Protective Effects of Gnetin C from Melinjo Seed Extract against High-Fat Diet-Induced Hepatic Steatosis and Liver Fibrosis in NAFLD Mice Model
Abstract
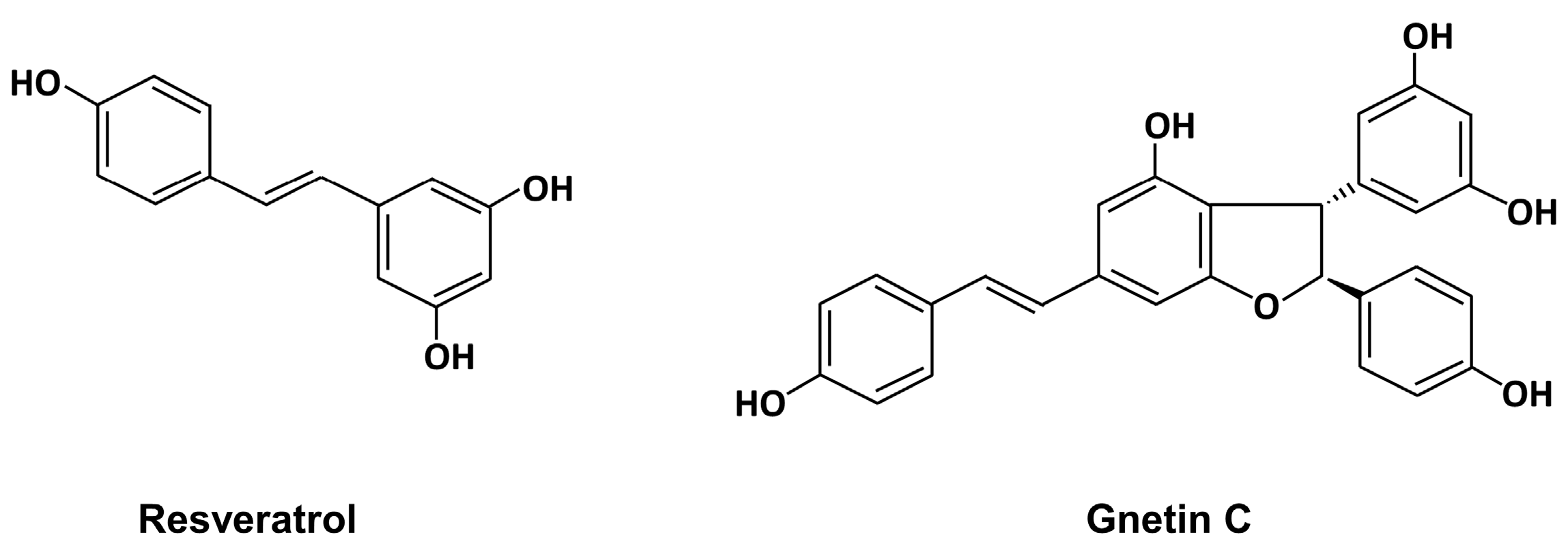
:1. Introduction
2. Materials and Methods
2.1. Preparation of MSE
2.2. Animals and Study Design
2.3. Blood Glucose, Insulin Tolerance Test (ITT), and Plasma Biochemical Parameters
2.4. Measurement of Hepatic Lipid Content
2.5. Liver Histopathology
2.6. RNA Extraction and Quantitative Reverse Transcriptase-Mediated Polymerase Chain Reaction
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
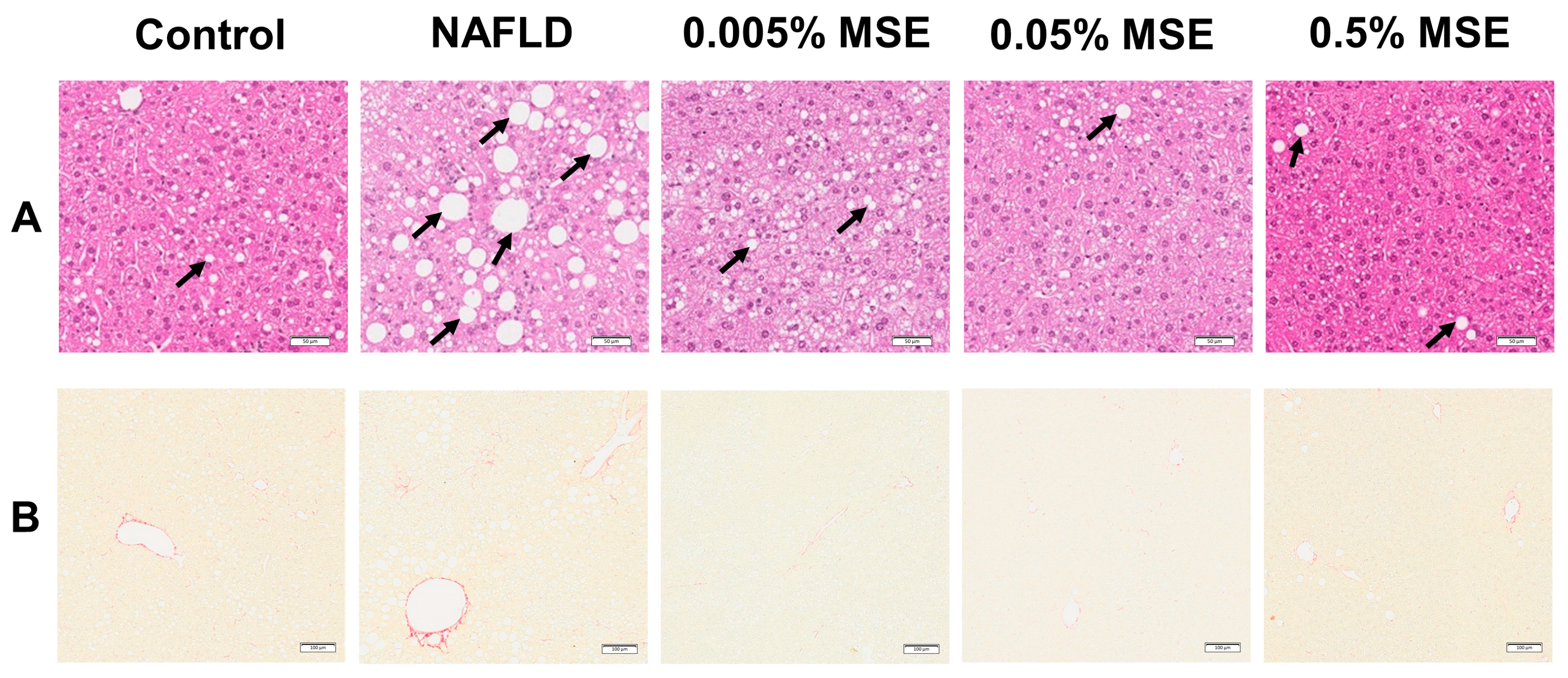
3.1. MSE Ameliorated Hepatic Steatosis and Inflammation in NAFLD Model Mice
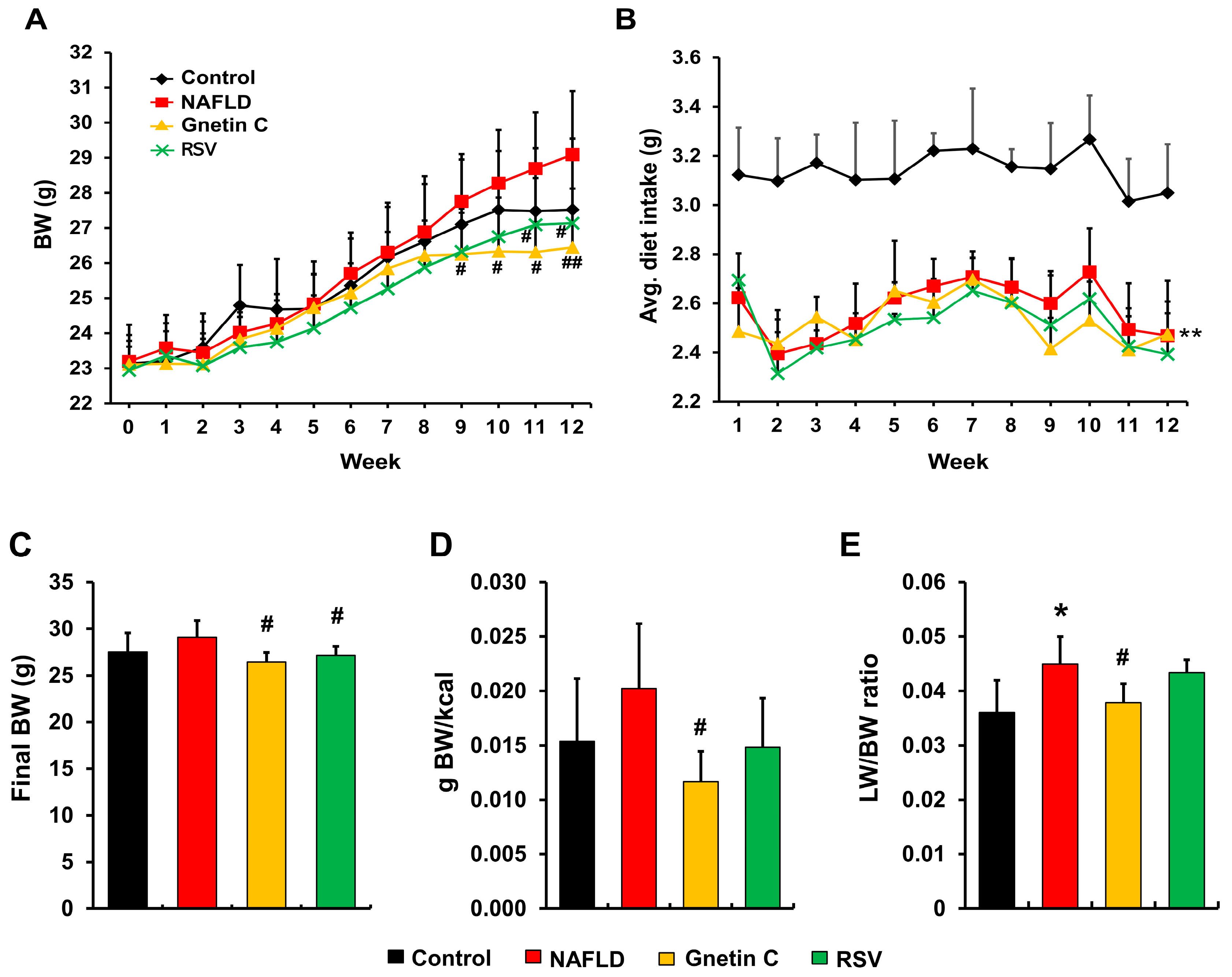
3.2. Effect of Gnetin C and Resveratrol Supplementation on Body Weight, Feeding Efficiency, and Liver Weight in NAFLD Model Mice
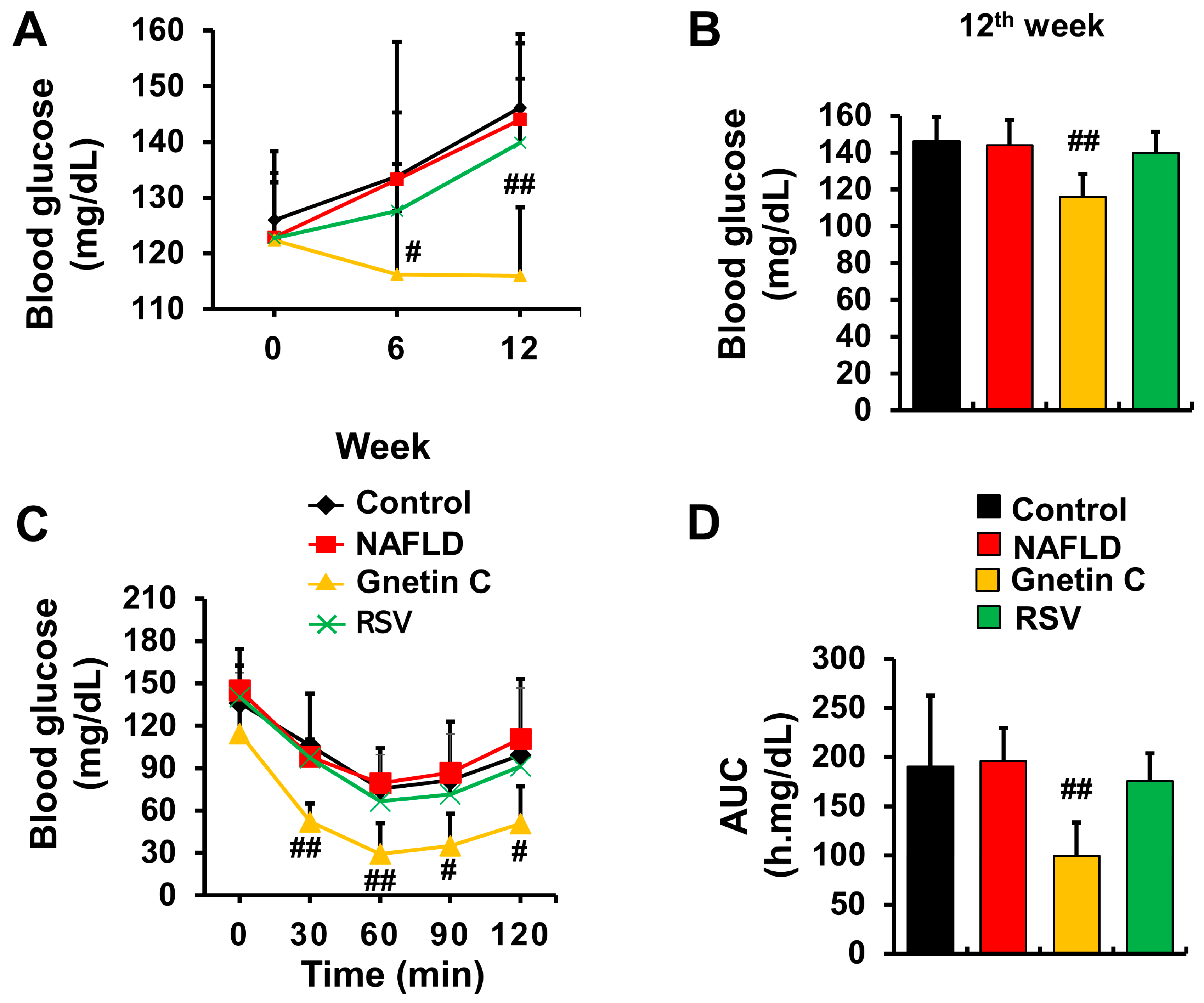
3.3. Gnetin C Ameliorated Blood Glucose Rise and Improved Insulin Sensitivity
3.4. Plasma Biochemical Parameters and Lipid Content
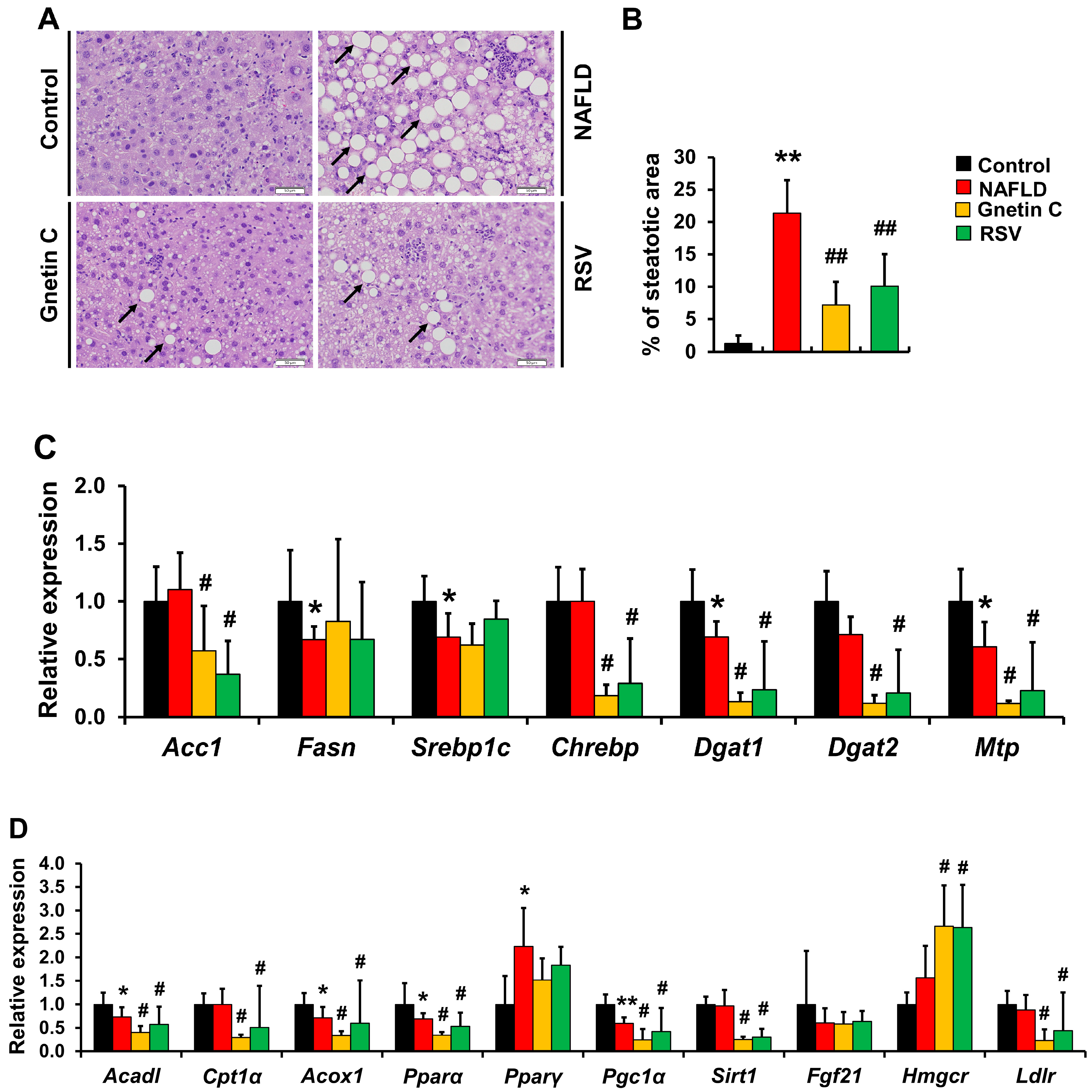
3.5. Effect of Gnetin C and Resveratrol Supplementation on HFCD-Induced Hepatic Steatosis and Inflammation
3.6. Effects of Gnetin C and Resveratrol Supplementation on mRNA Expression of Genes Involved in Lipid Synthesis and Metabolism
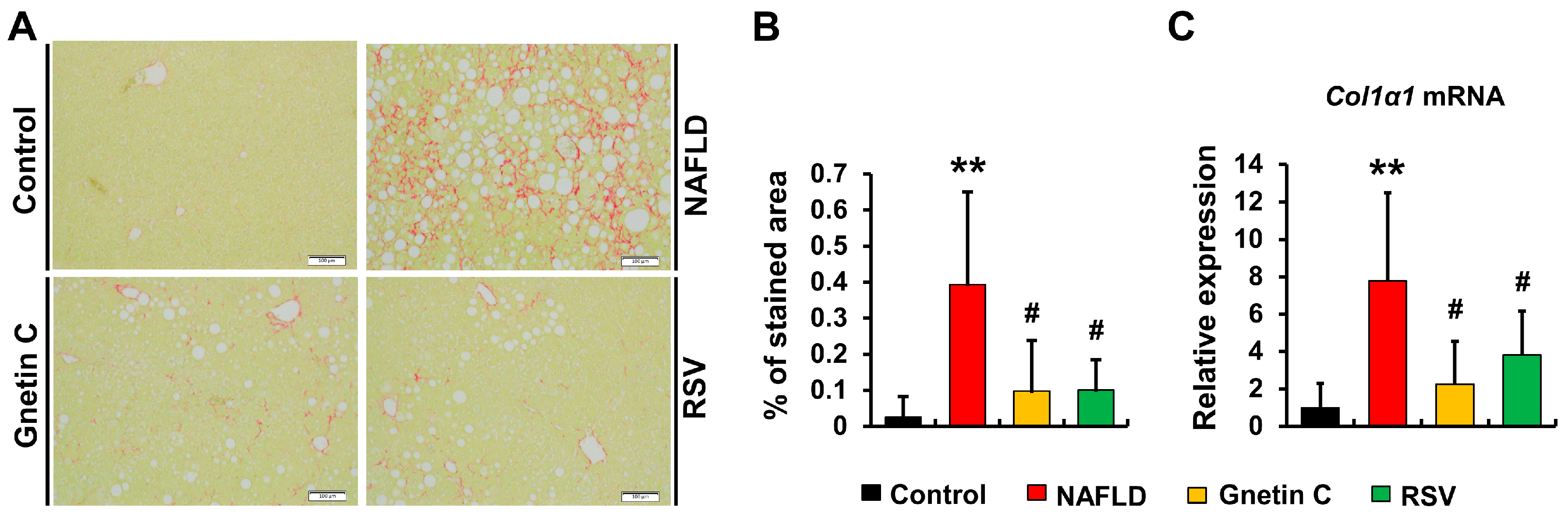
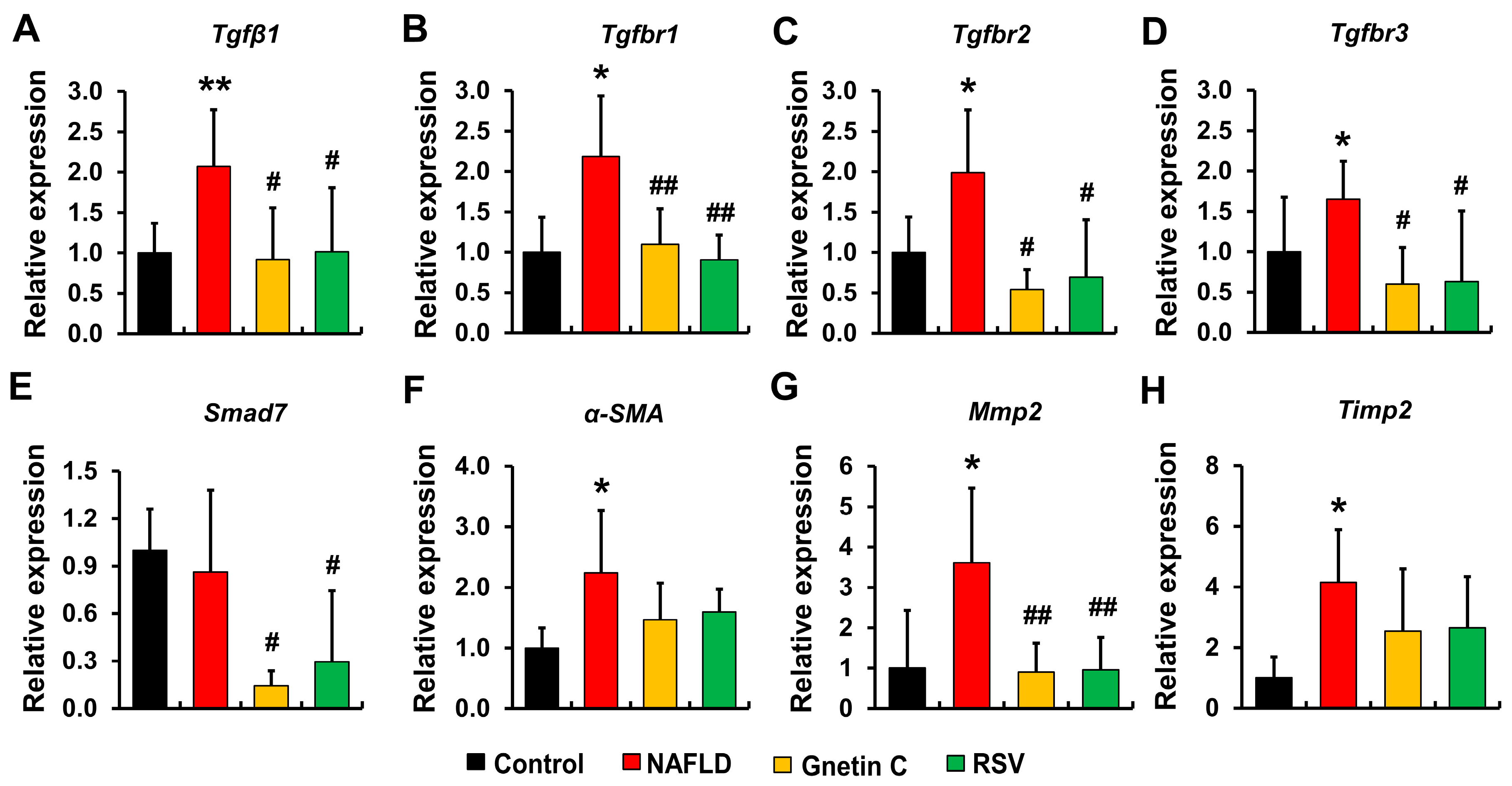
3.7. Effect of Gnetin C and Resveratrol Supplementation in Suppressing Hepatic Fibrosis Progression
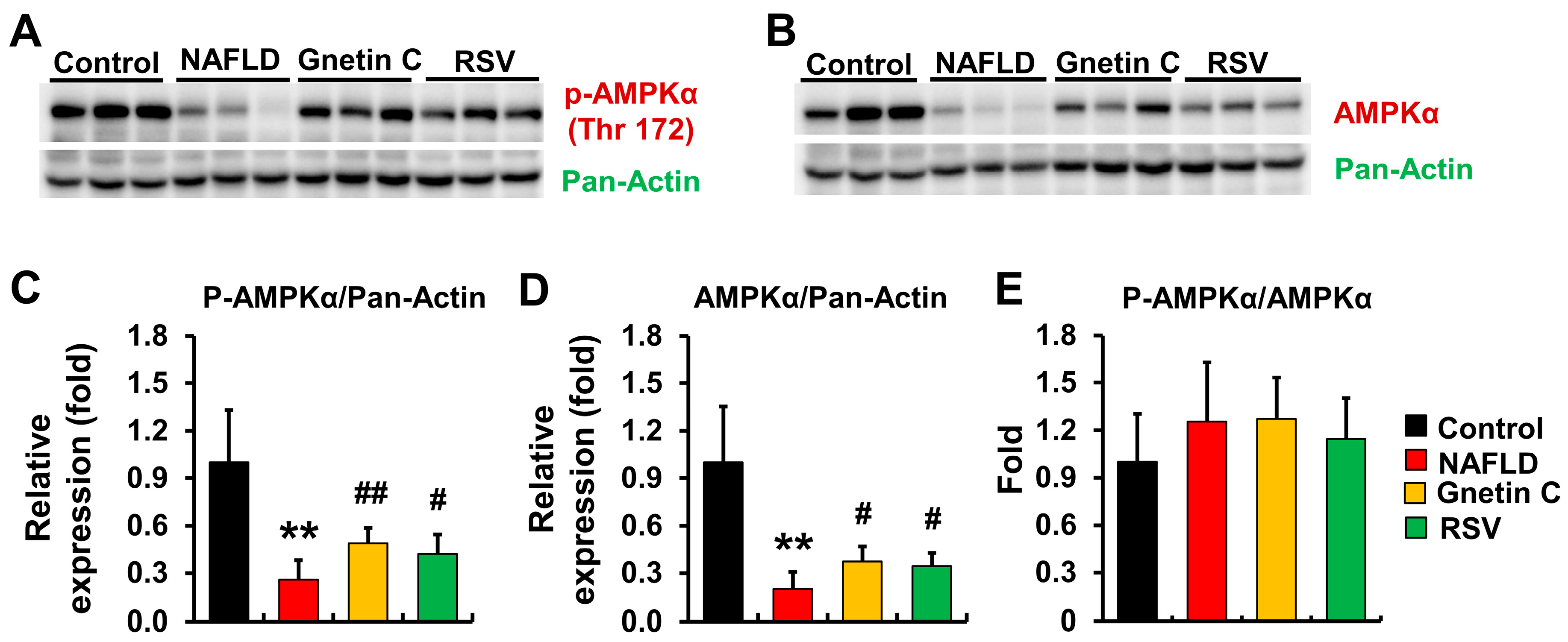
