Brown Fat and Nutrition: Implications for Nutritional Interventions
Abstract
:1. Introduction
2. Overview of Different Adipose Tissues
3. BAT Acts as a Therapeutic Target for Combating Metabolic Disorders
3.1. Distribution of BAT in the Human Body
3.2. Correlation between BAT, Nutritional Imbalance and Metabolic Disorders
3.3. Strategies for BAT Activation
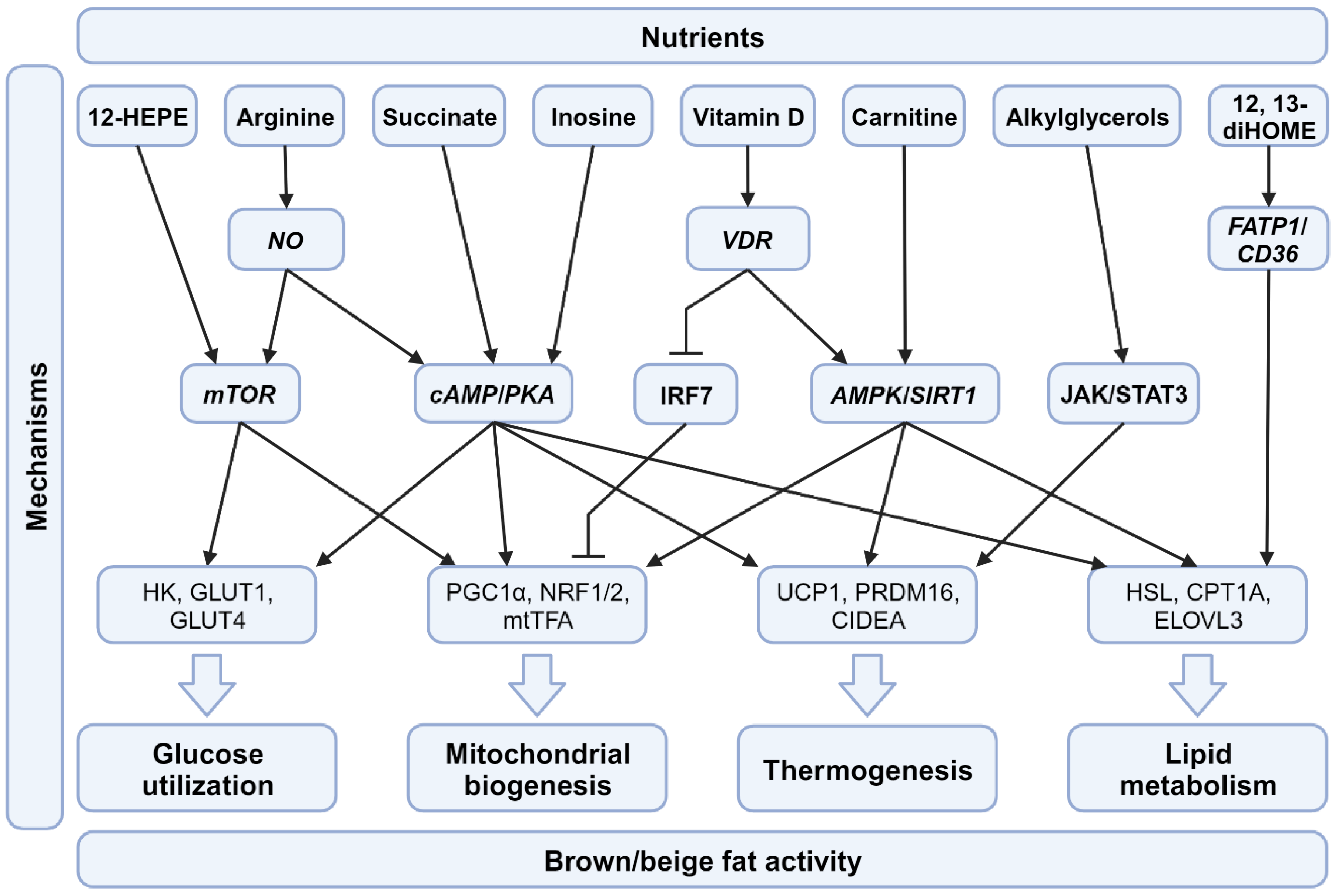
4. Nutritional Factors Regulate BAT Activity
4.1. Macronutrients and Their Impact on BAT
4.1.1. Proteins
4.1.2. Carbohydrates
4.1.3. Omega-3 Fatty Acids
4.1.4. Omega-6 Fatty Acids
4.2. Micronutrients and Their Impact on BAT
4.2.1. Vitamin D
4.2.2. Succinate
4.2.3. Inosine
4.2.4. Arginine
4.2.5. 12,13-diHOME
4.2.6. 12-HEPE
4.2.7. Alkylglycerols
4.2.8. Carnitine
5. Natural Products
5.1. Epigallocatechin Gallate (EGCG)
5.2. Resveratrol
5.3. Caffeine
5.4. Capsaicin and Capsinoids
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.L.; Lin, Z.J.; Li, C.C.; Lin, X.; Shan, S.K.; Guo, B.; Zheng, M.H.; Li, F.; Yuan, L.Q.; Li, Z.H. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 98. [Google Scholar] [CrossRef]
- Symonds, M.E.; Sebert, S.P.; Hyatt, M.A.; Budge, H. Nutritional programming of the metabolic syndrome. Nat. Rev. Endocrinol. 2009, 5, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Gallou-Kabani, C.; Junien, C. Nutritional Epigenomics of Metabolic Syndrome: New Perspective Against the Epidemic. Diabetes 2005, 54, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Suchacki, K.J.; Stimson, R.H. Nutritional Regulation of Human Brown Adipose Tissue. Nutrients 2021, 13, 1748. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.J.; Enerback, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Jung, S.M.; Sanchez-Gurmaches, J.; Guertin, D.A. Brown Adipose Tissue Development and Metabolism. In Brown Adipose Tissue; Pfeifer, A., Klingenspor, M., Herzig, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–36. [Google Scholar] [CrossRef]
- Zoico, E.; Rubele, S.; De Caro, A.; Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Coolbaugh, C.L.; Damon, B.M.; Bush, E.C.; Welch, E.B.; Towse, T.F. Cold exposure induces dynamic, heterogeneous alterations in human brown adipose tissue lipid content. Sci. Rep. 2019, 9, 13600. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Franquet Elia, E.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef]
- Heenan, K.A.; Carrillo, A.E.; Fulton, J.L.; Ryan, E.J.; Edsall, J.R.; Rigopoulos, D.; Markofski, M.M.; Flouris, A.D.; Dinas, P.C. Effects of Nutrition/Diet on Brown Adipose Tissue in Humans: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2752. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, F.; Wang, C.H.; Tseng, Y.H. The evolving view of thermogenic adipocytes—Ontogeny, niche and function. Nat. Rev. Endocrinol. 2021, 17, 726–744. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Harada, Y.; Yoshikawa, T.; Dai, P. Mitochondrial Energy Metabolism in the Regulation of Thermogenic Brown Fats and Human Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 1352. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, A.; Oh, K.J.; Lee, S.C.; Kim, W.K.; Bae, K.H. The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4924. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes. Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scime, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol 2020, 11, 498. [Google Scholar] [CrossRef]
