Sarcopenia as a Little-Recognized Comorbidity of Type II Diabetes Mellitus: A Review of the Diagnosis and Treatment
Abstract
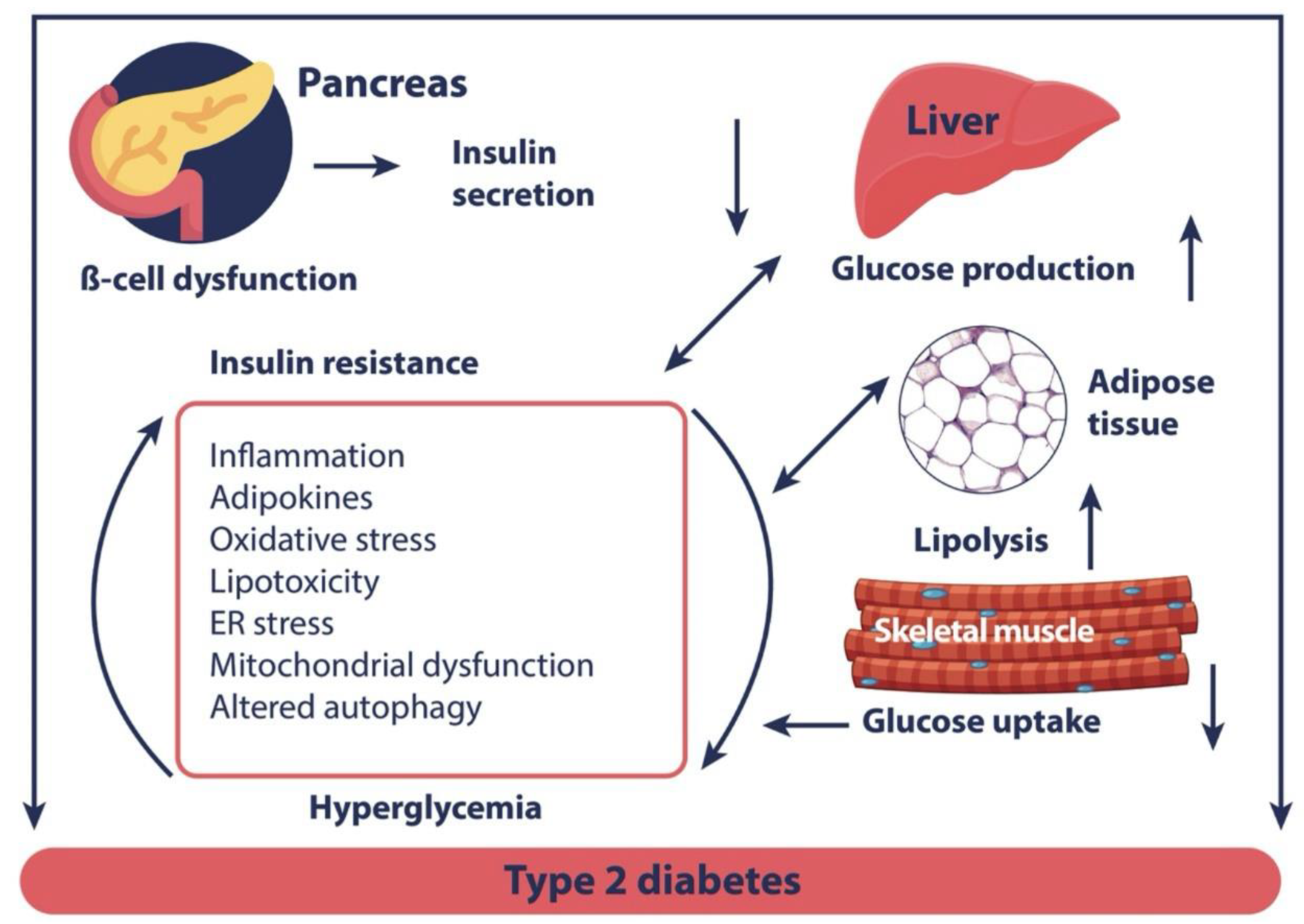
:1. Introduction
2. Methodology
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Review Process
2.4. Data Synthesis
3. Results
- (A)
- What is the pathophysiology and prevalence of a loss of muscle mass and function associated with T2DM?
- (B)
- What is the best treatment for a loss of muscle mass and function in T2DM patients?
