Effect of Moderate-Intense Training and Detraining on Glucose Metabolism, Lipid Profile, and Liver Enzymes in Male Wistar Rats: A Preclinical Randomized Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Animal Care
2.2. Sample Size Calculation
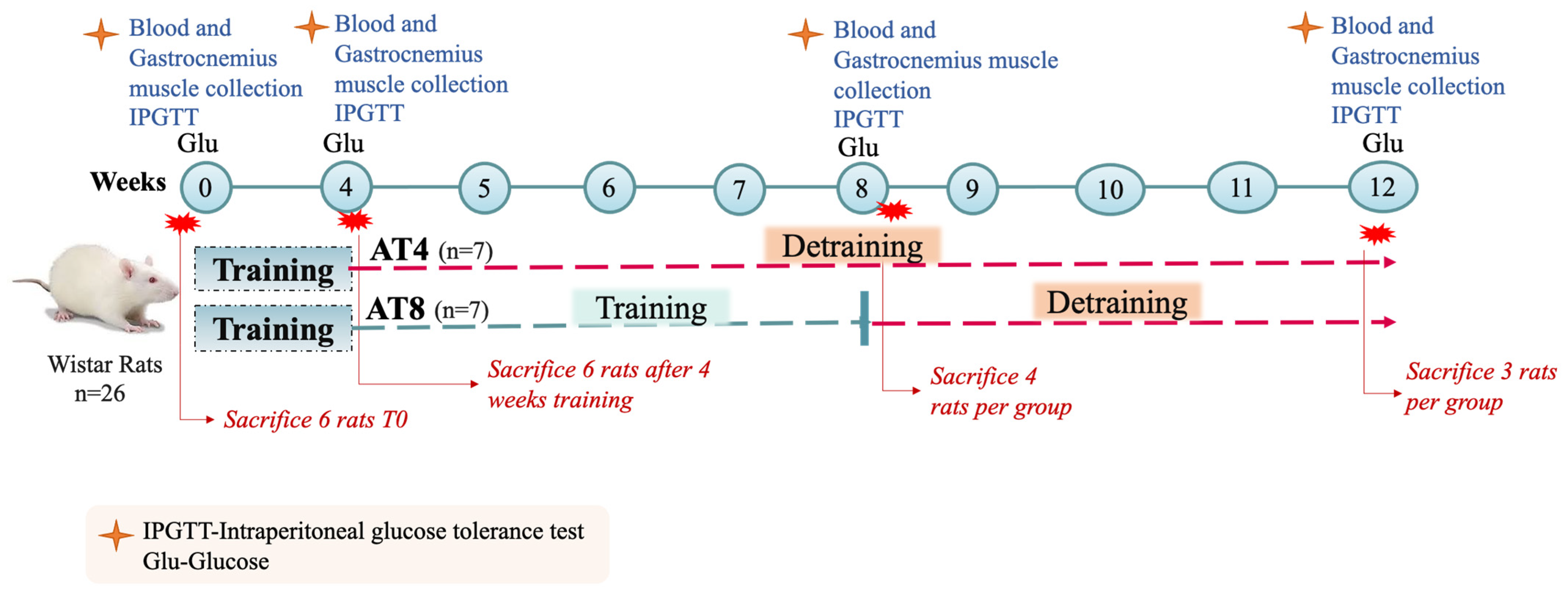
2.3. Training and Experimental Protocol
2.4. Blood Collection
2.5. Feed Intake, Body Weight, and Blood Glucose
2.6. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.7. Lipid Profile, Glucose, and Liver Enzyme
2.8. Serum Insulin
2.9. Enzymatic Activity
2.10. Muscle Glycogen and Pyruvate
2.11. Lactate
2.12. Immunoblot Analysis
2.13. Statistical Analysis
3. Results
3.1. Body Weight
3.2. Feed Intake
3.3. Intraperitoneal Glucose Tolerance Test (IPGTT)
3.4. Serum Glucose
3.5. Serum Insulin
3.6. Serum Lipid Profiles
3.6.1. Serum Total Cholesterol Concentration
3.6.2. Serum Triglycerides Concentration
3.6.3. Serum Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) Concentration
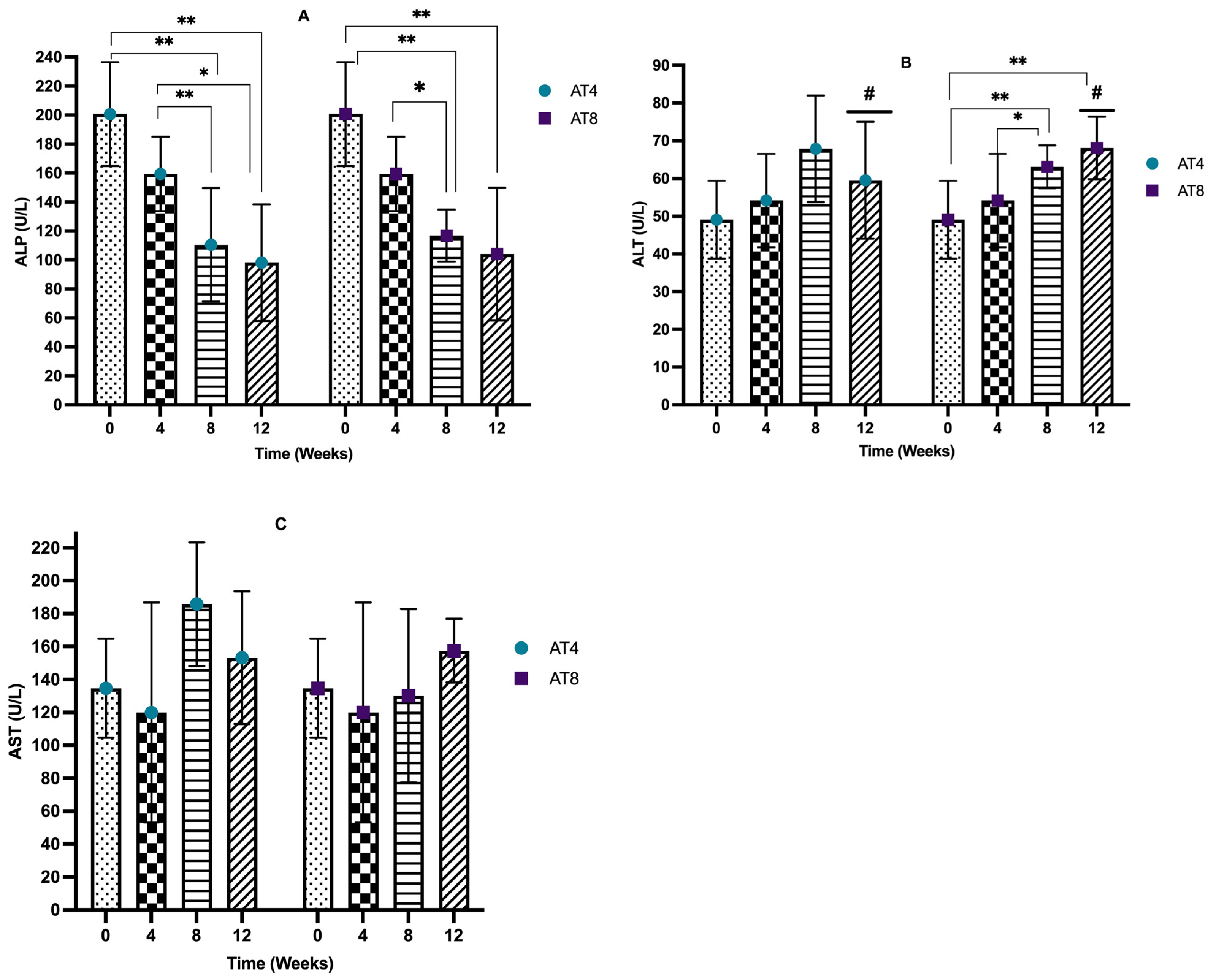
3.7. Serum Liver Enzymes
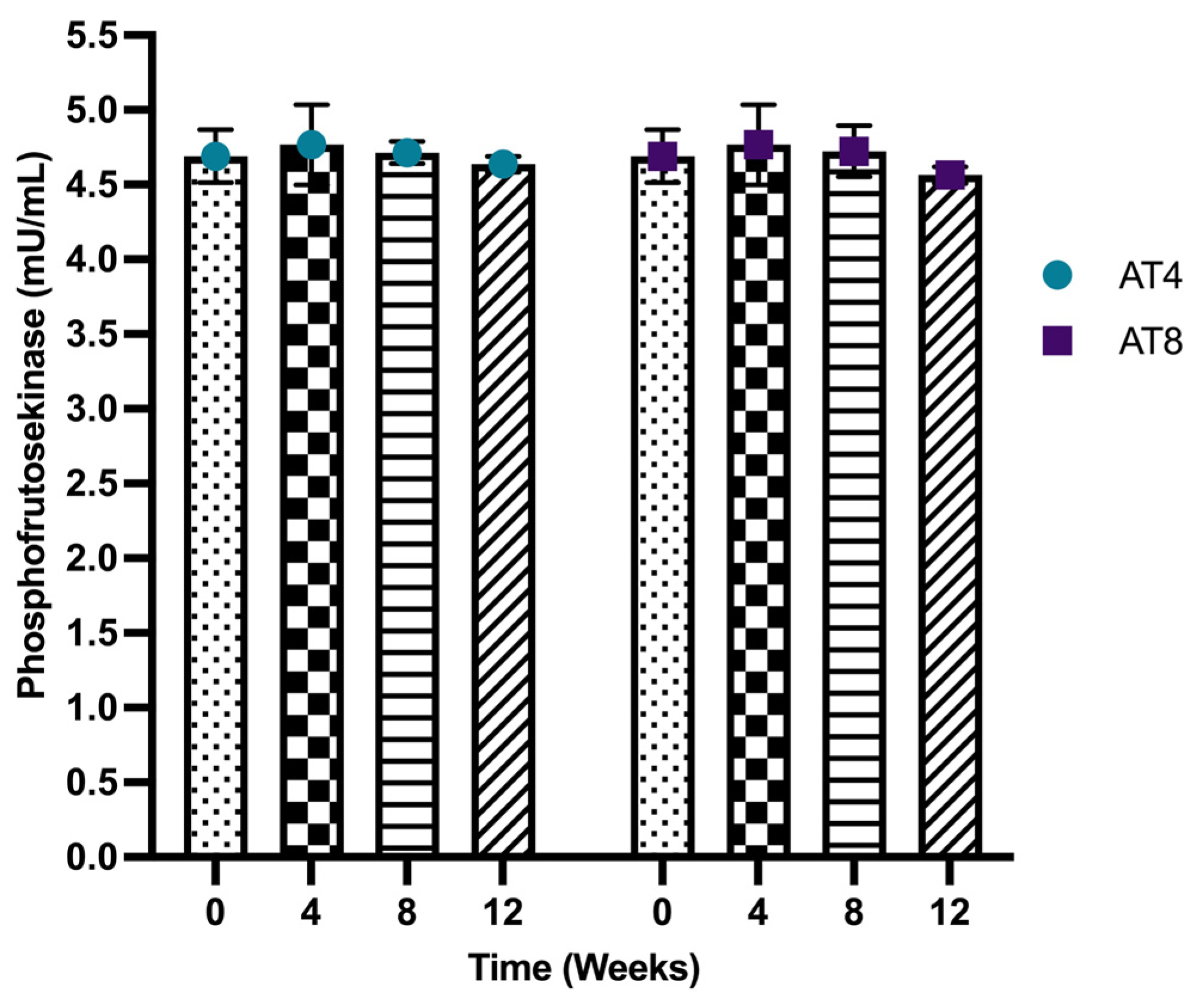
3.8. Phosphofructokinase (PFK)
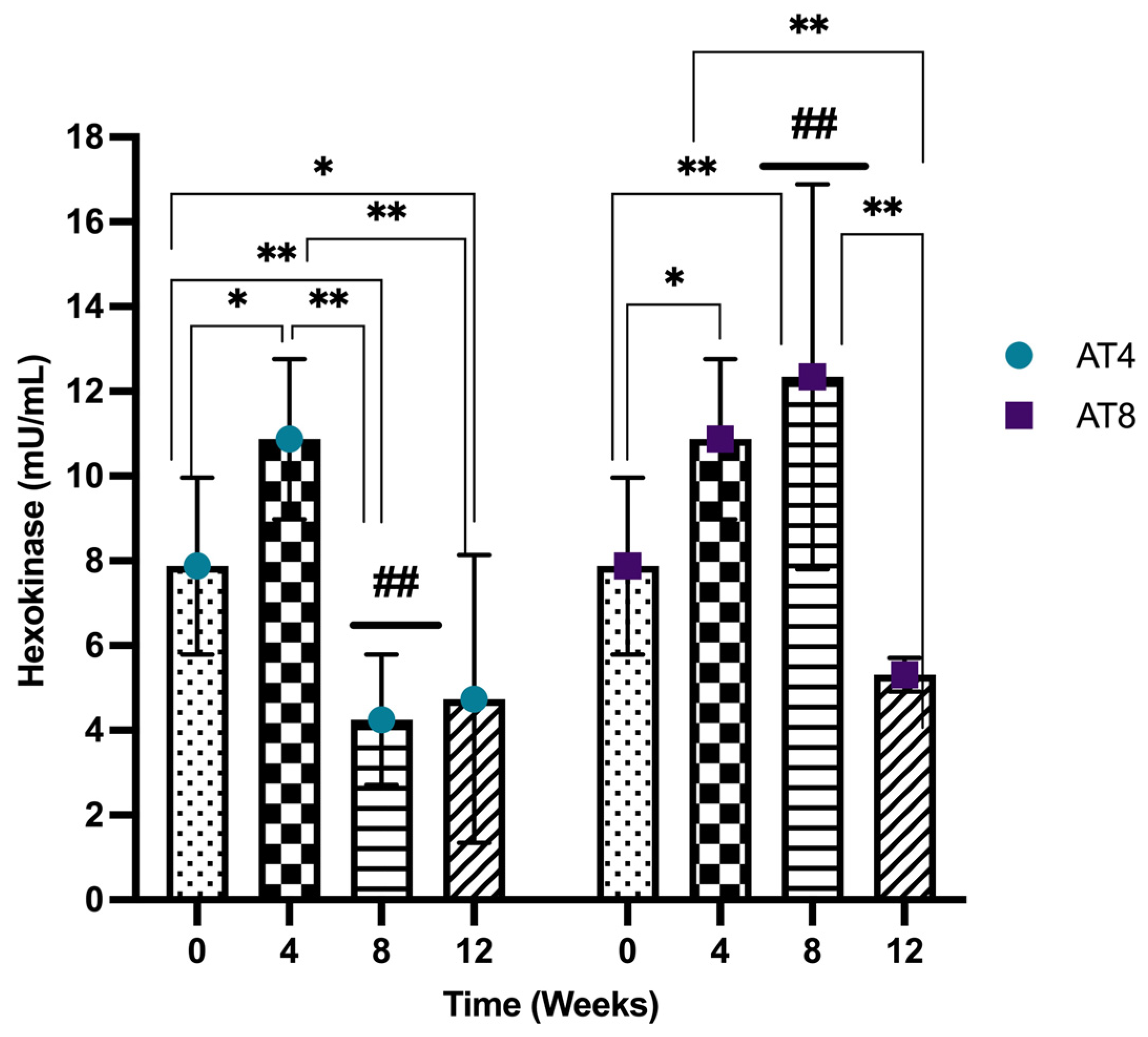
3.9. Hexokinase (HK)
3.10. Pyruvate
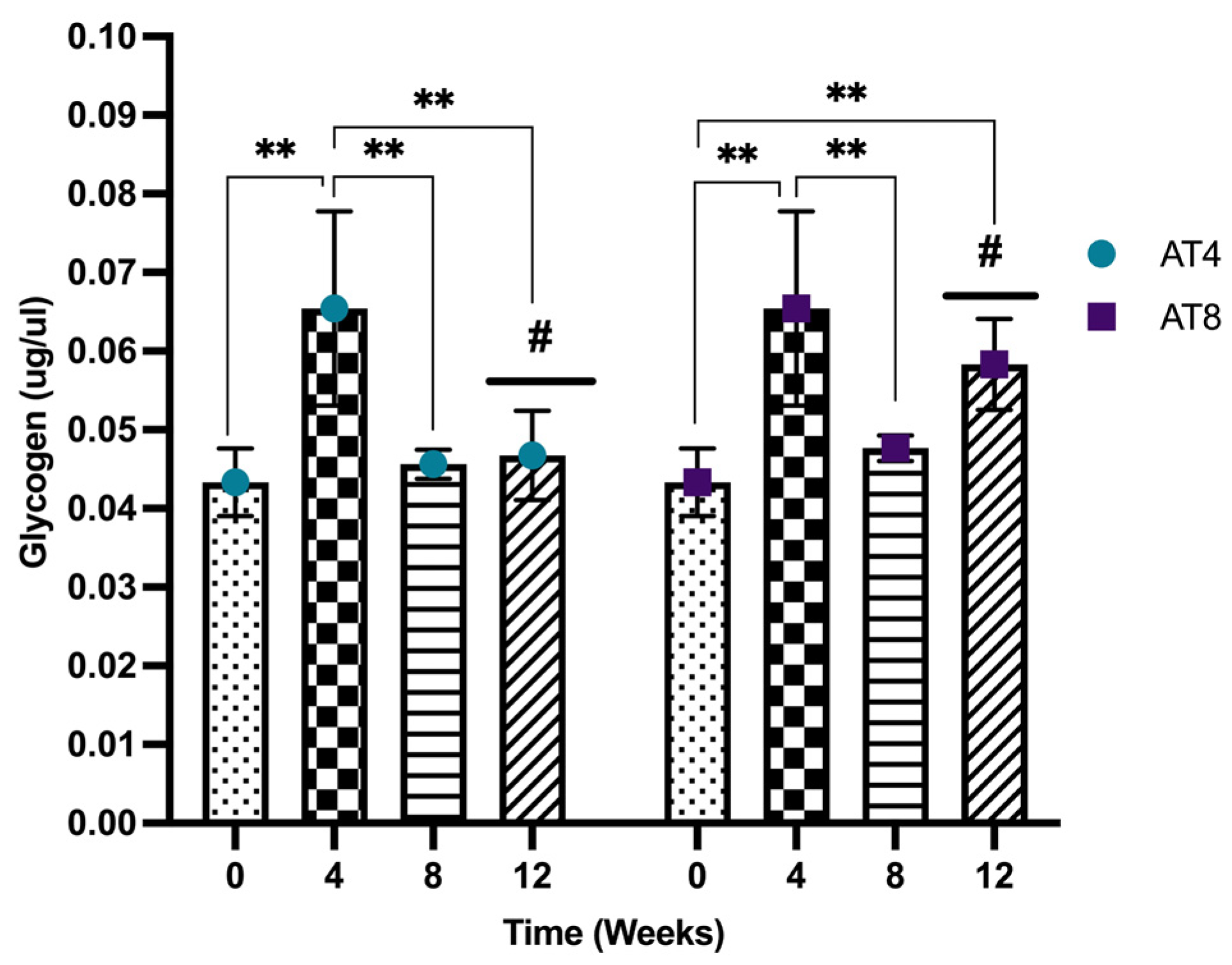
3.11. Glycogen
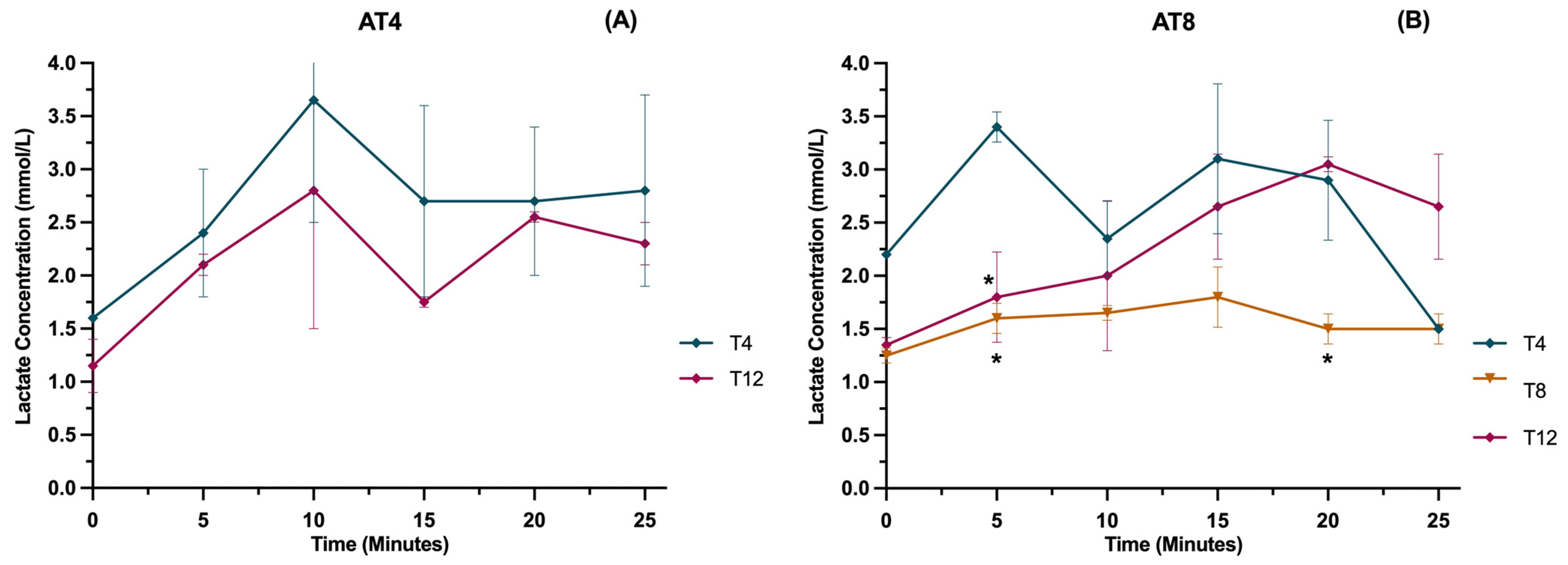
3.12. Lactate
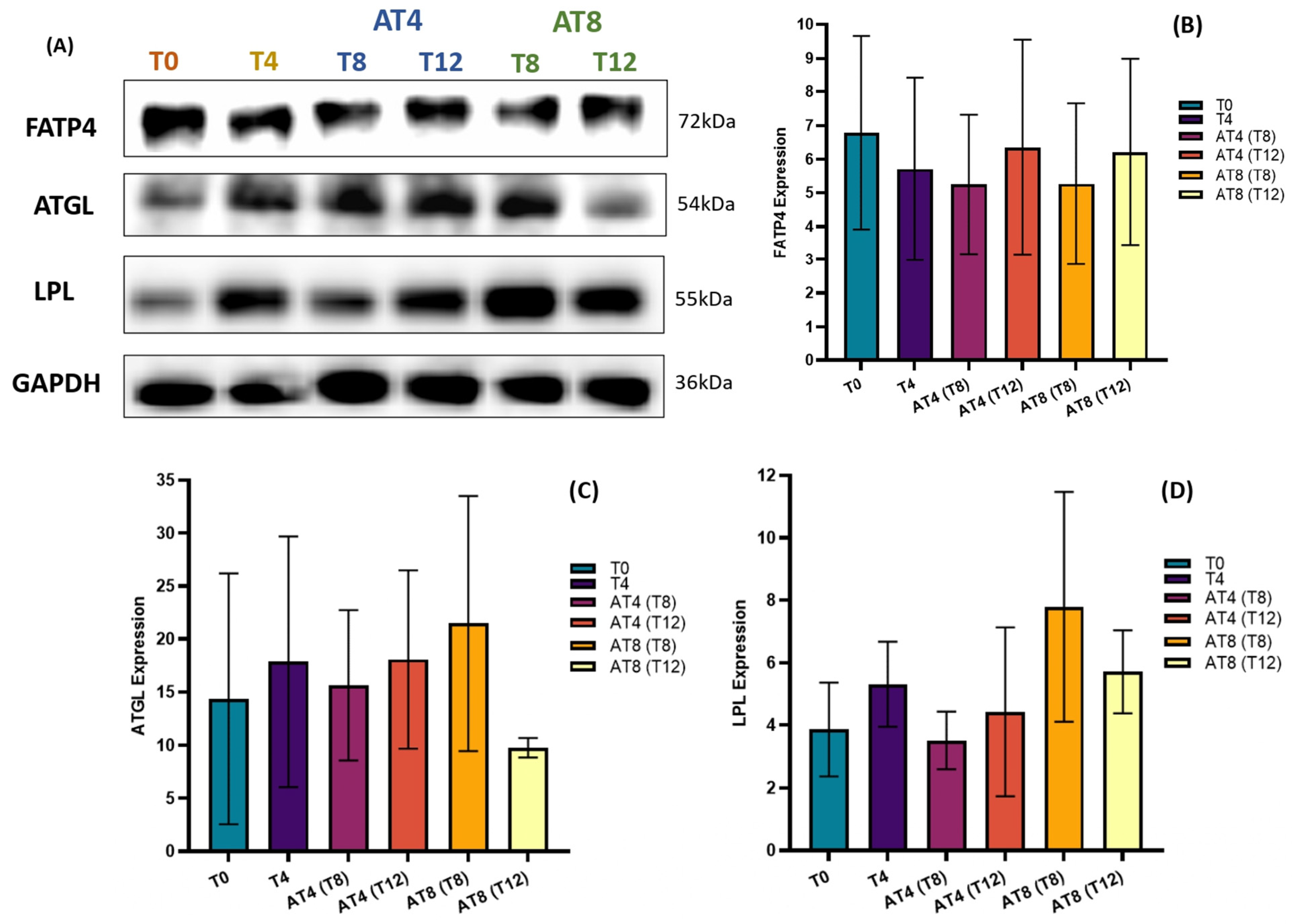
3.13. Protein Expressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization (WHO). Physical Inactivity. 2021. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3416 (accessed on 21 June 2023).