- Rabiee, A. Beige Fat Maintenance; Toward a Sustained Metabolic Health. Front. Endocrinol 2020, 11, 634. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, T.; Cui, X.; Yan, L.; Wang, Q.; Xu, X.; Zhao, Q.; Xu, X.; Tang, Q.-Q.; Tang, H.; et al. Asparagine reinforces mTORC1 signaling to boost thermogenesis and glycolysis in adipose tissues. EMBO J. 2021, 40, e108069. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.L.; Tseng, Y.H. Brown fat fuel utilization and thermogenesis. Trends Endocrinol. Metab. 2014, 25, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.-E.; Kozak, L.P. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Gaudry, M.J.; Jastroch, M.; Treberg, J.R.; Hofreiter, M.; Paijmans, J.L.A.; Starrett, J.; Wales, N.; Signore, A.V.; Springer, M.S.; Campbell, K.L. Inactivation of thermogenic UCP1 as a historical contingency in multiple placental mammal clades. Sci. Adv. 2017, 3, e1602878. [Google Scholar] [CrossRef]
- Wright, T.; Davis, R.W.; Pearson, H.C.; Murray, M.; Sheffield-Moore, M. Skeletal muscle thermogenesis enables aquatic life in the smallest marine mammal. Science 2021, 373, 223–225. [Google Scholar] [CrossRef]
- Keipert, S.; Kutschke, M.; Ost, M.; Schwarzmayr, T.; van Schothorst, E.M.; Lamp, D.; Brachthäuser, L.; Hamp, I.; Mazibuko, S.E.; Hartwig, S.; et al. Long-Term Cold Adaptation Does Not Require FGF21 or UCP1. Cell Metab. 2017, 26, 437–446.e435. [Google Scholar] [CrossRef]
- Young, T.K. Obesity, central fat patterning, and their metabolic correlates among the inuit of the central Canadian Arctic. Hum. Biol. 1996, 68, 245–263. [Google Scholar]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Zafrir, B. Brown adipose tissue: Research milestones of a potential player in human energy balance and obesity. Horm. Metab. Res. 2013, 45, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, Y.; Ruze, R.; Xu, R.; Song, J.; Wang, C.; Xu, Q. The evolving view of thermogenic fat and its implications in cancer and metabolic diseases. Signal Transduct. Target. Ther. 2022, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Zhu, X.; Maretich, P.; Chen, Y. Metabolic Improvement via Enhancing Thermogenic Fat-Mediated Non-shivering Thermogenesis: From Rodents to Humans. Front. Endocrinol 2020, 11, 633. [Google Scholar] [CrossRef]
- Scheele, C.; Nielsen, S. Metabolic regulation and the anti-obesity perspectives of human brown fat. Redox Biol. 2017, 12, 770–775. [Google Scholar] [CrossRef]
- Cinti, S. Between brown and white: Novel aspects of adipocyte differentiation. Ann. Med. 2011, 43, 104–115. [Google Scholar] [CrossRef]
- Palacios, E.; Neitzschman, H.R.; Nguyen, J. Madelung Disease: Multiple Symmetric Lipomatosis. Ear Nose Throat J. 2014, 93, 94–96. [Google Scholar] [CrossRef]
- Heaton, J.M. The distribution of brown adipose tissue in the human. J. Anat. 1972, 112 Pt 2, 35–39. [Google Scholar]
- Tanuma, Y.; Yamamoto, M.; Ito, T.; Yokochi, C. The Occurrence of Brown Adipose Tissue in Perirenal Fat in Japanese. Arch. Histol. Jpn. 1975, 38, 43–70. [Google Scholar] [CrossRef]