3.8. Effect of Gnetin C and Resveratrol Supplementation on AMPK Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, E.E.; Wong, V.W.S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Kessoku, T.; Imajo, K.; Honda, Y.; Kato, T.; Ogawa, Y.; Tomeno, W.; Kato, S.; Mawatari, H.; Fujita, K.; Yoneda, M.; et al. Resveratrol ameliorates fibrosis and inflammation in a mouse model of nonalcoholic steatohepatitis. Sci. Rep. 2016, 6, 22251. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Aguirre, L.; Gomez-Zorita, S.; Rolo, A.P.; Portillo, M.P. The influence of dietary conditions in the effects of resveratrol on hepatic steatosis. Food Funct. 2020, 11, 9432–9444. [Google Scholar] [CrossRef]
- Heebøll, S.; Thomsen, K.L.; Clouston, A.; Sundelin, E.I.; Radko, Y.; Christensen, L.P.; Ramezani-Moghadam, M.; Kreutzfeldt, M.; Pedersen, S.B.; Jessen, N.; et al. Effect of resveratrol on experimental non-alcoholic steatohepatitis. Pharmacol. Res. 2015, 95–96, 34–41. [Google Scholar] [CrossRef]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and non-alcoholic fatty liver disease: The Mediterranean way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef]
- Carneros, D.; López-Lluch, G.; Bustos, M. Physiopathology of lifestyle interventions in non-alcoholic fatty liver disease (NAFLD). Nutrients 2020, 12, 3472. [Google Scholar] [CrossRef]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary polyphenols and non-alcoholic fatty liver disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Hou, C.Y.; Tain, Y.L.; Yu, H.R.; Huang, L.T. The effects of resveratrol in the treatment of metabolic syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef]