- (C)
- How feasible is the measurement of muscle mass and function in T2DM?
3.1. Diagnostic Methods
3.2. Treatment
3.2.1. Choosing a Glucose-Lowering Drug
3.2.2. Nutritional Intervention
3.2.3. Physical Activity
3.2.4. Correct Daily Regime
4. Discussion
5. Future Directions
5.1. Pathophysiology
5.2. Diagnostic Methods
5.3. Treatment Options
5.4. Prevalence
5.5. Technology Integration
5.6. Patient-Centered Approaches
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Sanz Paris, A.; García, J.M.; Gómez-Candela, C.; Burgos, R.; Martín, A.; Matía, P.; Martín Palmero, Á. Malnutrition prevalence in hospitalized elderly patients. Nutr. Hosp. 2013, 28, 592–599. [Google Scholar] [PubMed]
- Gómez-Candela, C.; Pérez, L.; Sanz París, A.; Burgos, R.; Matía, P.; García, J.M.; Martín Palmero, Á. Análisis del perfil de los pacientes ancianos diabéticos hospitalizados que participaron en el estudio VIDA. Nutr. Hosp. 2016, 33, 31–36. [Google Scholar] [CrossRef]
- Álvarez Hernández, J.; León Sanz, M.; Planas Vilá, M.; Araujo, K.; García de Lorenzo, A.; Celaya Pérez, S. Prevalence and costs of malnutrition in hospitalized dysphagic patients: A subanalysis of the predyces study. Nutr. Hosp. 2015, 32, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.Z.; Petrina-Jáuregui, M.E.; Ripa-Ciáurriz, C.; Sánchez, R.S.; Villazón-González, F.; Faes, G.-D.; Fernández-López, C.; Calles-Romero, L.; Martín-Palmero, Á.; Riestra-Fernández, M.; et al. SeDREno study-prevalence of hospital malnutrition according to GLIM criteria, ten years after the PREDyCES study. Nutr. Hosp. 2021, 38, 1016–1025. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Bell, J.A.; Ng, S.Y.; Kemp, G.J.; Kivimaki, M.; Hamer, M. Dynapenic obesity and the risk of incident Type 2 diabetes: The English Longitudinal Study of Ageing. Diabet. Med. 2016, 33, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della Pepa, G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef]
- Landi, F.; Onder, G.; Bernabei, R. Sarcopenia and diabetes: Two sides of the same coin. J. Am. Med. Dir. Assoc. 2013, 14, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Gunendi, Z.; Meray, J.; Yetkin, İ. The evaluation of muscle strength and architecture in type 1 diabetes mellitus: A cross-sectional study. BMC Endocr. Disord. 2022, 22, 153. [Google Scholar] [CrossRef]
- Rocha, M.; Apostolova, N.; Diaz-Rua, R.; Muntane, J.; Victor, V.M. Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome. Trends Endocrinol. Metab. 2020, 31, 725–741. [Google Scholar] [PubMed]
- Çakmak, G.; Ganidağlı, S.; Efendioğlu, E.M.; Öztürk, E.; Öztürk, Z.A. Do Long-Term Complications of Type 2 Diabetes Increase Susceptibility to Geriatric Syndromes in Older Adults? Medicina 2021, 57, 968. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Fan, D. Diabetes mellitus is a risk factor for low bone mass-related fractures: A meta-analysis of cohort studies. Medicine 2017, 96, e8811. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, L.M.; Petterle, R.R.; Moreira, C.A.; Borba, V.Z.C. Osteosarcopenia and trabecular bone score in patients with type 2 diabetes mellitus. Arch. Endocrinol. Metab. 2021, 65, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pombo, A.; Rodríguez-Carnero, G.; Castro, A.I.; Cantón-Blanco, A.; Seoane, L.M.; Casanueva, F.F.; Crujeiras, A.B.; Martínez-Olmos, M.A. Relevance of nutritional assessment and treatment to counteract cardiac cachexia and sarcopenia in chronic heart failure. Clin. Nutr. 2021, 40, 5141–5155. [Google Scholar] [CrossRef]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; et al. Association between Geriatric Nutrition Risk Index and The Presence of Sarcopenia in People with Type 2 Diabetes Mellitus: A Cross-Sectional Study. Nutrients 2021, 13, 3729. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Ladang, A.; Beaudart, C.; Reginster, J.Y.; Al-Daghri, N.; Bruyère, O.; Burlet, N.; Cesari, M.; Cherubini, A.; da Silva, M.C.; Cooper, C.; et al. Biochemical Markers of Musculoskeletal Health and Aging to be Assessed in Clinical Trials of Drugs Aiming at the Treatment of Sarcopenia: Consensus Paper from an Expert Group Meeting Organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the Centre Académique de Recherche et d’Expérimentation en Santé (CARES SPRL), Under the Auspices of the World Health Organization Collaborating Center for the Epidemiology of Musculoskeletal Conditions and Aging. Calcif. Tissue Int. 2023, 112, 197–217. [Google Scholar]
- Massimino, E.; Izzo, A.; Riccardi, G.; Della Pepa, G. The Impact of Glucose-Lowering Drugs on Sarcopenia in Type 2 Diabetes: Current Evidence and Underlying Mechanisms. Cells 2021, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Koshizaka, M.; Ishikawa, K.; Ishibashi, R.; Takahashi, S.; Sakamoto, K.; Yokoh, H.; Baba, Y.; Ide, S.; Ide, K.; Ishi kawa, T.; et al. Comparison of Visceral Fat Reduction by Ipragliflozin and Metforminin Elderly Type 2 Diabetes Patients: Sub-Analysisofa Randomized-Controlled Study. Diabetes Ther. 2021, 12, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Nagai, Y.; Kato, H.; Fukuda, H.; Tanaka, Y. Effect of the Dipeptidyl Peptidase- 4 Inhibitor Sitagliptin on Muscle Mass and the Muscle/Fat Ratio in Patients with Type 2 Diabetes. J. Clin. Med. Res. 2020, 12, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T.; Yajima, K.; Takahashi, H.; Yasuda, K. The effect of dulaglutide on body composition in type 2 diabetes mellitus patients on hemodialysis. J. Diabetes Complicat. 2018, 32, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Guido, D.; Bologna, C.; Solerte, S.B.; Guerriero, F.; Isu, A.; Rondanelli, M. Liraglutide and obesity in elderly: Efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin. Exp. Res. 2016, 28, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perna, S.; Astrone, P.; Grugnetti, A.; Solerte, S.B.; Guido, D. Twenty-four-Week effects of liraglutide on body composition, adherence to appetite, and lipid profile in overweight and obese patients with type 2 diabetes mellitus. Patient Prefer. Adherence 2016, 10, 407–413. [Google Scholar] [PubMed]
- Hong, J.Y.; Park, K.Y.; Kim, B.J.; Hwang, W.M.; Kim, D.H.; Lim, D.M. Effects of Short Term Exenatide Treatment on Regional Fat Distribution, Glycated Hemoglobin Levels, and Aortic Pulse Wave Velocity of Obese Type 2 Diabetes Mellitus Patients. Endocrinol. Metab. 2016, 31, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Tsurutani, Y.; Nakai, K.; Inoue, K.; Azuma, K.; Mukai, S.; Maruyama, S.; Lizuka, T.; Matsuzawa, Y.; Saito, J.; Omura, M.; et al. Comparative study of the effects of ipragliflozin and sitagliptin on multiple metabolic variables in Japanese patients with type 2 diabetes: A multicentre, randomized, prospective, open-label, active-controlled study. Diabetes Obes. Metab. 2018, 20, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Meguro, S.; Kawai, T.; Suzuki, Y. Increased grip strength with sodium-glucose cotransporter 2. J. Diabetes 2016, 8, 736–737. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef]
- Sugiyama, S.; Jinnouchi, H.; Kurinami, N.; Hieshima, K.; Yoshida, A.; Jinnouchi, K.; Nishimura, H.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. Dapagliflozin Reduces Fat Mass without Affecting Muscle Mass in Type 2 Diabetes. J. Atheroscler. Thromb. 2018, 25, 467–476. [Google Scholar] [CrossRef]
- Yamakage, H.; Tanaka, M.; Inoue, T.; Odori, S.; Kusakabe, T.; Satoh Asahara, N. Effects of dapagliflozin on the serum levels of fibroblast growth factor 21 and myokines and muscle mass in Japanese patients with type 2 diabetes: A randomized, controlled trial. J. Diabetes Investig. 2020, 11, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Tobita, H.; Sato, S.; Miyake, T.; Ishihara, S.; Kinoshita, Y. Effects of Dapagliflozin on Body Composition and Liver Tests in Patients with Nonalcoholic Steatohepatitis Associated with Type 2 Diabetes Mellitus: A Prospective, Open-label, Uncontrolled Study. Curr. Ther. Res. Clin. Exp. 2017, 87, 13–19. [Google Scholar] [CrossRef]