- Trayhurn, P.; Arch, J.R.S. New Physiological Aspects of Brown Adipose Tissue. Curr. Obes. Rep. 2014, 3, 414–421. [Google Scholar] [CrossRef]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V.; et al. Human BAT Possesses Molecular Signatures That Resemble Beige/Brite Cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; de Jong, J.M.A.; Fischer, A.W.; Nedergaard, J.; Petrovic, N. Human brown adipose tissue: Classical brown rather than brite/beige? Exp. Physiol. 2020, 105, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Novak, M. Development of brown and white adipose tissue. J. Lipid Res. 1975, 16, 79–91. [Google Scholar] [CrossRef]
- Kim, S.H.; Plutzky, J. Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders. Diabetes Metab. J. 2016, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.E.; Bloor, I.; Ojha, S.; Budge, H. The Placenta, Maternal Diet and Adipose Tissue Development in the Newborn. Ann. Nutr. Metab. 2017, 70, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dilbaz, S.; Coßmann, J.; Hoang, A.C.; Diedrich, V.; Herwig, A.; Harauma, A.; Hoshi, Y.; Moriguchi, T.; Landgraf, K.; et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J. Clin. Investig. 2019, 129, 2485–2499. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, G.; Shao, M.; Nham, K.; An, Y.; Wang, Q.; Zhu, Y.; Kusminski, C.M.; Hassan, G.; Gupta, R.K.; et al. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab. 2018, 27, 252–262.e3. [Google Scholar] [CrossRef]
- Hoang, A.C.; Yu, H.; Röszer, T. Transcriptional Landscaping Identifies a Beige Adipocyte Depot in the Newborn Mouse. Cells 2021, 10, 2368. [Google Scholar] [CrossRef]
- Novak, M.; Monkus, E.; Wolf, H. The metabolism of subcutaneous adipose tissue in the immediate postnatal period of human neonates. 3. Role of fetal glycogen in lipolysis and fatty acid esterification in the first hours of life. Pediatr. Res. 1973, 7, 769–777. [Google Scholar] [CrossRef]
- Novak, M.; Monkus, E.; Pardo, V. Human neonatal subcutaneous adipose tissue. Function and ultrastructure. Biol. Neonate 1971, 19, 306–321. [Google Scholar] [CrossRef]
- Röszer, T. Co-Evolution of Breast Milk Lipid Signaling and Thermogenic Adipose Tissue. Biomolecules 2021, 11, 1705. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, D.; Landgraf, K.; Wagner, I.V.; Gesing, J.; Tauscher, R.; Lakowa, N.; Kiess, W.; Bühligen, U.; Wojan, M.; Till, H.; et al. Direct Evidence of Brown Adipocytes in Different Fat Depots in Children. PLoS ONE 2015, 10, e0117841. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Brown and Beige Adipose Tissues in Health and Disease. Compr. Physiol. 2017, 7, 1281–1306. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Gavalda-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tang, M.; Xiao, T.; Liu, H.; Liu, W.; Li, G.; Zhang, F.; Xiao, Y.; Zhou, Z.; Liu, F.; et al. Obesity-Associated miR-199a/214 Cluster Inhibits Adipose Browning via PRDM16–PGC-1α Transcriptional Network. Diabetes 2018, 67, 2585–2600. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, N.; Kotschi, S.; Bartelt, A. Fire up the pyre: Inosine thermogenic signaling for obesity therapy. Signal Transduct. Target. Ther. 2022, 7, 375. [Google Scholar] [CrossRef]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef]
