A Biphasic Glucose Response during an Oral Glucose Tolerance Test Is Associated with Greater Plasma Insulin and GLP-1 Responses and a Reduction in 1-Hour Glucose but Does Not Relate to the Rate of Gastric Emptying in Healthy, Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Biochemical Measurements
2.4. Insulin Secretion, Sensitivity and Oral Disposition Index
2.5. Gastric Emptying
2.6. Statistical Analysis
3. Results
3.1. Baseline Measurements
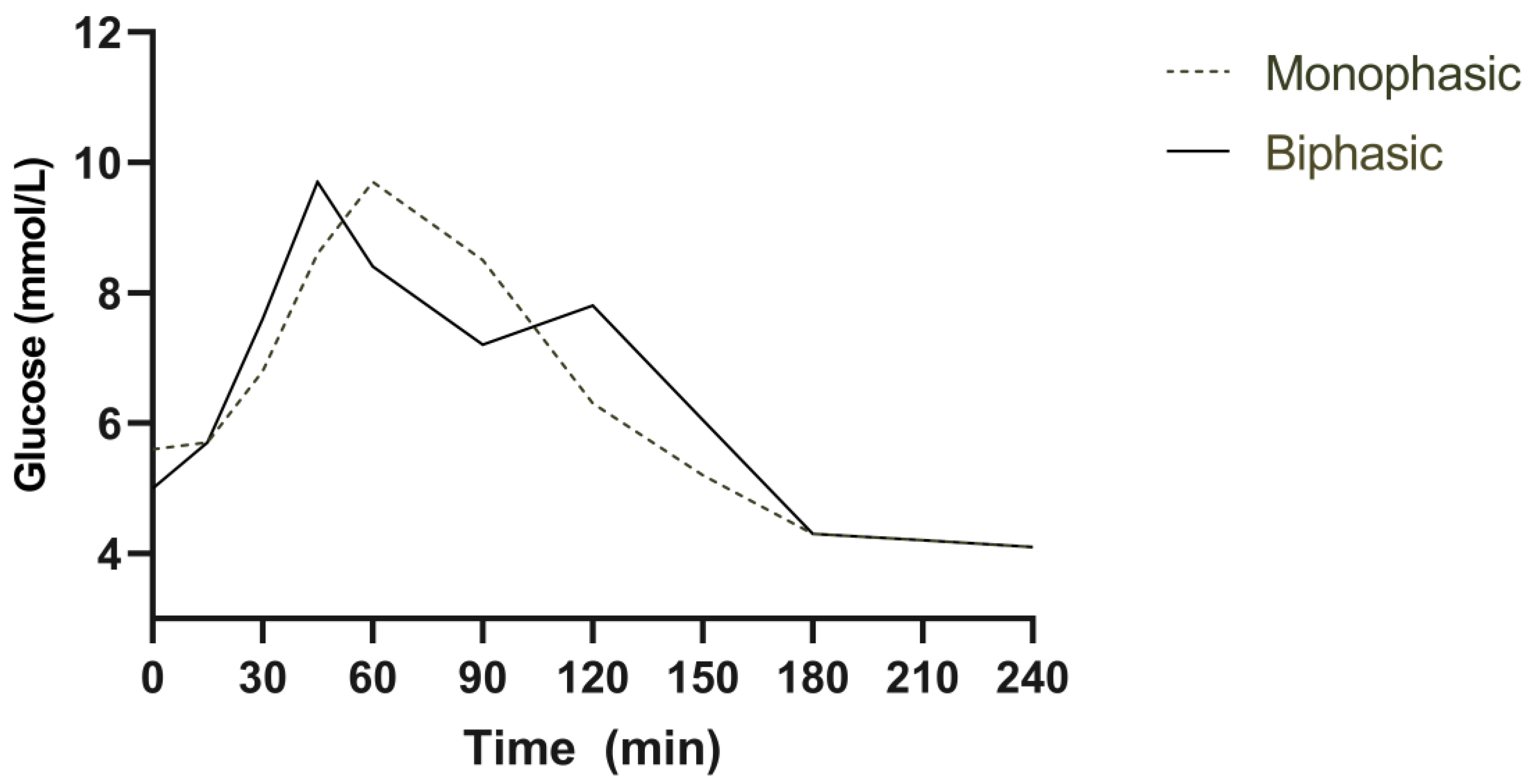
3.2. Plasma Glucose
3.3. Insulin Secretion and Sensitivity
3.4. Plasma GLP-1 and GIP
3.5. Gastric Emptying
3.6. Follow-Up
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergman, M.; Chetrit, A.; Roth, J.; Jagannathan, R.; Sevick, M.; Dankner, R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia: Observations from the 24 year follow-up of the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabetes Res. Clin. Pract. 2016, 120, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Nolfe, G.; Pataky, Z.; Monti, L.; Porcellati, F.; Gabriel, R.; Mitrakou, A.; Mingrone, G. Shape of the OGTT glucose curve and risk of impaired glucose metabolism in the EGIR-RISC cohort. Metabolism 2017, 70, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Coletta, D.K.; Mandarino, L.J.; Shaibi, G.Q. Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes Care 2012, 35, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Arslanian, S.; El Ghormli, L.; Young Kim, J.; Bacha, F.; Chan, C.; Ismail, H.M.; Levitt Katz, L.E.; Levitsky, L.; Tryggestad, J.B.; White, N.H.; et al. The Shape of the Glucose Response Curve During an Oral Glucose Tolerance Test: Forerunner of Heightened Glycemic Failure Rates and Accelerated Decline in beta-Cell Function in TODAY. Diabetes Care 2019, 42, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.M.; Xu, P.; Libman, I.M.; Becker, D.J.; Marks, J.B.; Skyler, J.S.; Palmer, J.P.; Sosenko, J.M.; Type 1 Diabetes TrialNet Study Group. The shape of the glucose concentration curve during an oral glucose tolerance test predicts risk for type 1 diabetes. Diabetologia 2018, 61, 84–92. [Google Scholar] [CrossRef]
- Tura, A.; Morbiducci, U.; Sbrignadello, S.; Winhofer, Y.; Pacini, G.; Kautzky-Willer, A. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: Any relationship with the degree of glucose tolerance? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R941–R948. [Google Scholar] [CrossRef]