- Sasaki, T.; Sugawara, M.; Fukuda, M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J. Diabetes Investig. 2019, 10, 108–117. [Google Scholar] [CrossRef]
- Bouchi, R.; Terashima, M.; Sasahara, Y.; Asakawa, M.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: A pilot study. Cardiovasc. Diabetol. 2017, 16, 32. [Google Scholar] [CrossRef]
- Inoue, M.; Hayashi, A.; Taguchi, T.; Arai, R.; Sasaki, S.; Takano, K.; Inoue, Y.; Shichiri, M. Effects of canagliflozin on body composition and hepatic fat content in type 2 diabetes patients with non- alcoholic fatty liver disease. J. Diabetes Investig. 2019, 10, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Morino, K.; Ugi, S.; Tanaka Mizuno, S.; Fuse, K.; Miyazawa, I.; Kondo, K.; Sato, D.; Ohashi, N.; Ida, S.; et al. SUMS-ADDIT-1 Research group. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces body weight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: A randomized clinical trial. J. Diabetes Investig. 2019, 10, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.; Miyoshi, H.; Ono, K.; Sugawara, H.; Kameda, R.; Ichiyama, M.; Yamamoto, K.; Nomoto, H.; Nakamura, A.; Atsumi, T. Ipragliflozin effectively reduced visceral fat in Japanese patients with type 2 diabetes under adequate diet therapy. Endocr. J. 2016, 63, 589–596. [Google Scholar] [CrossRef]
- Iemitsu, K.; Kawata, T.; Iizuka, T.; Takihata, M.; Takai, M.; Nakajima, S.; Minami, N.; Umezawa, S.; Kanamori, A.; Takeda, H.; et al. Efficacy and Safety of Ipragliflozin in Patients with Type 2 Diabetes ASSIGN-K Study. J. Endocrinol. Metab. 2019, 9, 51–62. [Google Scholar] [CrossRef]
- Kato, M.; Sakai, K.; Saito, K.; Tsutsui, K.; Yamashita, S.; Kato, N. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes receiving conventional therapy: Clinical implication of the importance of exercise habits during treatment with ipragliflozin. Diabetol. Int. 2017, 8, 275–285. [Google Scholar] [CrossRef]
- Miyake, T.; Yoshida, S.; Furukawa, S.; Sakai, T.; Tada, F.; Senba, H.; Yamamoto, S.; Koizumi, Y.; Yoshida, O.; H irooka, M.; et al. Ipragliflozin Ameliorates Liver Damage in Non-alcoholic Fatty Liver Disease. Open Med. 2018, 13, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Matsuba, R.; Matsuba, I.; Shimokawa, M.; Nagai, Y.; Tanaka, Y. Tofogliflozin decreases body fat mass and improves peripheral insulin resistance. Diabetes Obes. Metab. 2018, 20, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, U.; Then, C.; Rottenkolber, M.; Selte, C.; Seissler, J.; Conzade, R.; Linkohr, B.; Peters, A.; Drey, M.; Thorand, B. Longitudinal association of type 2 diabetes and insulin therapy with muscle parameters in the KORA-Age study. Acta Diabetol. 2020, 57, 1057–1063. [Google Scholar] [CrossRef]
- Burgos Peláez, R.; García Almeida, J.M.; Matía Martín, P.; Palma Milla, S.; Sanz Paris, A.; Zugasti Murillo, A.; Alfaro Martínez, J.J.; Artero-Fullana, A.; Calañas Continente, A.; Chinchetru, M.J.; et al. Abordaje de la desnutrición en pacientes hospitalizados con diabetes/hiperglucemia y otras patologías [Malnutrition management of hospitalized patients with diabetes/hyperglycemia and concurrent pathologies]. Nutr. Hosp. 2022, 39, 1–8. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Sanz Paris, A.; Álvarez Hernandez, J.; Ballesteros-Pomar, M.A.; Botella-Romero, F.; León-Sanz, M.; Martín-Palmero, A.; Olmos, M.M.; Olveira, G. Evidence-based recommendations and expert consensus on enteral nutrition in the adult patient with diabetes mellitus or hyperglycemia. Nutrition 2017, 41, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Deutz, N.E.P.; Biolo, G.