- Kooijman, S.; van den Berg, R.; Ramkisoensing, A.; Boon, M.R.; Kuipers, E.N.; Loef, M.; Zonneveld, T.C.; Lucassen, E.A.; Sips, H.C.; Chatzispyrou, I.A.; et al. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc. Natl. Acad. Sci. USA 2015, 112, 6748–6753. [Google Scholar] [CrossRef]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- Finlin, B.S.; Memetimin, H.; Zhu, B.; Confides, A.L.; Vekaria, H.J.; El Khouli, R.H.; Johnson, Z.R.; Westgate, P.M.; Chen, J.; Morris, A.J.; et al. The beta3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Investig. 2020, 130, 2319–2331. [Google Scholar] [CrossRef]
- Payab, M.; Abedi, M.; Foroughi Heravani, N.; Hadavandkhani, M.; Arabi, M.; Tayanloo-Beik, A.; Sheikh Hosseini, M.; Gerami, H.; Khatami, F.; Larijani, B.; et al. Brown adipose tissue transplantation as a novel alternative to obesity treatment: A systematic review. Int. J. Obes. 2021, 45, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Baxa, U.; Niu, G.; Chen, X.; Veech, R.L. A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life 2013, 65, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Xia, F.; Chen, L.; Lv, Y.; Lv, S.; Yu, J.; Liu, J.; Ding, G. Differential Responses of White Adipose Tissue and Brown Adipose Tissue to Calorie Restriction During Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary Factors Promoting Brown and Beige Fat Development and Thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef]
- Mercer, S.W.; Trayhurn, P. Effect of High Fat Diets on the Thermogenic Activity of Brown Adipose Tissue in Cold-Acclimated Mice1. J. Nutr. 1984, 114, 1151–1158. [Google Scholar] [CrossRef]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef]
- Madsen, L.; Myrmel, L.S.; Fjære, E.; Øyen, J.; Kristiansen, K. Dietary Proteins, Brown Fat, and Adiposity. Front. Physiol. 2018, 9, 1792. [Google Scholar] [CrossRef]
- Maliszewska, K.; Adamska-Patruno, E.; Miniewska, K.; Bauer, W.; Buczyńska, A.; Mojsak, M.; Kretowski, A. Different Protein Sources Enhance 18FDG-PET/MR Uptake of Brown Adipocytes in Male Subjects. Nutrients 2022, 14, 3411. [Google Scholar] [CrossRef]
- Maliszewska, K.; Adamska-Patruno, E.; Miniewska, K.; Bauer, W.; Mojsak, M.; Kretowski, A. PET/MRI-evaluated brown adipose tissue activity may be related to dietary MUFA and omega-6 fatty acids intake. Sci. Rep. 2022, 12, 4112. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Chang, E.; Kim, Y. Vitamin D Insufficiency Exacerbates Adipose Tissue Macrophage Infiltration and Decreases AMPK/SIRT1 Activity in Obese Rats. Nutrients 2017, 9, 338. [Google Scholar] [CrossRef]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Niemann, B.; Haufs-Brusberg, S.; Puetz, L.; Feickert, M.; Jaeckstein, M.Y.; Hoffmann, A.; Zurkovic, J.; Heine, M.; Trautmann, E.-M.; Müller, C.E.; et al. Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine. Nature 2022, 609, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Jobgen, W.; Meininger, C.J.; Jobgen, S.C.; Li, P.; Lee, M.-J.; Smith, S.B.; Spencer, T.E.; Fried, S.K.; Wu, G. Dietary l-Arginine Supplementation Reduces White Fat Gain and Enhances Skeletal Muscle and Brown Fat Masses in Diet-Induced Obese Rats. J. Nutr. 2008, 139, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Leiria, L.O.; Wang, C.-H.; Lynes, M.D.; Yang, K.; Shamsi, F.; Sato, M.; Sugimoto, S.; Chen, E.Y.; Bussberg, V.; Narain, N.R.; et al. 12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat. Cell Metab. 2019, 30, 768–783.e767. [Google Scholar] [CrossRef]