- Helmrich, S.; Ragland, D.; Paffenbarger, R. Prevention of non—Insulin-dependent diabetes mellitus with physical activity. Med. Sci. Sports Exerc. 1994, 26, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M. Physical activity and cancer prevention—Data from epidemiologic studies. Med. Sci. Sports Exerc. 2003, 35, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Activity. 2020. Available online: https://www.who.int/health-topics/physical-activity#tab=tab_1 (accessed on 14 May 2023).
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Apostolopoulos, V.; Feehan, J.; Ali, H.I.; Ismail, L.C.; Al Dhaheri, A.S.O.S.; Stojanovska, L. Effect of Calorie Restriction and Exercise on Type 2 Diabetes. Prilozi (Makedon. Akad. Nauk. Umet. Odd. Med. Nauki.) 2021, 42, 109–126. [Google Scholar] [CrossRef]
- Baba, C.S.; Alexander, G.; Kalyani, B.; Pandey, R.; Rastogi, S.; Pandey, A.; Choudhuri, G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2006, 21, 191–198. [Google Scholar] [CrossRef]
- Guo, R.; Liong, E.C.; So, K.F.; Fung, M.-L.; Tipoe, G.L. Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 139–144. [Google Scholar] [CrossRef]
- Georgakouli, K.; Manthou, E.; Fatouros, I.G.; Deli, C.K.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D.; Koutedakis, Y.; Theodorakis, Y.; Jamurtas, A.Z. Effects of acute exercise on liver function and blood redox status in heavy drinkers. Exp. Ther. Med. 2015, 10, 2015–2022. [Google Scholar] [CrossRef]
- Smart, N.A.; King, N.; McFarlane, J.R.; Graham, P.L.; Dieberg, G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 834–843. [Google Scholar] [CrossRef]