- Andrade, J.M.O.; Paraíso, A.F.; de Oliveira, M.V.M.; Martins, A.M.E.; Neto, J.F.; Guimarães, A.L.S.; de Paula, A.M.; Qureshi, M.; Santos, S.H.S. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 2014, 30, 915–919. [Google Scholar] [CrossRef]
- Shang, J.; Chen, L.L.; Xiao, F.X.; Sun, H.; Ding, H.C.; Xiao, H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol. Sin. 2008, 29, 698–706. [Google Scholar] [CrossRef]
- Alberdi, G.; Rodríguez, V.M.; Macarulla, M.T.; Miranda, J.; Churruca, I.; Portillo, M.P. Hepatic lipid metabolic pathways modified by resveratrol in rats fed an obesogenic diet. Nutrition 2013, 29, 562–567. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Fernández-Quintela, A.; MacArulla, M.T.; Aguirre, L.; Hijona, E.; Bujanda, L.; Milagro, F.; Martínez, J.A.; Portillo, M.P. Resveratrol attenuates steatosis in obese zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br. J. Nutr. 2012, 107, 202–210. [Google Scholar] [CrossRef]
- Choi, Y.J.; Suh, H.R.; Yoon, Y.; Lee, K.J.; Kim, D.G.; Kim, S.; Lee, B.H. Protective effect of resveratrol derivatives on high-fat diet induced fatty liver by activating AMP-activated protein kinase. Arch. Pharm. Res. 2014, 37, 1169–1176. [Google Scholar] [CrossRef]
- Di Pascoli, M.; Diví, M.; Rodríguez-Vilarrupla, A.; Rosado, E.; Gracia-Sancho, J.; Vilaseca, M.; Bosch, J.; García-Pagán, J.C. Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J. Hepatol. 2013, 58, 904–910. [Google Scholar] [CrossRef]
- Barua, C.C. Gnetum gnemon Linn.: A comprehensive review on its biological, pharmacological and pharmacognostical potentials. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 531–539. [Google Scholar]