- Ozaki, K.; Sano, T.; Tsuji, N.; Matsuura, T.; Narama, I. Carnitine is necessary to maintain the phenotype and function of brown adipose tissue. Lab. Investig. 2011, 91, 704–710. [Google Scholar] [CrossRef]
- Liu, X.; Fan, W.; Zhang, X.; Zhan, S.; Zhong, T.; Guo, J.; Wang, Y.; Cao, J.; Li, L.; Zhang, H.; et al. Maternal L-carnitine supplementation promotes brown adipose tissue thermogenesis of newborn goats after cold exposure. Faseb. J. 2022, 36, e22461. [Google Scholar] [CrossRef]
- Sae-tan, S.; Grove, K.A.; Lambert, J.D. Weight control and prevention of metabolic syndrome by green tea. Pharmacol. Res. 2011, 64, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, K.; Wayne, D.; Leija, H.A.L.; Bloor, I.; Morris, D.E.; Law, J.; Budge, H.; Sacks, H.; Symonds, M.E.; Sottile, V. Caffeine exposure induces browning features in adipose tissue in vitro and in vivo. Sci. Rep. 2019, 9, 9104. [Google Scholar] [CrossRef] [PubMed]
- Bracco, D.; Ferrarra, J.M.; Arnaud, M.J.; Jéquier, E.; Schutz, Y. Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am. J. Physiol. 1995, 269, E671–E678. [Google Scholar] [CrossRef]
- Martins, B.C.; Soares, A.C.; Martins, F.F.; Resende, A.C.; Inada, K.O.P.; Souza-Mello, V.; Nunes, N.M.; Daleprane, J.B. Coffee consumption prevents obesity-related comorbidities and attenuates brown adipose tissue whitening in high-fat diet-fed mice. J. Nutr. Biochem. 2023, 117, 109336. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Kawai, Y.; Iwanaga, T.; Saito, M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr. 2012, 95, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A. Safety and efficacy of high-protein diets for weight loss. Proc. Nutr. Soc. 2012, 71, 339–349. [Google Scholar] [CrossRef]
- Glick, Z.; Wickler, S.J.; Stern, J.S.; Horwitz, B.A. Blood Flow into Brown Fat of Rats Is Greater after a High Carbohydrate than after a High Fat Test Meal. J. Nutr. 1984, 114, 1934–1939. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Saville, M.E.; Stock, M.J. Brown fat activity in fasted and refed rats. Biosci. Rep. 1984, 4, 351–357. [Google Scholar] [CrossRef]
- Becerril, S.; Rodríguez, A.; Catalán, V.; Sáinz, N.; Ramírez, B.; Collantes, M.; Peñuelas, I.; Gómez-Ambrosi, J.; Frühbeck, G. Deletion of inducible nitric-oxide synthase in leptin-deficient mice improves brown adipose tissue function. PLoS ONE 2010, 5, e10962. [Google Scholar] [CrossRef]
- Park, J.H.; Hur, W.; Lee, S.B. Intricate Transcriptional Networks of Classical Brown and Beige Fat Cells. Front. Endocrinol. 2015, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Quesada-López, T.; Cereijo, R.; Turatsinze, J.-V.; Planavila, A.; Cairó, M.; Gavaldà-Navarro, A.; Peyrou, M.; Moure, R.; Iglesias, R.; Giralt, M.; et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat. Commun. 2016, 7, 13479. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chen, C.-Y.; Kang, J.; Nie, Y. Endogenous Omega-3 Polyunsaturated Fatty Acids Preserved Morphology and Function of Brown Fat Impaired by High-Fat Diet Feeding in Mice. Curr. Dev. Nutr. 2021, 5, 1214. [Google Scholar] [CrossRef]