- Costill, D.L.; Coyle, E.F.; Fink, W.F.; Lesmes, G.R.; Witzmann, F.A. Adaptations in skeletal muscle following strength training. J. Appl. Physiol. 1979, 46, 96–99. [Google Scholar] [CrossRef]
- Roberts, A.D.; Billeter, R.; Howald, H. Anaerobic muscle enzyme changes after interval training. Int. J. Sports Med. 1982, 3, 18–21. [Google Scholar] [CrossRef]
- Simoneau, J.A.; Lortie, G.; Boulay, M.R.; Marcotte, M.; Thibault, M.C.; Bouchard, C. Effects of two high-intensity intermittent training programs interspaced by detraining on human skeletal muscle and performance. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Evertsen, F.; Medbo, J.I.; Jebens, E.; Gjovaag, T.F. Effect of training on the activity of five muscle enzymes studied on elite cross-country skiers. Acta Physiol. Scand. 1999, 167, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, H.; Andersen, P.H.; Lund, S.; Schmitz, O.; Junker, S.; Pedersen, O. Pre-and posttranslational upregulation of muscle-specific glycogen synthase in athletes. Am. J. Physiol.-Endocrinol. Metab. 1994, 266, E92–E101. [Google Scholar] [CrossRef] [PubMed]
- Pilegaard, H.; Keller, C.; Steensberg, A.; Wulff Helge, J.; Klarlund Pedersen, B.; Saltin, B.; Neufer, P.D. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J. Physiol. 2002, 541, 261–271. [Google Scholar] [CrossRef]