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cuerda, C.; Delzenne, N.; Leon Sanz, M.; Ljungqvist, O.; et al. Carbohydrates and insulin resistance in clinical nutrition: Recommendations from the ESPEN expert group. Clin. Nutr. 2017, 36, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Vaquerizo Alonso, C.; Grau Carmona, T.; Juan Díaz, M.; Spanish Society of Intensive Care Medicine and Coronary Units-Spanish Society of Parenteral and Enteral Nutrition (SEMICYUC-SENPE). Recomendaciones para el soporte nutricional y metabólico especializado del paciente crítico. Actualización. Consenso SEMICYUC-SENPE: Hiperglucemia y diabetes mellitus [Guidelines for specialized nutritional and metabolic support in the critically-ill patient. Update. Consensus of the Spanish Society of Intensive Care Medicine and Coronary Units-Spanish Society of Parenteral and Enteral Nutrition (SEMICYUC-SENPE): Hyperglycemia and diabetes mellitus]. Med. Intensiva 2011, 35 (Suppl. S1), 48–52. (In Spanish) [Google Scholar] [CrossRef]
- Chan, L.C.; Yang, Y.C.; Lin, H.C.; Wahlqvist, M.L.; Hung, Y.J.; Lee, M.S. Nutrition counseling is associated with less sarcopenia in diabetes: A cross-sectional and retrospective cohort study. Nutrition 2021, 91–92, 111269. [Google Scholar] [CrossRef]
- Velázquez-Alva, M.C.; Irigoyen-Camacho, M.E.; Zepeda-Zepeda, M.A.; Lazarevich, I.; Arrieta-Cruz, I.; D’Hyver, C. Sarcopenia, nutritional status and type 2 diabetes mellitus: A cross-sectional study in a group of Mexican women residing in a nursing home. Nutr. Diet. 2020, 77, 515–522. [Google Scholar] [CrossRef]
- Holeček, M.; Vodeničarovová, M.; Fingrová, R. Dual Effects of Beta-Hydroxy-Beta-Methylbutyrate (HMB) on Amino Acid, Energy, and Protein Metabolism in the Liver and Muscles of Rats with Streptozotocin-Induced Type 1 Diabetes. Biomolecules 2020, 10, 1475. [Google Scholar] [CrossRef]
- de Luis Román, D.; Garrachón Vallo, F.; Carretero Gómez, J.; López Gómez, J.J.; Tarazona Santabalbina, F.J.; Guzmán Rolo, G.; García Almeida, J.M.; Sanz Paris, A. La masa muscular disminuida en la diabetes de tipo 2. Una comorbididad oculta que debemos tener en cuenta [Decreased muscle mass in type-2 diabetes. A hidden comorbidity to consider]. Nutr. Hosp. 2023, 11, 59–66. (In Spanish) [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Umpierre, D.; Ribeiro, P.A.B.; Kramer, C.K.; Leitão, C.B.; Zucatti, A.T.N.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.; Chan, E.; Giallauria, F.; Graham, P.L.; Smart, N.A. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: A systematic review and meta- analysis. Cardiovasc. Diabetol. 2017, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Viecelli, C.; Aguayo, D. May the Force and Mass Be with You-Evidence-Based Contribution of MechanoBiological Descriptors of Resistance Exercise. Front. Physiol. 2022, 12, 686119. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Imataka, K.; Murata, K. Relationship between frailty and mortality, hospitalization, and cardiovascular diseases in diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2018, 18, 81. [Google Scholar] [CrossRef]
- Yu, J.H.; Yun, C.H.; Ahn, J.H.; Suh, S.; Cho, H.J.; Lee, S.K.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Choi, K.M.; et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J. Clin. Endocrinol. Metab. 2015, 100, 1494–1502. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, J.; No, M.H.; Heo, J.W.; Cho, E.J.; Chang, E.; Park, D.H.; Kang, J.H.; Kwak, H.B. Re-Setting the Circadian Clock Using Exercise against Sarcopenia. Int. J. Mol. Sci. 2020, 21, 3106. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A. Special Issue Diabetes and Muscle: From the Assessment to Treatment. Nutrients. Available online: https://www.mdpi.com/journal/nutrients/special_issues/3A1M1755Y5 (accessed on 13 September 2023).