- Amelia, E.; Wahyuni, I.; Marianingsih, P. The diversification use of Melinjo (Gnetum gnemon) in Banten local food. In Joint Proceedings of the 2nd and the 3rd International Conference on Food Security Innovation (ICFSI 2018–2019); Atlantis Press: Amsterdam, The Netherlands, 2021; Volume 9, pp. 203–206. [Google Scholar] [CrossRef]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids isolated from the seeds of Melinjo (Gnetum gnemon L.) and their biological activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef]
- Tani, H.; Koshino, H.; Taniguchi, T.; Yoshimatsu, M.; Hikami, S.; Takahashi, S. Structural studies on stilbene oligomers isolated from the seeds of Melinjo (Gnetum gnemon L.). ACS Omega 2020, 5, 12245–12250. [Google Scholar] [CrossRef]
- Tatefuji, T.; Yanagihara, M.; Fukushima, S.; Hashimoto, K. Safety assessment of Melinjo (Gnetum gnemon L.) seed extract: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2014, 67, 230–235. [Google Scholar] [CrossRef]
- Tani, H.; Hikami, S.; Iizuna, S.; Yoshimatsu, M.; Asama, T.; Ota, H.; Kimura, Y.; Tatefuji, T.; Hashimoto, K.; Higaki, K. Pharmacokinetics and safety of resveratrol derivatives in humans after oral administration of Melinjo (Gnetum gnemon L.) seed extract powder. J. Agric. Food Chem. 2014, 62, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; An, D.T.; Trung, L.Q.; Yamada, K.; Nakao, S.; Takami, A. Stilbene derivatives from Melinjo extract have antioxidant and immune modulatory effects in healthy individuals. Integr. Mol. Med. 2015, 2, 405–413. [Google Scholar] [CrossRef]