- Pilegaard, H.; Ordway, G.A.; Saltin, B.; Neufer, P.D. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am. J. Physiol.-Endocrinol. Metab. 2000, 279, E806–E814. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Simon, J.; Huang, T.Y.; Batdorf, H.; Scott, M.; Waskom, C.; Essajee, N.; Burke, S.; Collier, J.; Noland, R. Moderate Intensity Endurance Exercise Training Increases Muscle Glycogen Content but Does Not Alter Substrate Oxidation in C57BL6 Mice. FASEB J. 2018, 32, 855.30. [Google Scholar] [CrossRef]
- Morville, T.; Rosenkilde, M.; Munch-Andersen, T.; Andersen, P.R.; Groenbæk, K.K.; Helbo, S.; Kristensen, M.; Hansen, A.V.; Mattsson, N.; Rasmusen, H.K. Repeated prolonged exercise decreases maximal fat oxidation in older men. Med. Sci. Sports Exerc. 2017, 49, 308–316. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Detraining: Loss of training-induced physiological and performance adaptations. Part I. Sports Med. 2000, 30, 79–87. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Detraining: Loss of training-induced physiological and performance adaptations. Part II. Sports Med. 2000, 30, 145–154. [Google Scholar] [CrossRef]
- Neufer, P.D.; Shinebarger, M.H.; Dohm, G.L. Effect of training and detraining on skeletal muscle glucose transporter (GLUT4) content in rats. Can. J. Physiol. Pharmacol. 1992, 70, 1286–1290. [Google Scholar] [CrossRef]
- King, D.S.; Dalsky, G.P.; Clutter, W.E.; Young, D.A.; Staten, M.A.; Cryer, P.E.; Holloszy, J.O. Effects of exercise and lack of exercise on insulin sensitivity and responsiveness. J. Appl. Physiol. 1988, 64, 1942–1946. [Google Scholar] [CrossRef] [PubMed]
- Petibois, C.; Cassaigne, A.; Gin, H.; Déléris, G. Lipid profile disorders induced by long-term cessation of physical activity in previously highly endurance-trained subjects. J. Clin. Endocrinol. Metab. 2004, 89, 3377–3384. [Google Scholar] [CrossRef] [PubMed]
