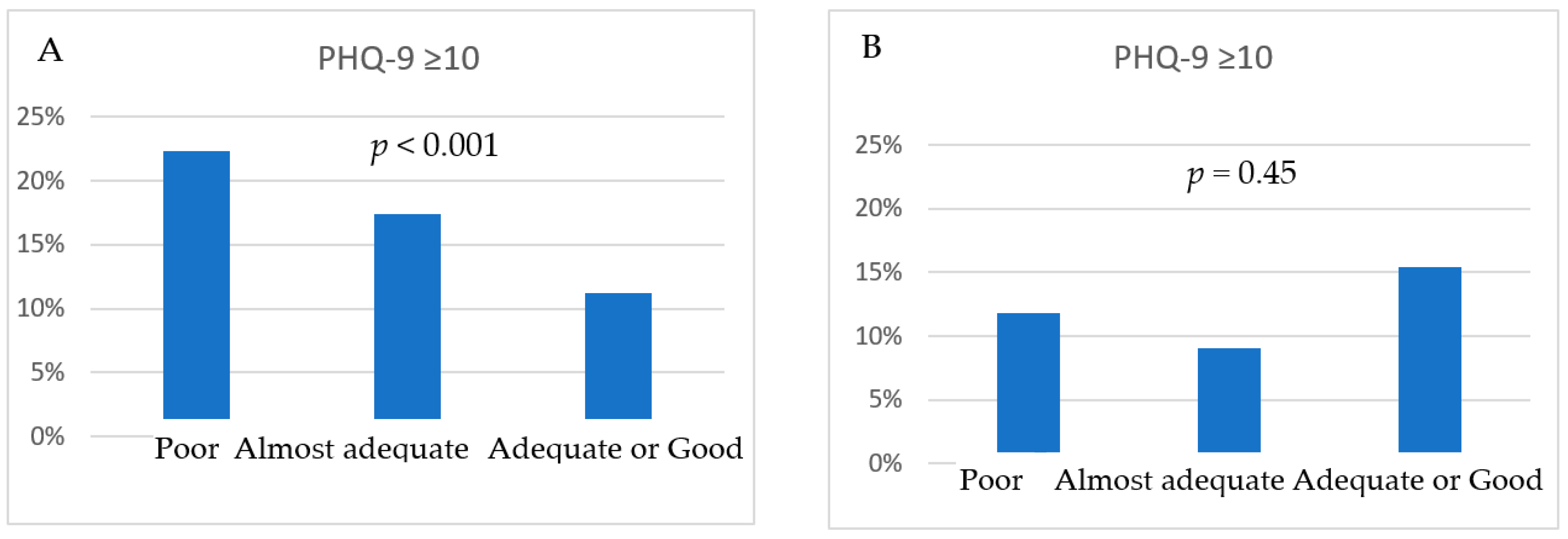
| Variables | Female (n = 2201) | Male (n = 1551) | p |
|---|---|---|---|
| n (%) | n (%) | ||
| Number of meals per day: | |||
| 4–5 meals | 1320 (60) | 659 (41.8) | <0.001 |
| 3–2 meals | 828 (37.6) | 843 (54.4) | |
| Fewer than 3 meals | 53 (2.4) | 59 (3.8) | |
| Number of meals with products providing animal protein: | |||
| In all meals | 208 (9.5) | 182 (11.7) | <0.001 |
| In 75% of meals | 942 (42.8) | 843 (54.4) | |
| In fewer meals | 1051 (47.8) | 526 (33.9) | |
| Frequency of milk or cheese: | |||
| In at least 2 meals | 239 (10.9) | 129 (8.3) | <0.001 |
| Daily, at least in 1 meal and on 50% of days in 2 meals | 802 (36.4) | 422 (27.2) | |
| Less frequently | 1160 (52.7) | 1000 (64.5) | |
| Frequency of fruit and vegetables: | |||
| Daily, at least in 3 meals | 206 (9.4) | 74 (4.8) | <0.001 |
| Daily, in at least 2 meals | 1073 (48.8) | 581 (37.5) | |
| Less frequently | 922 (41.9) | 896 (57.8) | |
| Frequency of fruit vegetables in raw form: | |||
| Daily | 675 (30.7) | 286 (18.4) | <0.001 |
| In 75% of days | 644 (29.3) | 460 (29.7) | |
| Less frequently | 882 (40.1) | 805 (51.9) | |
| Frequency of wholemeal bread, cereals and dry legumes: | |||
| Daily, at least one of the products listed | 534 (24.3) | 254 (16.4) | <0.001 |
| On 75% of days, one of the products listed | 763 (34.7) | 513 (33.1) | |
| Less frequently | 904 (41.1) | 784 (50.5) | |
| Variables | Dietary Assessment | |||||||
|---|---|---|---|---|---|---|---|---|
| Female (n = 2201) | p | Male (n = 1551) | p | |||||
| Poor Diet | Almost Adequate Diet | Adequate Diet/Good Diet | Poor Diet | Almost Adequate Diet | Adequate Diet/Good Diet | |||
| Age [years]: | 51 ± 8.02 | 52 ± 8.3 | 51 ± 8.2 | 0.17 | 52 ± 8.0 | 53 ± 8.0 | 53 ± 7.5 | 0.03 |
| Place of living: | ||||||||
| Rural areas | 858 (59.7) | 477 (33.2) | 103 (7.2) | <0.001 | 757 (70.7) | 275 (25.7) | 39 (3.6) | 0.145 |
| Urban areas | 362 (47.4) | 325 (42.6) | 76 (10.0) | 320 (66.7) | 134 (27.9) | 26 (5.4) | ||
| Marital status: | ||||||||
| Married | 1052 (54.9) | 707 (36.9) | 158 (8.2) | 0.43 | 951 (68.8) | 370 (26.8) | 62 (4.5) | 0.3 |
| Single (bachelor/bachelorette) | 76 (60.8) | 43 (34.4) | 6 (4.8) | 111 (75.5) | 34 (23.1) | 2 (1.4) | ||
| Widow/widower | 92 (57.9) | 52 (32.7) | 15 (9.4) | 15 (71.4) | 5 (23.8) | 1 (4.8) | ||
| Education: | ||||||||
| Primary | 130 (58.3) | 75 (33.6) | 18 (8.1) | 0.002 | 129 (67.9) | 54 (28.4) | 7 (3.7) | 0.27 |
| Vocation | 409 (61.3) | 212 (31.8) | 46 (6.9) | 517 (71.5) | 181 (25.0) | 25 (3.5) | ||
| Secondary | 422 (54.4) | 291 (37.5) | 63 (8.1) | 295 (68.9) | 115 (26.9) | 18 (4.2) | ||
| University | 259 (48.4) | 224 (41.9) | 52 (9.7) | 136 (64.8) | 59 (28.1) | 15 (7.1) | ||
| Smoking status: | ||||||||
| Yes | 149 (59.6) | 89 (35.6) | 12 (4.8) | 0.14 | 260 (75.4) | 77 (22.3) | 8 (2.3) | 0.035 |