- Khoirunisa, V.; Rusydi, F.; Boli, L.S.P.; Puspitasari, I.; Rachmawati, H.; Dipojono, H.K. The significance of long-range correction to the hydroperoxyl radical-scavenging reaction of trans-resveratrol and gnetin C. R. Soc. Open Sci. 2021, 8, 201127. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Kunimasa, K.; Yamori, Y.; Mori, M.; Mori, H.; Nakamura, K.; Miller, G.; Manne, U.; Tiwari, A.K.; Narayanan, B. Antitumor activity of Melinjo (Gnetum gnemon L.) seed extract in human and murine tumor models in vitro and in a colon-26 tumor-bearing mouse model in vivo. Cancer Med. 2015, 4, 1767–1780. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Elbadry, M.I.; Taniwaki, M.; Harada, K.; Trung, L.Q.; Nakagawa, N.; Takami, A.; Ishiyama, K.; Yamauchi, T.; Takenaka, K.; et al. The simultaneous inhibition of the MTOR and MAPK pathways with gnetin-C induces apoptosis in acute myeloid leukemia. Cancer Lett. 2017, 400, 127–136. [Google Scholar] [CrossRef]
- Matsuno, Y.; Atsumi, Y.; Alauddin, M.; Rana, M.M.; Fujimori, H.; Hyodo, M.; Shimizu, A.; Ikuta, T.; Tani, H.; Torigoe, H.; et al. Resveratrol and its related polyphenols contribute to the maintenance of genome stability. Sci. Rep. 2020, 10, 5388. [Google Scholar] [CrossRef]
- Gadkari, K.; Kolhatkar, U.; Hemani, R.; Campanelli, G.; Cai, Q.; Kumar, A.; Levenson, A.S. Therapeutic potential of gnetin C in prostate cancer: A pre-clinical study. Nutrients 2020, 12, 3631. [Google Scholar] [CrossRef]
- Nakagami, Y.; Suzuki, S.; Espinoza, J.L.; Quang, L.V.; Enomoto, M.; Takasugi, S.; Nakamura, A.; Nakayama, T.; Tani, H.; Hanamura, I.; et al. Immunomodulatory and metabolic changes after gnetin-C supplementation in humans. Nutrients 2019, 11, 1403. [Google Scholar] [CrossRef]