- Ratel, S.; Gryson, C.; Rance, M.; Penando, S.; Bonhomme, C.; Le Ruyet, P.; Duclos, M.; Boirie, Y.; Walrand, S. Detraining-induced alterations in metabolic and fitness markers after a multicomponent exercise-training program in older men. Appl. Physiol. Nutr. Metab. 2012, 37, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Blanc, S.P.; Normand, S.; Pachiaudi, C.; Fortrat, J.-O.; Laville, M.; Gharib, C. Fuel homeostasis during physical inactivity induced by bed rest. J. Clin. Endocrinol. Metab. 2000, 85, 2223–2233. [Google Scholar] [CrossRef]
- Dolkas, C.; Greenleaf, J. Insulin and glucose responses during bed rest with isotonic and isometric exercise. J. Appl. Physiol. 1977, 43, 1033–1038. [Google Scholar] [CrossRef]
- Lipman, R.L.; Schnure, J.J.; Bradley, E.M.; Lecocq, F.R. Impairment of peripheral glucose utilization in normal subjects by prolonged bed rest. J. Lab. Clin. Med. 1970, 76, 221–230. [Google Scholar]
- Mikines, K.J.; Richter, E.A.; Dela, F.; Galbo, H. Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. J. Appl. Physiol. 1991, 70, 1245–1254. [Google Scholar] [CrossRef]
- Stuart, C.A.; Shangraw, R.E.; Prince, M.J.; Peters, E.J.; Wolfe, R.R. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism 1988, 37, 802–806. [Google Scholar] [CrossRef]
- Lipman, R.L.; Raskin, P.; Love, T.; Triebwasser, J.; Lecocq, F.R.; Schnure, J.J. Glucose intolerance during decreased physical activity in man. Diabetes 1972, 21, 101–107. [Google Scholar] [CrossRef]