- Marathe, C.S.; Horowitz, M.; Trahair, L.G.; Wishart, J.M.; Bound, M.; Lange, K.; Rayner, C.K.; Jones, K.L. Relationships of early and late glycemic responses with gastric emptying during an oral glucose tolerance test. J. Clin. Endocrinol. Metab. 2015, 100, 3565–3571. [Google Scholar] [CrossRef]
- Wang, X.; Xie, C.; Marathe, C.S.; Malbert, C.H.; Horowitz, M.; Jones, K.L.; Rayner, C.K.; Sun, Z.; Wu, T. Disparities in gastric emptying and postprandial glycaemia between Han Chinese and Caucasians with type 2 diabetes. Diabetes Res. Clin. Pract. 2020, 159, 107951. [Google Scholar] [CrossRef]
- Phillips, W.T. Gastric emptying in ethnic populations: Possible relationship to development of diabetes and metabolic syndrome. Ethn. Dis. 2006, 16, 682–692. [Google Scholar]
- Watson, L.E.; Xie, C.; Wang, X.; Li, Z.; Phillips, L.K.; Sun, Z.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Gastric emptying in patients with well-controlled type 2 diabetes compared with young and older control subjects without diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3311–3319. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Pilichiewicz, A.N.; Chaikomin, R.; Brennan, I.M.; Wishart, J.M.; Rayner, C.K.; Jones, K.L.; Smout, A.J.; Horowitz, M.; Feinle-Bisset, C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E743–E753. [Google Scholar] [CrossRef]
- Pham, H.; Marathe, C.S.; Phillips, L.K.; Trahair, L.G.; Hatzinikolas, S.; Huynh, L.; Wu, T.; Nauck, M.A.; Rayner, C.K.; Horowitz, M.; et al. Longitudinal changes in fasting and glucose-stimulated GLP-1 and GIP in healthy older subjects. J. Clin. Endocrinol. Metab. 2019, 104, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Deane, A.M.; Nguyen, N.Q.; Stevens, J.E.; Fraser, R.J.; Holloway, R.H.; Besanko, L.K.; Burgstad, C.; Jones, K.L.; Chapman, M.J.; Rayner, C.K.; et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J. Clin. Endocrinol. Metab. 2010, 95, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Goetze, O.; Anstipp, J.; Hagemann, D.; Holst, J.J.; Schmidt, W.E.; Gallwitz, B.; Nauck, M.A. Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E621–E625. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Phillips, L.; Trahair, L.; Hatzinikolas, S.; Horowitz, M.; Jones, K.L. Longitudinal changes in the blood pressure responses to, and gastric emptying of, an oral glucose load in healthy older subjects. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 244–248. [Google Scholar] [CrossRef]