- Gamgaram, V.K.D.; Olivares, M.G.; Fuster, G.O. Olveira Fuster, Diabetes y sarcopenia. Nutr. Clin. Med. 2023, XVII, 75–88. [Google Scholar]
- Hashimoto, Y.; Takahashi, F.; Okamura, T.; Hamaguchi, M.; Fukui, M. Diet, exercise, and pharmacotherapy for sarcopenia in people with diabetes. Metabolism 2023, 144, 155585. [Google Scholar] [CrossRef]


| Diagnostic Method | Objective |
| Medical history | Assess history for malabsorption: previous GI procedures, taste disorders, anorexia, nausea, vomiting, gastrointestinal motility disorders, dysphagia, oral health issues, history of pulmonary aspiration, diabetic gastroparesis, allergies, drug use, nutritional supplements, and medications, etc. |
| Physical examination | Assess loss of fat and muscle in specific body regions: orbital, temporal, and intercostal spaces, etc Muehrcke’s lines in the fingernails suggest hypoalbuminemia, alopecia is associated with protein deficiency, and scaling of the scalp results from essential fatty acid deficiency. |
| Anthropometric data | Calculate body mass index (BMI) and percentage of unintentional weight loss. |
| Nutrition assessment | Use questionnaires to assess nutritional risk: Nutritional Risk Screening 2002 (NRS 2002), Subjective Global Assessment (SGA), Mini Nutritional Assessment (MNA), Malnutrition Universal Screening Tool (MUST), and Short Nutritional Assessment Questionnaire (SNAQ). |
| Bioelectrical methods | Bioelectrical impedance analysis (BIA) provides a detailed description of body composition (fat mass, fat-free mass, body water, lean body mass, and vector analysis). |
| Imaging tests | The use of muscle ultrasonography to assess the quadriceps provides a simple, quick, and cost-effective way of estimating total body muscle quantity and quality, but the cut-off points for this tool have not yet been validated in the population. |
| Biochemical laboratory markers | Changes in biochemical parameters such as ghrelin, leptin, adiponectin, myostatin, cancer-associated fibroblast (CAF), tumor necrosis factor-alpha (TNF-a), Interleukin-1 (IL-1), Interleukin-6 (IL-6), growth hormone (GH)/Insulin-like growth factor I (IGF-1), and testosterone. |
| Aerobic activities | • Walking |
| • Swimming | |
| • Dancing | |
| • Cycling | |
| • Ellipticals | |
| • Low-impact aerobics | |
| • Water aerobics | |
| Strength exercises | • Resistance band exercises |
| • Self-loading or with a load | |
| • Climbing stairs | |
| • Sit-to-stand | |
| • Carrying things | |
| • Some tai chi exercises | |
| • Yoga | |
| To improve balance or neuromotor fitness | • Balance |
| • Agility | |
| • Coordination | |
| • Gait | |
| • Proprioceptive training | |
| • Multifaceted activities: tai chi and yoga | |
| Flexibility |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salom Vendrell, C.; García Tercero, E.; Moro Hernández, J.B.; Cedeno-Veloz, B.A. Sarcopenia as a Little-Recognized Comorbidity of Type II Diabetes Mellitus: A Review of the Diagnosis and Treatment. Nutrients 2023, 15, 4149. https://doi.org/10.3390/nu15194149
Salom Vendrell C, García Tercero E, Moro Hernández JB, Cedeno-Veloz BA. Sarcopenia as a Little-Recognized Comorbidity of Type II Diabetes Mellitus: A Review of the Diagnosis and Treatment. Nutrients. 2023; 15(19):4149. https://doi.org/10.3390/nu15194149
Chicago/Turabian StyleSalom Vendrell, Christian, Elisa García Tercero, Juan Bautista Moro Hernández, and Bernardo Abel Cedeno-Veloz. 2023. "Sarcopenia as a Little-Recognized Comorbidity of Type II Diabetes Mellitus: A Review of the Diagnosis and Treatment" Nutrients 15, no. 19: 4149. https://doi.org/10.3390/nu15194149