- Mankowitz, K.; Seipa, R.; Semenkovich, C.F.; Daugherty, A.; Schonfeld, G. Short-term interruption of training affects both fasting and post-prandial lipoproteins. Atherosclerosis 1992, 95, 181–189. [Google Scholar] [CrossRef]
- Barzegarzadeh, H.; Dabidi, R.V. Effects of a 12-Week aerobic Training Course Followed by a 4-Week Detraining Period on Alanine Aminotransferase, Aspartate Aminotransferase, Alkaline Phosphatase and Blood Lipids Level Changes in Menopausal Rats. J. Rafsanjan Univ. Med. Sci. 2012, 11, 207–218. [Google Scholar]
- Ravussin, E. Energy metabolism in obesity: Studies in the Pima Indians. Diabetes Care 1993, 16, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Wendorf, M.; Goldfine, I.D. Archaeology of NIDDM: Excavation of the “thrifty” genotype. Diabetes 1991, 40, 161–165. [Google Scholar] [CrossRef]
- O’Kane, M.; Parretti, H.M.; Pinkney, J.; Welbourn, R.; Hughes, C.A.; Mok, J.; Walker, N.; Thomas, D.; Devin, J.; Coulman, K.D. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery—2020 update. Obes. Rev. 2020, 21, e13087. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Vogel, R.; Lavie, C.J.; Cordain, L. Exercise like a hunter-gatherer: A prescription for organic physical fitness. Prog. Cardiovasc. Dis. 2011, 53, 471–479. [Google Scholar] [CrossRef]
- Center for disease control and prevention (CDC). Physical Activity and Health: A Report of the Surgeon General; US Department of Health and Human Services: Washington, DC, USA, 1996. Available online: https://www.cdc.gov/nccdphp/sgr/index.htm (accessed on 2 May 2023).