- Johnson, R.D.; Horowitz, M.; Maddox, A.F.; Wishart, J.M.; Shearman, D.J. Cigarette smoking and rate of gastric emptying: Effect on alcohol absorption. BMJ 1991, 302, 20–23. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Prigeon, R.L.; Faulenbach, M.V.; Tong, J.; Carr, D.B.; Boyko, E.J.; Leonetti, D.L.; McNeely, M.J.; Fujimoto, W.Y.; Kahn, S.E. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009, 32, 335–341. [Google Scholar] [CrossRef]
- Trahair, L.G.; Nauck, M.A.; Wu, T.; Stevens, J.E.; Buttfield, M.D.; Hatzinikolas, S.; Pham, H.; Meier, J.J.; Rayner, C.K.; Horowitz, M.; et al. Measurement of Gastric Emptying Using a 13C-octanoic Acid Breath Test with Wagner-Nelson Analysis and Scintigraphy in Type 2 Diabetes. Exp. Clin. Endocrinol. Diabetes 2022, 130, 751–757. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef]
- Chang, A.M.; Halter, J.B. Aging and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E7–E12. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Huang, W.; Watson, L.E.; Soenen, S.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Plasma GLP-1 response to oral and intraduodenal nutrients in health and type 2 diabetes—Impact on gastric emptying. J. Clin. Endocrinol. Metab. 2021, 107, e1643–e1652. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Michaliszyn, S.F.; Nasr, A.; Lee, S.; Tfayli, H.; Hannon, T.; Hughan, K.S.; Bacha, F.; Arslanian, S. The Shape of the Glucose Response Curve During an Oral Glucose Tolerance Test Heralds Biomarkers of Type 2 Diabetes Risk in Obese Youth. Diabetes Care 2016, 39, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Faerch, K.; Torekov, S.S.; Vistisen, D.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; Sandbaek, A.; et al. GLP-1 Response to Oral Glucose Is Reduced in Prediabetes, Screen-Detected Type 2 Diabetes, and Obesity and Influenced by Sex: The ADDITION-PRO Study. Diabetes 2015, 64, 2513–2525. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- McDonald, G.W.; Fisher, G.F.; Burnham, C. Reproducibility of the Oral Glucose Tolerance Test. Diabetes 1965, 14, 473–480. [Google Scholar] [CrossRef]