- Ikuta, T.; Saito, S.; Tani, H.; Tatefuji, T.; Hashimoto, K. Resveratrol derivative-rich Melinjo (Gnetum gnemon L.) seed extract improves obesity and survival of C57BL/6 mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2015, 79, 2044–2049. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Kaede, R.; Nagaya, K.; Saito, M.; Aoyama, J.; Elfeky, M.; Okamatsu-Ogura, Y.; Kimura, K.; Terao, A. Melinjo (Gnetum gnemon L.) seed extract induces uncoupling protein 1 expression in brown fat and protects mice against diet-induced obesity, inflammation, and insulin resistance. Nutr. Res. 2018, 58, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Oniki, K.; Kawakami, T.; Nakashima, A.; Miyata, K.; Watanabe, T.; Fujikawa, H.; Nakashima, R.; Nasu, A.; Eto, Y.; Takahashi, N.; et al. Melinjo seed extract increases adiponectin multimerization in physiological and pathological conditions. Sci. Rep. 2020, 10, 4313. [Google Scholar] [CrossRef]
- Chiba, T.; Kimura, Y.; Suzuki, S.; Tatefuji, T.; Umegaki, K. Trans-resveratrol enhances the anticoagulant activity of warfarin in a mouse model. J. Atheroscler. Thromb. 2016, 23, 1099. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Agista, A.Z.; Rusbana, T.B.; Islam, J.; Ohsaki, Y.; Sultana, H.; Hirakawa, R.; Watanabe, K.; Nochi, T.; Ardiansyah; Budijanto, S.; et al. Fermented rice bran supplementation prevents the development of intestinal fibrosis due to Dss-induced inflammation in mice. Nutrients 2021, 13, 1869. [Google Scholar] [CrossRef]
- POLYTRON® PT 2500 E Kinemetica AG. Available online: https://www.kinematica.ch/documents/07_product_brochures/EN/RZ_KIN_Brochure_Polytron_PT_2500E_EN_211214.pdf (accessed on 1 August 2023).