- Eaton, S.B.; Konner, M. Paleolithic nutrition: A consideration of its nature and current implications. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef]
- Gastebois, C.; Chanon, S.; Rome, S.; Durand, C.; Pelascini, E.; Jalabert, A.; Euthine, V.; Pialoux, V.; Blanc, S.; Simon, C. Transition from physical activity to inactivity increases skeletal muscle miR-148b content and triggers insulin resistance. Physiol. Rep. 2016, 4, e12902. [Google Scholar] [CrossRef]
- Laye, M.J.; Rector, R.S.; Borengasser, S.J.; Naples, S.P.; Uptergrove, G.M.; Ibdah, J.A.; Booth, F.W.; Thyfault, J.P. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J. Appl. Physiol. 2009, 106, 161–168. [Google Scholar] [CrossRef]
- Bergouignan, A.; Momken, I.; Lefai, E.; Antoun, E.; Schoeller, D.A.; Platat, C.; Chery, I.; Zahariev, A.; Vidal, H.; Gabert, L. Activity energy expenditure is a major determinant of dietary fat oxidation and trafficking, but the deleterious effect of detraining is more marked than the beneficial effect of training at current recommendations. Am. J. Clin. Nutr. 2013, 98, 648–658. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. MJMS 2017, 24, 101. [Google Scholar]
- Lutwak, L.; Whedon, G.D. The effect of physical conditioning on glucose tolerance. Clin. Res. 1959, 7, 143–144. [Google Scholar]
- Abdolmaleki, F.; Heidarianpour, A. The response of serum Glypican-4 levels and its potential regulatory mechanism to endurance training and chamomile flowers’ hydroethanolic extract in streptozotocin–nicotinamide-induced diabetic rats. Acta Diabetol. 2018, 55, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Omidi, M.; Yousefi, M. The effect of 8 weeks of aerobic exercise and 4 weeks detraining on serum fast blood sugar, insulin and glycosylated hemoglobin in. J. Pract. Stud. Biosci. Sport 2019, 7, 55–64. [Google Scholar]
- Eizadi, M.; Bagheri, G.; Kasparast, J.M.; Zahedmanesh, F.; Afsharmand, Z. Effects of training on body composition, blood lipids, and glucose homeostasis assessed by the homeostasis model assessment. Sci. Sports 2013, 28, 75–80. [Google Scholar] [CrossRef]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef]
- Akçakoyun, F. Changes in serum lipid profile following moderate exercise. Afr. J. Pharm. Pharmacol. 2010, 4, 829–833. [Google Scholar]
- Banz, W.J.; Maher, M.A.; Thompson, W.G.; Bassett, D.R.; Moore, W.; Ashraf, M.; Keefer, D.J.; Zemel, M.B. Effects of resistance versus aerobic training on coronary artery disease risk factors. Exp. Biol. Med. 2003, 228, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S. Effects of aerobic exercise on lipids and lipoproteins in adults with type 2 diabetes: A meta-analysis of randomized-controlled trials. Public Health 2007, 121, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, S.J.; Yousefi, M.S. The effect of interval recovery periods during HIIT on liver enzymes and lipid profile in overweight women. Sci. Sports 2015, 30, 147–154. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- LeMura, L.M.; von Duvillard, S.P.; Andreacci, J.; Klebez, J.M.; Chelland, S.A.; Russo, J. Lipid and lipoprotein profiles, cardiovascular fitness, body composition, and diet during and after resistance, aerobic and combination training in young women. Eur. J. Appl. Physiol. 2000, 82, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Sertie, R.A.L.; Andreotti, S.; Proença, A.R.G.; Campana, A.B.; Lima-Salgado, T.M.; Batista, M.L., Jr.; Seelaender, M.C.L.; Curi, R.; Oliveira, A.C.; Lima, F.B. Cessation of physical exercise changes metabolism and modifies the adipocyte cellularity of the periepididymal white adipose tissue in rats. J. Appl. Physiol. 2013, 115, 394–402. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; Van Loon, L.J.C. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef]
- Zhao, X.-G.; Huang, H.-M.; Du, C.-Y. Effect of a combination of aerobic exercise and dietary modification on liver function in overweight and obese men. J. Mens Health 2021, 17, 176–182. [Google Scholar]
- Hanley, A.J.; Wagenknecht, L.E.; Festa, A.; D’Agostino Jr, R.B.; Haffner, S.M. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: The Insulin Resistance Atherosclerosis Study. Diabetes care 2007, 30, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Hazar, S.; Hazar, M.; Korkmaz, Ş.; Bayil, S.; Gürkan, A.C. The effect of graded maximal aerobic exercise on some metabolic hormones, muscle damage and some metabolic end products in sportsmen. Sci. Res. Essays 2011, 6, 1337–1343. [Google Scholar]
- Cho, N.H.; Jang, H.C.; Choi, S.H.; Kim, H.R.; Lee, H.K.; Chan, J.C.; Lim, S. Abnormal liver function test predicts type 2 diabetes: A community-based prospective study. Diabetes Care 2007, 30, 2566–2568. [Google Scholar] [CrossRef]
- O’Doherty, R.M.; Bracy, D.P.; Osawa, H.; Wasserman, D.H.; Granner, D.K. Rat skeletal muscle hexokinase II mRNA and activity are increased by a single bout of acute exercise. Am. J. Physiol.-Endocrinol. Metab. 1994, 266, E171–E178. [Google Scholar] [CrossRef]
- Koval, J.A.; DeFronzo, R.A.; O’Doherty, R.M.; Printz, R.; Ardehali, H.; Granner, D.K.; Mandarino, L.J. Regulation of hexokinase II activity and expression in human muscle by moderate exercise. Am. J. Physiol.-Endocrinol. Metab. 1998, 274, E304–E308. [Google Scholar] [CrossRef] [PubMed]
- Biensø, R.S.; Olesen, J.; Gliemann, L.; Schmidt, J.F.; Matzen, M.S.; Wojtaszewski, J.F.P.; Hellsten, Y.; Pilegaard, H. Effects of exercise training on regulation of skeletal muscle glucose metabolism in elderly men. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 866–872. [Google Scholar] [CrossRef]