- Peddinti, G.; Bergman, M.; Tuomi, T.; Groop, L. 1-Hour Post-OGTT Glucose Improves the Early Prediction of Type 2 Diabetes by Clinical and Metabolic Markers. J. Clin. Endocrinol. Metab. 2019, 104, 1131–1140. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef]
| Monophasic | Biphasic | p-Value | |
|---|---|---|---|
| Age (years) | 77.3 ± 0.7 | 69.2 ± 0.9 | <0.001 * |
| Height (m) | 1.68 ± 0.02 | 1.67 ± 0.03 | 0.94 |
| Weight (kg) | 72.0 ± 2.9 | 73.4 ± 3.4 | 0.77 |
| Body mass index (kg/m2) | 25.6 ± 0.7 | 26.0 ± 0.6 | 0.62 |
| Monophasic (n = 22) | Biphasic (n = 14) | p Value | ||
|---|---|---|---|---|
| Glucose (mmol/L) | Fasting | 5.8 ± 0.9 | 5.7 ± 1.0 | 0.67 |
| 1 h | 9.5 ± 0.5 | 8.0 ± 0.5 | 0.04 * | |
| 2 h | 7.5 ± 0.4 | 7.3 ± 0.4 | 0.66 | |
| Peak | 10.6 ± 0.4 | 9.6 ± 0.3 | 0.01 * | |
| AUC0–60 | 522 ± 15 | 472 ± 16 | 0.04 * | |
| AUC0–120 | 1032 ± 43 | 920 ± 37 | 0.08 | |
| Insulin (pmol/L) | Fasting | 3.8 ± 0.4 | 4.9 ± 0.6 | 0.13 |
| 1 h | 54.5 ± 5.4 | 67.2 ± 13.7 | 0.40 | |
| 2 h | 44.9 ± 5.5 | 54.1 ± 9.1 | 0.36 | |
| Peak | 63.2 ± 7.0 | 75.7 ± 13.3 | 0.37 | |
| AUC0–60 | 1942 ± 211 | 2712 ± 488 | 0.17 | |
| AUC0–120 | 3568 ± 352 | 4522 ± 859 | 0.32 | |
| Insulin secretion | 10.4 ± 1.1 | 20.9 ± 4.3 | 0.03 * | |
| Insulin sensitivity | 0.32 ± 0.04 | 0.24 ± 0.02 | 0.14 | |
| Oral disposition index | 3.2 ± 0.4 | 4.5 ± 0.8 | 0.13 | |
| GLP-1 (pmol/L) | Fasting | 20.8 ± 1.2 | 21.3 ± 1.5 | 0.82 |
| 1 h | 30.7 ± 1.8 | 33.3 ± 2.4 | 0.37 | |
| 2 h | 24.3 ± 1.4 | 30.3 ± 2.3 | 0.02 * | |
| Peak | 36.2 ± 2.1 | 49.4 ± 4.8 | 0.007 * | |
| AUC0–60 | 1846 ± 97 | 2290 ± 171 | 0.02 * | |
| AUC0–120 | 3421 ± 165 | 4190 ± 288 | 0.02 * | |
| GIP (pmol/L) | Fasting | 20.0 ± 1.7 | 19.5 ± 1.8 | 0.83 |
| 1 h | 52.1 ± 3.8 | 49.2 ± 3.7 | 0.60 | |
| 2 h | 52.7 ± 3.9 | 53.8 ± 3.5 | 0.84 | |
| Peak | 56.4 ± 4.1 | 55.7 ± 3.7 | 0.91 | |
| AUC0–60 | 2668 ± 189 | 2436 ± 148 | 0.39 | |
| AUC0–120 | 5863 ± 424 | 5546 ± 354 | 0.60 |
| Monophasic (n = 24) | Biphasic (n = 12) | p Value | ||
|---|---|---|---|---|
| Glucose (mmol/L) | Fasting conc. | 5.6 ± 0.1 | 5.4 ± 0.2 | 0.09 |
| 1 h | 10.0 ± 0.4 | 7.9 ± 0.3 | 0.01 * | |
| 2 h | 7.4 ± 0.4 | 7.6 ± 0.4 | 0.78 | |
| Peak | 10.6 ± 0.3 | 9.2 ± 0.4 | 0.02 * | |
| AUC0–60 | 498 ± 13 | 457 ± 14 | 0.06 | |