- Chiba, T.; Suzuki, S.; Sato, Y.; Itoh, T.; Umegaki, K. Evaluation of methionine content in a high-fat and choline-deficient diet on body weight gain and the development of non-alcoholic steatohepatitis in mice. PLoS ONE 2016, 11, e0164191. [Google Scholar] [CrossRef]
- Morris, E.M.; Meers, G.M.E.; Booth, F.W.; Fritsche, K.L.; Hardin, C.D.; Thyfault, J.P.; Ibdah, J.A. Pgc-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 979–992. [Google Scholar] [CrossRef]
- Lim, J.Y.; Oh, M.A.; Kim, W.H.; Sohn, H.Y.; Park, S.I. AMP-activated protein kinase inhibits TGF-β-induced fibrogenic responses of hepatic stellate cells by targeting transcriptional coactivator P300. J. Cell Physiol. 2012, 227, 1081–1089. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Luo, X.Y.; Takahara, T.; Kawai, K.; Fujino, M.; Sugiyama, T.; Tsuneyama, K.; Tsukada, K.; Nakae, S.; Zhong, L.; Li, X.K. IFN-γ deficiency attenuates hepatic inflammation and fibrosis in a steatohepatitis model induced by a methionine- and choline-deficient high-fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, 891–899. [Google Scholar] [CrossRef]
- Li, Z.; Vance, D.E. Thematic review series: Glycerolipids. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.M.; Corrales, F.J.; Lu, S.C.; Avila, M.A. S-adenosylmethionine: A control switch that regulates liver function. FASEB J. 2002, 16, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Gluchowski, N.L.; Gabriel, K.R.; Chitraju, C.; Bronson, R.T.; Mejhert, N.; Boland, S.; Wang, K.; Lai, Z.W.; Farese, R.V.; Walther, T.C. Hepatocyte deletion of triglyceride-synthesis enzyme acyl CoA: Diacylglycerol acyltransferase 2 reduces steatosis without increasing inflammation or fibrosis in mice. Hepatology 2019, 70, 1972–1985. [Google Scholar] [CrossRef]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor–deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Honsawek, S.; Poovorawan, Y. Adiponectin as a novel biomarker for liver fibrosis. World J. Hepatol. 2018, 10, 708–718. [Google Scholar] [CrossRef]
- Lu, C.; Xing, H.; Yang, L.; Chen, K.; Shu, L.; Zhao, X.; Song, G. Resveratrol ameliorates high-fat-diet-induced abnormalities in hepatic glucose metabolism in mice via the AMP-activated protein kinase pathway. Evid.-Based Complement. Altern. Med. 2021, 2021, 6616906. [Google Scholar] [CrossRef]
- Aljobaily, N.; Krutsinger, K.; Viereckl, M.J.; Joly, R.; Menlove, B.; Cone, B.; Suppes, A.; Han, Y. Low-dose administration of cannabigerol attenuates inflammation and fibrosis associated with methionine/choline deficient diet-induced NASH model via modulation of cannabinoid receptor. Nutrients 2023, 15, 178. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in hepatic stellate cell activation and liver fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Bakir, A.S.; Shabana, S.S. Serum TGF-β, serum MMP-1, and HOMA-IR as non-invasive predictors of fibrosis in egyptian patients with NAFLD. Saudi J. Gastroenterol. 2012, 18, 327–333. [Google Scholar] [CrossRef]
- Vonghia, L.; Magrone, T.; Verrijken, A.; Michielsen, P.; Van Gaal, L.; Jirillo, E.; Francque, S. Peripheral and hepatic vein cytokine levels in correlation with non-alcoholic fatty liver disease (NAFLD)-related metabolic, histological, and haemodynamic features. PLoS ONE 2015, 10, e0143380. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Blobe, G.C.; Schiemann, W.P.; Pepin, M.C.; Beauchemin, M.; Moustakas, A.; Lodish, H.F.; O’Connor-McCourt, M.D. Functional roles for the cytoplasmic domain of the type III transforming growth factor β receptor in regulating transforming growth factor β signaling. J. Biol. Chem. 2001, 276, 24627–24637. [Google Scholar] [CrossRef] [PubMed]
- Conidi, A.; Cazzola, S.; Beets, K.; Coddens, K.; Collart, C.; Cornelis, F.; Cox, L.; Joke, D.; Dobreva, M.P.; Dries, R.; et al. Few smad proteins and many smad-interacting proteins yield multiple functions and action modes in TGFβ/BMP signaling in vivo. Cytokine Growth Factor Rev. 2011, 22, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, Z.; Van Dam, H.; Zhang, L.; Zhou, F. Regulation of TGF-β superfamily signaling by SMAD mono-ubiquitination. Cells 2014, 3, 981–993. [Google Scholar] [CrossRef]
- Roeb, E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018, 68–69, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Naim, A.; Pan, Q.; Baig, M.S. Matrix metalloproteinases (MMPs) in liver diseases. J. Clin. Exp. Hepatol. 2017, 7, 367–372. [Google Scholar] [CrossRef]