- Moradi, S.; Alivand, M.; KhajeBishak, Y.; AsghariJafarabadi, M.; Alipour, M.; Faghfouri, A.; Alipour, B. The Effect of ω3 Fatty Acids Supplementation on Levels of PPARγ and UCP2 Genes Expression, Serum Level of UCP2 Protein, Metabolic Status, and Appetite in Elite male Athletes: Protocol for a Randomized Control Trial. Int. J. Surg. Protoc. 2021, 25, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J. Omega 3 supplements do not reduce risk of heart disease, stroke, or death, finds review. BMJ 2018, 362, k3149. [Google Scholar] [CrossRef]
- Simopoulos, A. The FTO Gene, Browning of Adipose Tissue and Omega-3 Fatty Acids. J. Nutr. Nutr. 2016, 9, 123–126. [Google Scholar] [CrossRef]
- Rice, S.; Mikes, M.; Drew, K.; Bibus, D. Impacts of a Balanced Omega 6:3 Diet on Fatty Acid Deposition in White and Brown Adipose Tissue and Circulating Plasma in the Hibernating Arctic Ground Squirrel. FASEB J. 2019, 33, lb321. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Miazek, K.; Selmi, A.; Balcerczyk, A.; Śliwińska, A. The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders? Int. J. Mol. Sci. 2022, 23, 956. [Google Scholar] [CrossRef]
- Nimitphong, H.; Park, E.; Lee, M.-J. Vitamin D regulation of adipogenesis and adipose tissue functions. Nutr. Res. Pract. 2020, 14, 553–567. [Google Scholar] [CrossRef]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E. Vitamin D deficiency in the aetiology of obesity-related insulin resistance. Diabetes Metab. Res. Rev. 2019, 35, e3146. [Google Scholar] [CrossRef]
- de Oliveira, L.F.; de Azevedo, L.G.; da Mota Santana, J.; de Sales, L.P.C.; Pereira-Santos, M. Obesity and overweight decreases the effect of vitamin D supplementation in adults: Systematic review and meta-analysis of randomized controlled trials. Rev. Endocr. Metab. Disord. 2020, 21, 67–76. [Google Scholar] [CrossRef]
- Hoang, A.C.; Sasi-Szabó, L.; Pál, T.; Szabó, T.; Diedrich, V.; Herwig, A.; Landgraf, K.; Körner, A.; Röszer, T. Mitochondrial RNA stimulates beige adipocyte development in young mice. Nat. Metab. 2022, 4, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Gupta, M.; Feldman, B.J. Vitamin D Regulates Fatty Acid Composition in Subcutaneous Adipose Tissue Through Elovl3. Endocrinology 2016, 157, 91–97. [Google Scholar] [CrossRef]
- Mutt, S.J.; Hyppönen, E.; Saarnio, J.; Järvelin, M.R.; Herzig, K.H. Vitamin D and adipose tissue-more than storage. Front. Physiol. 2014, 5, 228. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I.; Young, J.B. Birth weight, climate at birth and the risk of obesity in adult life. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 281–287. [Google Scholar] [CrossRef]
- Wattie, N.; Ardern, C.I.; Baker, J. Season of birth and prevalence of overweight and obesity in Canada. Early Hum. Dev. 2008, 84, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Futawaka, K.; Koyama, R.; Fukuda, Y.; Hayashi, M.; Imamoto, M.; Miyawaki, T.; Kasahara, M.; Tagami, T.; Moriyama, K. Vitamin D3/VDR resists diet-induced obesity by modulating UCP3 expression in muscles. J. Biomed. Sci. 2016, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Wu, Z.; Satterfield, M.C.; Bazer, F.W.; Wu, G. Regulation of brown adipose tissue development and white fat reduction by L-arginine. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 529–538. [Google Scholar] [CrossRef]