| AUC0–120 | 1027 ± 32 | 936 ± 39 | 0.10 | |
| Insulin (pmol/L) | Fasting | 5.2 ± 0.5 | 4.9 ± 0.8 | 0.76 |
| 1 h | 76.4 ± 10.2 | 50.6 ± 11.2 | 0.11 | |
| 2 h | 72.0 ± 12.3 | 52.1 ± 8.5 | 0.19 | |
| Peak | 101.4 ± 14.9 | 80.3 ± 13.3 | 0.42 | |
| AUC0–60 | 2500 ± 300 | 2676 ± 587 | 0.79 | |
| AUC0–120 | 7472 ± 1044 | 5920 ± 923 | 0.27 | |
| Insulin secretion | 12.4 ± 1.6 | 17.4 ± 3.4 | 0.20 | |
| Insulin sensitivity | 0.23 ± 0.02 | 0.28 ± 0.07 | 0.44 | |
| Oral disposition index | 2.7 ± 0.4 | 3.9 ± 0.7 | 0.15 | |
| GLP-1 (pmol/L) | Fasting | 14.6 ± 0.9 | 16.8 ± 1.2 | 0.16 |
| 1 h | 25.5 ± 1.4 | 31.7 ± 3.1 | 0.04 * | |
| 2 h | 20.2 ± 1.3 | 25.2 ± 2.3 | 0.06 | |
| Peak | 30.9 ± 1.8 | 44.4 ± 4.7 | 0.003 * | |
| AUC0–60 | 1422 ± 73 | 2036 ± 188 | 0.001 * | |
| AUC0–120 | 2759 ± 116 | 3837 ± 335 | 0.001 * | |
| GIP (pmol/L) | Fasting | 16.4 ± 1.2 | 18.3 ± 1.5 | 0.36 |
| 1 h | 49.1 ± 2.6 | 49.3 ± 4.3 | 0.97 | |
| 2 h | 49.0 ± 3.0 | 53.6 ± 3.7 | 0.36 | |
| Peak | 53.0 ± 3.0 | 56.7 ± 4.0 | 0.47 | |
| AUC0–60 | 2384 ± 127 | 2534 ± 189 | 0.51 | |
| AUC0–120 | 5365 ± 298 | 5662 ± 426 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalleh, R.J.; Marathe, C.S.; Trahair, L.G.; Jones, K.L.; Horowitz, M. A Biphasic Glucose Response during an Oral Glucose Tolerance Test Is Associated with Greater Plasma Insulin and GLP-1 Responses and a Reduction in 1-Hour Glucose but Does Not Relate to the Rate of Gastric Emptying in Healthy, Older Adults. Nutrients 2023, 15, 3889. https://doi.org/10.3390/nu15183889
Jalleh RJ, Marathe CS, Trahair LG, Jones KL, Horowitz M. A Biphasic Glucose Response during an Oral Glucose Tolerance Test Is Associated with Greater Plasma Insulin and GLP-1 Responses and a Reduction in 1-Hour Glucose but Does Not Relate to the Rate of Gastric Emptying in Healthy, Older Adults. Nutrients. 2023; 15(18):3889. https://doi.org/10.3390/nu15183889
Chicago/Turabian StyleJalleh, Ryan J., Chinmay S. Marathe, Laurence G. Trahair, Karen L Jones, and Michael Horowitz. 2023. "A Biphasic Glucose Response during an Oral Glucose Tolerance Test Is Associated with Greater Plasma Insulin and GLP-1 Responses and a Reduction in 1-Hour Glucose but Does Not Relate to the Rate of Gastric Emptying in Healthy, Older Adults" Nutrients 15, no. 18: 3889. https://doi.org/10.3390/nu15183889