- Park, P.H.; Sanz-Garcia, C.; Nagy, L.E. Adiponectin as an anti-fibrotic and anti-inflammatory adipokine in the liver. Curr. Pathobiol. Rep. 2015, 3, 243–252. [Google Scholar] [CrossRef]
- Wozniak, S.E.; Gee, L.L.; Wachtel, M.S.; Frezza, E.E. Adipose tissue: The new endocrine organ? A review article. Dig. Dis. Sci. 2009, 54, 1847–1856. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, Y.; Yang, S.J. Involvement of resveratrol in crosstalk between adipokine adiponectin and hepatokine fetuin-A in vivo and in vitro. J. Nutr. Biochem. 2015, 26, 1254–1260. [Google Scholar] [CrossRef]
| Control | NAFLD | 0.005% MSE | 0.05% MSE | 0.5% MSE | |
|---|---|---|---|---|---|
| BW (g) | 32.8 ± 1.7 | 29.3 ± 3.1 * | 29.4 ± 2.6 | 28.4 ± 2.3 | 25.3 ± 1.7 ## |
| Liver (g) | 1.25 ± 0.08 | 1.46 ± 0.17 * | 1.45 ± 0.11 | 1.46 ± 0.11 | 1.20 ± 0.08 ## |
| % of BW | 3.83 ± 0.17 | 4.99 ± 0.28 ** | 4.95 ± 0.23 | 5.14 ± 0.25 | 4.75 ± 0.20 |
| Plasma lipids | |||||
| TC (mg/dL) | 114.6 ± 10.5 | 86.1 ± 7.1 ** | 88.0 ± 8.5 | 88.9 ± 6.5 | 79.3 ± 11.6 |
| TG (mg/dL) | 101.9 ± 20.1 | 89.6 ± 11.0 | 99.8 ± 22.9 | 84.8 ± 9.1 | 65.7 ± 11.0 # |
| NEFA (mEq/L) | 1.30 ± 0.14 | 1.20 ± 0.08 | 1.25 ± 0.11 | 1.11 ± 0.11 | 0.89 ± 0.08 ## |
| Plasma liver function markers | |||||
| AST (IU/L | 25.9 ± 4.5 | 48.6 ± 12.2 * | 43.4 ± 5.1 | 57.8 ± 21.2 | 37.5 ± 5.1 |
| ALT (IU/L) | 5.9 ± 1.4 | 20.9 ± 6.2 ** | 20.8 ± 5.1 | 20.3 ± 2.3 | 8.7 ± 2.0 ## |
| ALP (IU/L) | 33.1 ± 3.7 | 38.3 ± 2.8 * | 39.1 ± 3.4 | 37.3 ± 2.6 | 35.2 ± 3.9 |
| Liver histology | |||||
| Steatosis | 0.63 ± 0.74 | 2.00 ± 1.07 ** | 1.38 ± 0.74 | 0.88 ± 0.65 # | 0.25 ± 0.45 ## |
| Fibrosis | 0.38 ± 0.51 | 1.88 ± 0.65 ** | 1.63 ± 0.51 | 1.25 ± 0.88 | 1.00 ± 0.54 |
| Control | NAFLD | Gnetin C | Resveratrol | |
|---|---|---|---|---|
| Plasma Liver injury markers | ||||
| AST (IU/L) | 57.43 ± 15.56 | 90.00 ± 10.61 ** | 83.63 ± 13.77 | 88.50 ± 16.58 |
| ALT (IU/L) | 2.93 ± 0.049 | 9.61 ± 8.03 * | 3.98 ± 1.22 | 6.86 ± 6.72 |
| LDH (IU/L) | 146.29 ± 29.97 | 289.13 ± 74.65 ** | 220.13 ± 70.00 | 255.50 ± 36.08 |
| Plasma adiponectin (ng/mL) | 7187± 615 | 5065 ± 405 ** | 5943 ± 716 # | 5704 ± 644 |
| Plasma lipids | ||||
| TG (mg/dL) | 26.71 ± 7.71 | 20.13 ± 9.43 | 10.50 ± 1.70 # | 12.75 ± 3.85 # |
| TC (mg/dL) | 85.00 ± 11.90 | 70.13 ± 10.92 * | 47.50 ± 7.62 ## | 62.00 ± 10.21 |
| F-CHO (mg/dL) | 24.00 ± 3.16 | 20.63 ± 2.97 | 14.63 ± 2.07 ## | 17.25 ± 2.61 # |
| E-CHO (mg/dL) | 61.00 ± 8.83 | 49.50 ± 8.03 * | 32.88 ± 5.74 ## | 44.75 ± 7.74 |
| LDL-C (mg/dL) | 5.00 ± 1.00 | 3.38 ± 0.74 * | 2.13 ± 0.99 # | 2.63 ± 0.74 # |
| HDL-C (mg/dL) | 43.43 ± 6.85 | 38.00 ± 5.07 | 25.50 ± 4.07 ## | 33.50 ± 5.10 |
| NEFA (mEq/L) | 0.4 ± 0.09 | 0.28 ± 0.04 * | 0.28 ± 0.09 | 0.37 ± 0.04 # |
| Liver lipids | ||||
| Total lipid (mg/g tissue) | 72.28 ± 20.56 | 176.75 ± 36.26 ** | 130.67 ± 28.27 | 162.84 ± 52.21 |
| TG (mg/g tissue) | 21.65 ± 8.19 | 58.70 ± 13.54 ** | 49.59 ± 13.43 | 76.16 ± 7.34 # |
| TC (mg/g tissue) | 2.29 ± 0.91 | 3.29 ± 0.58 * | 2.72 ± 0.50 | 3.67 ± 0.65 |
| NEFA (mEq/L·g tissue) | 0.047 ± 0.01 | 0.080 ± 0.01 ** | 0.055 ± 0.01 ## | 0.062 ± 0.01 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabir, T.; Yoshiba, H.; Agista, A.Z.; Sultana, H.; Ohsaki, Y.; Yeh, C.-L.; Hirakawa, R.; Tani, H.; Ikuta, T.; Nochi, T.; et al. Protective Effects of Gnetin C from Melinjo Seed Extract against High-Fat Diet-Induced Hepatic Steatosis and Liver Fibrosis in NAFLD Mice Model. Nutrients 2023, 15, 3888. https://doi.org/10.3390/nu15183888
Kabir T, Yoshiba H, Agista AZ, Sultana H, Ohsaki Y, Yeh C-L, Hirakawa R, Tani H, Ikuta T, Nochi T, et al. Protective Effects of Gnetin C from Melinjo Seed Extract against High-Fat Diet-Induced Hepatic Steatosis and Liver Fibrosis in NAFLD Mice Model. Nutrients. 2023; 15(18):3888. https://doi.org/10.3390/nu15183888
Chicago/Turabian StyleKabir, Tohfa, Haruki Yoshiba, Afifah Zahra Agista, Halima Sultana, Yusuke Ohsaki, Chiu-Li Yeh, Ryota Hirakawa, Hiroko Tani, Tomoki Ikuta, Tomonori Nochi, and et al. 2023. "Protective Effects of Gnetin C from Melinjo Seed Extract against High-Fat Diet-Induced Hepatic Steatosis and Liver Fibrosis in NAFLD Mice Model" Nutrients 15, no. 18: 3888. https://doi.org/10.3390/nu15183888