- Boon, M.R.; Hanssen, M.J.W.; Brans, B.; Hulsman, C.J.M.; Hoeks, J.; Nahon, K.J.; Bakker, C.; van Klinken, J.B.; Havekes, B.; Schaart, G.; et al. Effect of L-arginine on energy metabolism, skeletal muscle and brown adipose tissue in South Asian and Europid prediabetic men: A randomised double-blinded crossover study. Diabetologia 2019, 62, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1357. [Google Scholar] [CrossRef] [PubMed]
- Wolfs, D.; Lynes, M.D.; Tseng, Y.H.; Pierce, S.; Bussberg, V.; Darkwah, A.; Tolstikov, V.; Narain, N.R.; Rudolph, M.C.; Kiebish, M.A.; et al. Brown Fat-Activating Lipokine 12,13-diHOME in Human Milk Is Associated with Infant Adiposity. J. Clin. Endocrinol. Metab. 2021, 106, e943–e956. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Larsen, L.H.; Lauritzen, L. Fish oil as a potential activator of brown and beige fat thermogenesis. Adipocyte 2018, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Nagatake, T.; Shibata, Y.; Morimoto, S.; Node, E.; Sawane, K.; Hirata, S.-I.; Adachi, J.; Abe, Y.; Isoyama, J.; Saika, A.; et al. 12-Hydroxyeicosapentaenoic acid inhibits foam cell formation and ameliorates high-fat diet-induced pathology of atherosclerosis in mice. Sci. Rep. 2021, 11, 10426. [Google Scholar] [CrossRef]
- Iannitti, T.; Palmieri, B. An update on the therapeutic role of alkylglycerols. Mar. Drugs 2010, 8, 2267–2300. [Google Scholar] [CrossRef]
- Tsukada, A.; Okamatsu-Ogura, Y.; Futagawa, E.; Habu, Y.; Takahashi, N.; Kato-Suzuki, M.; Kato, Y.; Ishizuka, S.; Sonoyama, K.; Kimura, K. White adipose tissue undergoes browning during preweaning period in association with microbiota formation in mice. iScience 2023, 26, 107239. [Google Scholar] [CrossRef]
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfäffle, R.; Kiess, W.; Körner, A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 2018, 379, 1303–1312. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Cave, M.C.; Hurt, R.T.; Frazier, T.H.; Matheson, P.J.; Garrison, R.N.; McClain, C.J.; McClave, S.A. Obesity, inflammation, and the potential application of pharmaconutrition. Nutr. Clin. Pract. 2008, 23, 16–34. [Google Scholar] [CrossRef]
- Zhang, E.; Jin, L.; Wang, Y.; Tu, J.; Zheng, R.; Ding, L.; Fang, Z.; Fan, M.; Al-Abdullah, I.; Natarajan, R.; et al. Intestinal AMPK modulation of microbiota mediates crosstalk with brown fat to control thermogenesis. Nat. Commun. 2022, 13, 1135. [Google Scholar] [CrossRef]
- Gleason, J.L.; Sundaram, R.; Mitro, S.D.; Hinkle, S.N.; Gilman, S.E.; Zhang, C.; Newman, R.B.; Hunt, K.J.; Skupski, D.W.; Grobman, W.A.; et al. Association of Maternal Caffeine Consumption during Pregnancy with Child Growth. JAMA Netw. Open 2022, 5, e2239609. [Google Scholar] [CrossRef]
- Voerman, E.; Jaddoe, V.W.; Hulst, M.E.; Oei, E.H.; Gaillard, R. Associations of maternal caffeine intake during pregnancy with abdominal and liver fat deposition in childhood. Pediatr. Obes. 2020, 15, e12607. [Google Scholar] [CrossRef]
- Janssens, P.L.; Hursel, R.; Martens, E.A.; Westerterp-Plantenga, M.S. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS ONE 2013, 8, e67786. [Google Scholar] [CrossRef] [PubMed]