| No | 868 (54.1) | 598 (37.3) | 137 (8.5) | 513 (66.5) | 222 (28.8) | 37 (4.8) | ||
| In the past | 203 (58.3) | 115 (33.0) | 30 (8.6) | 304 (70.0) | 110 (25.3) | 20 (4.6) | ||
| Living alone: | ||||||||
| Yes | 1156 (55.4) | 760 (36.5) | 169 (8.1) | 0.98 | 1031 (69.1) | 401 (26.9) | 61 (4.1) | 0.04 |
| No | 64 (55.2) | 42 (36.2) | 10 (8.6) | 46 (79.3) | 8 (13.8) | 4 (6.9) | ||
| BMI [kg/m2]: | ||||||||
| Norm | 363 (57.3) | 209 (33.0) | 61 (9.6) | 0.2 | 197 (72.4) | 68 (25.0) | 7 (2.6) | 0.31 |
| Overweight | 432 (54.4) | 302 (38.0) | 60 (7.6) | 497 (69.4) | 183 (25.6) | 36 (5.0) | ||
| Obese | 418 (54.9) | 287 (37.7) | 57 (7.5) | 381 (68.0) | 158 (28.2) | 21 (3.8) | ||
| Comorbidities: # | ||||||||
| Yes | 344 (52.0) | 263 (39.8) | 54 (8.2) | 0.088 | 277 (64.9) | 128 (30.0) | 22 (5.2) | 0.045 |
| No | 876 (56.9) | 539 (35.0) | 125 (8.1) | 800 (71.2) | 281 (25.0) | 43 (3.8) | ||
| Dietary Assessment | Women | Men | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | ||
| Model A | Good diet/Adequate diet | 1 | 1 | ||||
| Almost adequate diet | 1.64 | (0.99–2.71) | 0.05 | 0.54 | (0.25–1.15) | 0.11 | |
| Poor diet | 2.29 | (1.41–3.72) | <0.001 | 0.76 | (0.38–1.53) | 0.44 | |
| Model B | Good diet/Adequate diet | 1 | |||||
| Almost adequate diet | 1.64 | (0.99–2.71) | 0.06 | 0.54 | (0.25–1.16) | 0.12 | |
| Poor diet | 2.16 | 1.33–3.53) | 0.002 | 0.7 | (0.34–1.43) | 0.33 | |
| Model C | Good diet/Adequate diet | 1 | |||||
| Almost adequate diet | 1.62 | (0.98–2.689) | 0.002 | 0.54 | (0.25–1.18) | 0.12 | |
| Poor diet | 2.18 | (1.39–3.56) | 0.002 | 0.73 | (0.35–1.50) | 0.39 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicki, G.J.; Polak, M.; Ślusarska, B.; Czernecki, K. The Relationship between Diet and the Occurrence of Depressive Symptoms in a Community Example with High Rates of Social Deprivation: A Cross-Sectional Study. Nutrients 2023, 15, 3778. https://doi.org/10.3390/nu15173778
Nowicki GJ, Polak M, Ślusarska B, Czernecki K. The Relationship between Diet and the Occurrence of Depressive Symptoms in a Community Example with High Rates of Social Deprivation: A Cross-Sectional Study. Nutrients. 2023; 15(17):3778. https://doi.org/10.3390/nu15173778
Chicago/Turabian StyleNowicki, Grzegorz Józef, Maciej Polak, Barbara Ślusarska, and Karol Czernecki. 2023. "The Relationship between Diet and the Occurrence of Depressive Symptoms in a Community Example with High Rates of Social Deprivation: A Cross-Sectional Study" Nutrients 15, no. 17: 3778. https://doi.org/10.3390/nu15173778
APA StyleNowicki, G. J., Polak, M., Ślusarska, B., & Czernecki, K. (2023). The Relationship between Diet and the Occurrence of Depressive Symptoms in a Community Example with High Rates of Social Deprivation: A Cross-Sectional Study. Nutrients, 15(17), 3778. https://doi.org/10.3390/nu15173778