- Biensø, R.S.; Ringholm, S.; Kiilerich, K.; Aachmann-Andersen, N.-J.; Krogh-Madsen, R.; Guerra, B.; Plomgaard, P.; Van Hall, G.; Treebak, J.T.; Saltin, B. GLUT4 and glycogen synthase are key players in bed rest–induced insulin resistance. Diabetes 2012, 61, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Fell, R.D.; Terblanche, S.E.; Ivy, J.L.; Young, J.C.; Holloszy, J.O. Effect of muscle glycogen content on glucose uptake following exercise. J. Appl. Physiol. 1982, 52, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Bogardus, C.; Thuillez, P.; Ravussin, E.; Vasquez, B.; Narimiga, M.; Azhar, S. Effect of muscle glycogen depletion on in vivo insulin action in man. J. Clin. Investig. 1983, 72, 1605–1610. [Google Scholar] [CrossRef]
- Blomstrand, E.; Saltin, B. Effect of muscle glycogen on glucose, lactate and amino acid metabolism during exercise and recovery in human subjects. J. Physiol. 1999, 514, 293. [Google Scholar] [CrossRef] [PubMed]
- Weltan, S.M.; Bosch, A.N.; Dennis, S.C.; Noakes, T.D. Preexercise muscle glycogen content affects metabolism during exercise despite maintenance of hyperglycemia. Am. J. Physiol.-Endocrinol. Metab. 1998, 274, E83–E88. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sarkar, J.; Yamada, J.; Matsunaga, Y.; Nonaka, Y.; Banjo, M.; Sakaguchi, R.; Shinya, T.; Hatta, H. Enhanced skeletal muscle glycogen repletion after endurance exercise is associated with higher plasma insulin and skeletal muscle hexokinase 2 protein levels in mice: Comparison of level running and downhill running model. J. Physiol. Biochem. 2021, 77, 469–480. [Google Scholar] [CrossRef]
- Ryan, B.J.; Schleh, M.W.; Ahn, C.; Ludzki, A.C.; Gillen, J.B.; Varshney, P.; Van Pelt, D.W.; Pitchford, L.M.; Chenevert, T.L.; Gioscia-Ryan, R.A. Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. J. Clin. Endocrinol. Metab. 2020, 105, e2941–e2959. [Google Scholar] [CrossRef]
- Henriksson, J.; Reitman, J.S. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol. Scand. 1976, 97, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ranallo, R.F.; Rhodes, E.C. Lipid metabolism during exercise. Sports Med. 1998, 26, 29–42. [Google Scholar] [CrossRef]
- Zierler, K.L. Fatty acids as substrates for heart and skeletal muscle. Circ. Res. 1976, 38, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Pirahanchi, Y.; Anoruo, M.; Sharma, S. Biochemistry, Lipoprotein Lipase; StatPearls: Orlando, FL, USA, 2021. [Google Scholar]
- Mohammad, P.; Esfandiar, K.Z.; Abbas, S.; Ahoora, R. Effects of moderate-intensity continuous training and high-intensity interval training on serum levels of resistin, chemerin and liver enzymes in streptozotocin-nicotinamide induced type-2 diabetic rats. J. Diabetes Metab. Disord. 2019, 18, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Bey, L.; Hamilton, M.T. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: A molecular reason to maintain daily low-intensity activity. J. Physiol. 2003, 551, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Trites, M.J.; Clugston, R.D. The role of adipose triglyceride lipase in lipid and glucose homeostasis: Lessons from transgenic mice. Lipids Health Dis. 2019, 18, 204. [Google Scholar] [CrossRef]
- Alsted, T.J.; Nybo, L.; Schweiger, M.; Fledelius, C.; Jacobsen, P.; Zimmermann, R.; Zechner, R.; Kiens, B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E445–E453. [Google Scholar] [CrossRef] [PubMed]
- Kiens, B.; Lithell, H.; Mikines, K.J.; Richter, E.A. Effects of insulin and exercise on muscle lipoprotein lipase activity in man and its relation to insulin action. J. Clin. Investig. 1989, 84, 1124–1129. [Google Scholar] [CrossRef]
- Seip, R.L.; Angelopoulos, T.J.; Semenkovich, C.F. Exercise induces human lipoprotein lipase gene expression in skeletal muscle but not adipose tissue. Am. J. Physiol.-Endocrinol. Metab. 1995, 268, E229–E236. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, P.C.; Longo, A.B.; Ramos, S.V.; Roy, B.D.; Ward, W.E.; Peters, S.J. Increases in skeletal muscle ATGL and its inhibitor G0S2 following 8 weeks of endurance training in metabolically different rat skeletal muscles. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R125–R133. [Google Scholar] [CrossRef]
- International Diabetes Federation (IDF). IDF Diabetes Atlas 2021, 10th ed.; International Diabetes Federation (IDF): Brussels, Belgium, 2021; Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 11 June 2023).







| T0 | T4 | T8 | T12 | ||
|---|---|---|---|---|---|
| Body Weight | AT4 | 170.03 ± 20.80 | 258.05 ± 17.34 a | 324.30 ± 36.34 ab | 351.40 ± 46.86 ab |
| AT8 | 173.77 ± 27.71 | 258.25 ± 13.63 a | 330.16 ± 22.26 ab | 356.57 ± 8.86 ab | |
| Feed Intake | AT4 | 24.35 ± 3.18 | 25.86 ± 4.47 | 23.73 ± 2.30 | 21.41 ± 1.33 |
| AT8 | 24.35 ± 3.18 | 25.86 ± 4.47 | 23.39 ± 4.43 | 23.33 ± 1.85 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakoor, H.; Kizhakkayil, J.; Khalid, M.; Mahgoub, A.; Platat, C. Effect of Moderate-Intense Training and Detraining on Glucose Metabolism, Lipid Profile, and Liver Enzymes in Male Wistar Rats: A Preclinical Randomized Study. Nutrients 2023, 15, 3820. https://doi.org/10.3390/nu15173820
Shakoor H, Kizhakkayil J, Khalid M, Mahgoub A, Platat C. Effect of Moderate-Intense Training and Detraining on Glucose Metabolism, Lipid Profile, and Liver Enzymes in Male Wistar Rats: A Preclinical Randomized Study. Nutrients. 2023; 15(17):3820. https://doi.org/10.3390/nu15173820
Chicago/Turabian StyleShakoor, Hira, Jaleel Kizhakkayil, Mariyam Khalid, Amar Mahgoub, and Carine Platat. 2023. "Effect of Moderate-Intense Training and Detraining on Glucose Metabolism, Lipid Profile, and Liver Enzymes in Male Wistar Rats: A Preclinical Randomized Study" Nutrients 15, no. 17: 3820. https://doi.org/10.3390/nu15173820
APA StyleShakoor, H., Kizhakkayil, J., Khalid, M., Mahgoub, A., & Platat, C. (2023). Effect of Moderate-Intense Training and Detraining on Glucose Metabolism, Lipid Profile, and Liver Enzymes in Male Wistar Rats: A Preclinical Randomized Study. Nutrients, 15(17), 3820. https://doi.org/10.3390/nu15173820