- Kida, R.; Noguchi, T.; Murakami, M.; Hashimoto, O.; Kawada, T.; Matsui, T.; Funaba, M. Supra-pharmacological concentration of capsaicin stimulates brown adipogenesis through induction of endoplasmic reticulum stress. Sci. Rep. 2018, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, F.; Piper, M.; Ho, L.L.; Huang, T.L.; Gupta, A.; Streets, A.; Lynes, M.D.; Tseng, Y.H. Vascular smooth muscle-derived Trpv1(+) progenitors are a source of cold-induced thermogenic adipocytes. Nat. Metab. 2021, 3, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, J.; Qi, F. Identification of key candidate genes and molecular pathways in white fat browning: An anti-obesity drug discovery based on computational biology. Hum. Genom. 2019, 13, 55. [Google Scholar] [CrossRef]
- Tsuji, T.; Bussberg, V.; MacDonald, A.M.; Narain, N.R.; Kiebish, M.A.; Tseng, Y.H. Transplantation of Brown Adipose Tissue with the Ability of Converting Omega-6 to Omega-3 Polyunsaturated Fatty Acids Counteracts High-Fat-Induced Metabolic Abnormalities in Mice. Int. J. Mol. Sci. 2022, 23, 5321. [Google Scholar] [CrossRef] [PubMed]

| Types | Models | Impacts/Effects | Refs |
|---|---|---|---|
| Macronutrients | |||
| Proteins | healthy men | ↑ BAT activity | [69,70,71] |
| Carbohydrates | male mice | ↑ thermogenesis and blood flow | [40] |
| Intermittent fasting | mice | ↓ body weight; ↑ beige fat activity | [63] |
| Caloric restriction | mice | ↓ body weight, leptin, glucose; ↑ beige fat and BAT activity | [66] |
| Omega-3 fatty acids | healthy men | ↓ body weight; ↑ BAT activity | [72] |
| Omega-6 fatty acids | healthy men | ↑ body weight; ↓ BAT activity | [73] |
| Micronutrients | |||
| Vitamin D | obese rats | ↓ body weight; ↑ WAT browning | [74] |
| Succinate | male mice | ↓ body weight; ↑ glucose tolerance | [75] |
| Inosine | male mice | ↓ body weight; ↑ glucose tolerance | [76] |
| Arginine | obese rats | ↓ body fat; ↑ skeletal muscle and BAT mass | [77] |
| 12,13-diHome | healthy men and women | ↓ BMI and triglycerides; ↑ BAT activity | [78] |
| 12-HEPE | male mice | ↑ glucose tolerance | [79] |
| Alkylglycerols | infant mice | ↓ fat accumulation; ↑ beige fat activity | [47] |
| Carnitine | juvenile visceral steatosis mice | ↑ thermogenesis and BAT activity | [80] |
| newborn goats | ↑ thermogenesis and BAT activity | [81] | |
| Natural Product | |||
| Epigallocatechin gallate (EGCG) | male mice | ↑ insulin sensitivity, thermogenesis, UCP1 level; ↓ blood glucose | [82] |
| Resveratrol | obese men | ↓ triglycerides, glucose, energy expenditure | [83] |
| Caffeine | healthy men and women | ↑ thermogenesis | [84] |
| obese men | ↑ energy expenditure and lipid oxidation | [85] | |
| obese mice | ↓ body weight; ↑ glucose tolerance, UCP1 level | [86] | |
| Capsaicin | healthy men | ↑ Energy Expenditure, BAT activity | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noriega, L.; Yang, C.-Y.; Wang, C.-H. Brown Fat and Nutrition: Implications for Nutritional Interventions. Nutrients 2023, 15, 4072. https://doi.org/10.3390/nu15184072
Noriega L, Yang C-Y, Wang C-H. Brown Fat and Nutrition: Implications for Nutritional Interventions. Nutrients. 2023; 15(18):4072. https://doi.org/10.3390/nu15184072
Chicago/Turabian StyleNoriega, Lloyd, Cheng-Ying Yang, and Chih-Hao Wang. 2023. "Brown Fat and Nutrition: Implications for Nutritional Interventions" Nutrients 15, no. 18: 4072. https://doi.org/10.3390/nu15184072
APA StyleNoriega, L., Yang, C.-Y., & Wang, C.-H. (2023). Brown Fat and Nutrition: Implications for Nutritional Interventions. Nutrients, 15(18), 4072. https://doi.org/10.3390/nu15184072






