A Novel Albumin-Related Nutrition Biomarker Predicts Breast Cancer Prognosis in Neoadjuvant Chemotherapy: A Two-Center Cohort Study
Abstract
:1. Introduction
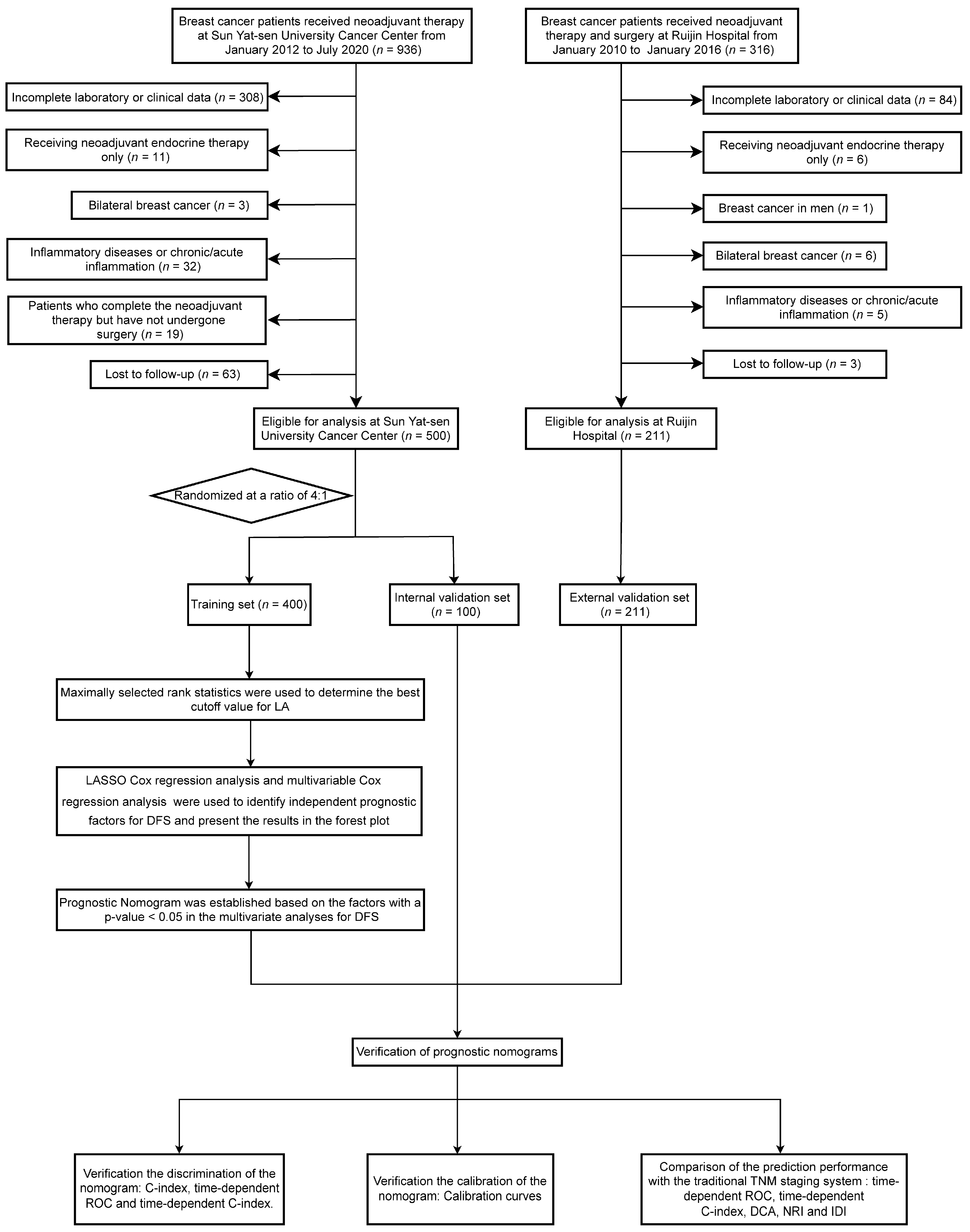
2. Materials and Methods
2.1. Patients
2.2. Data Collection and Classification
2.3. Follow-Up and Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
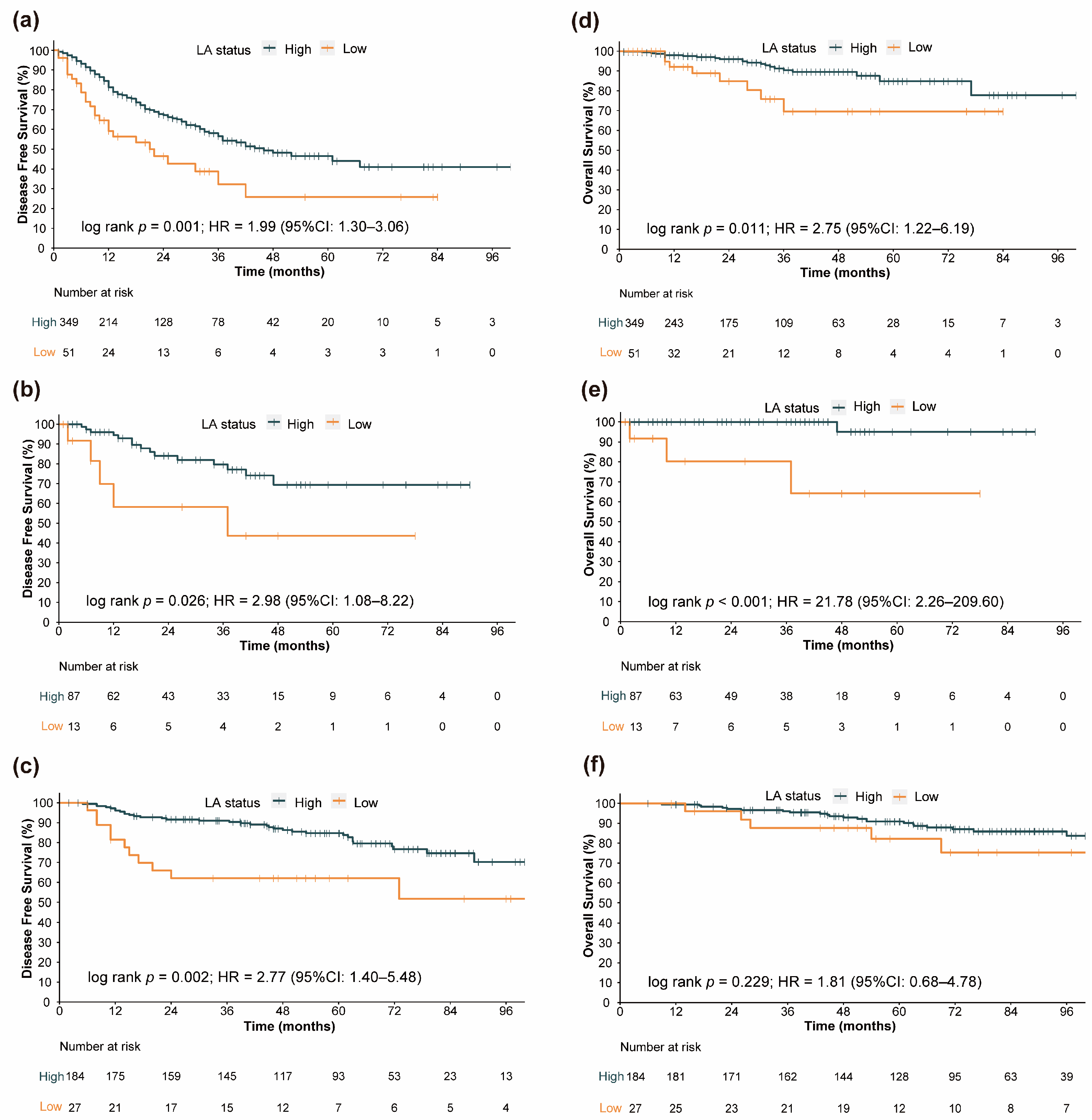
3.2. Prognostic Value of LA
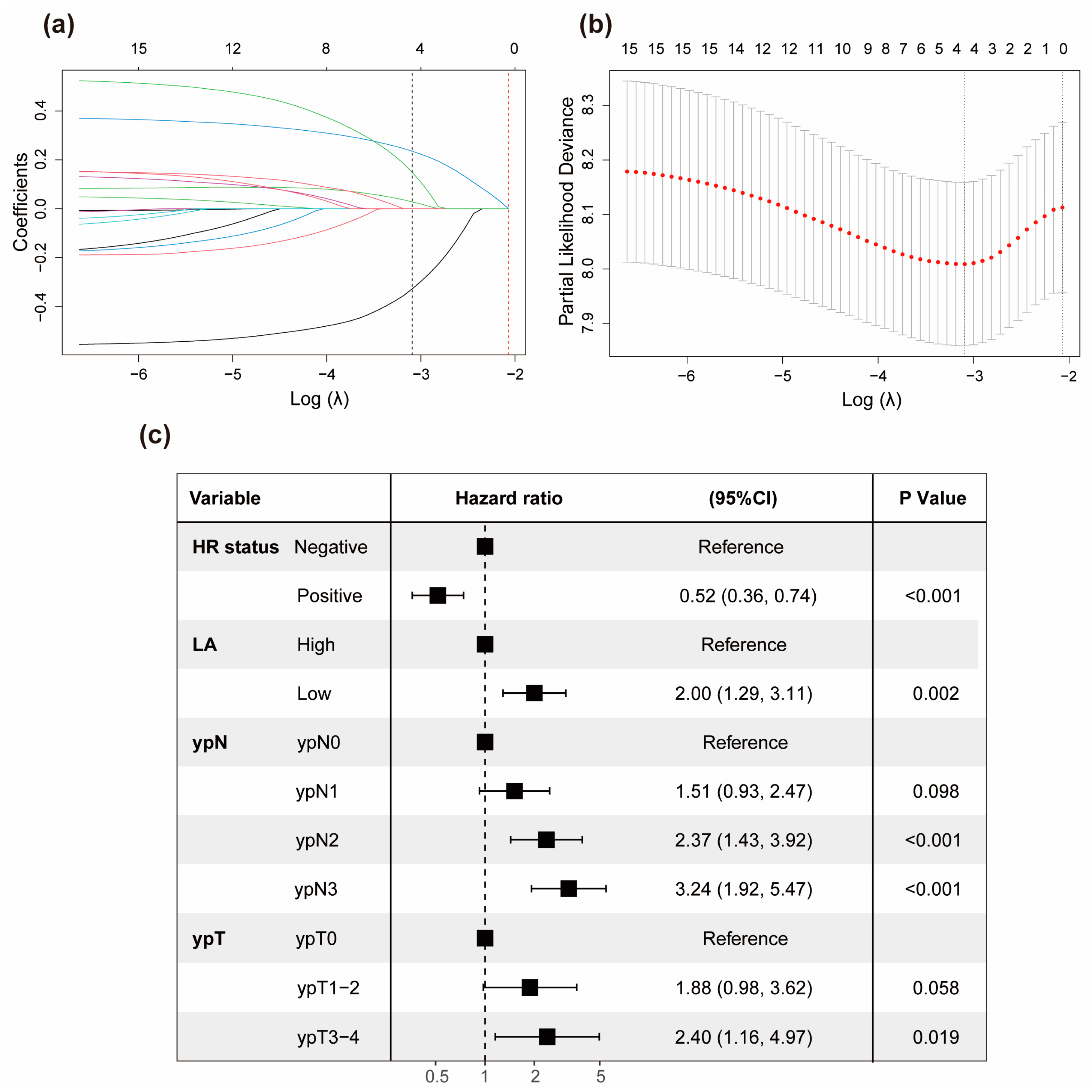
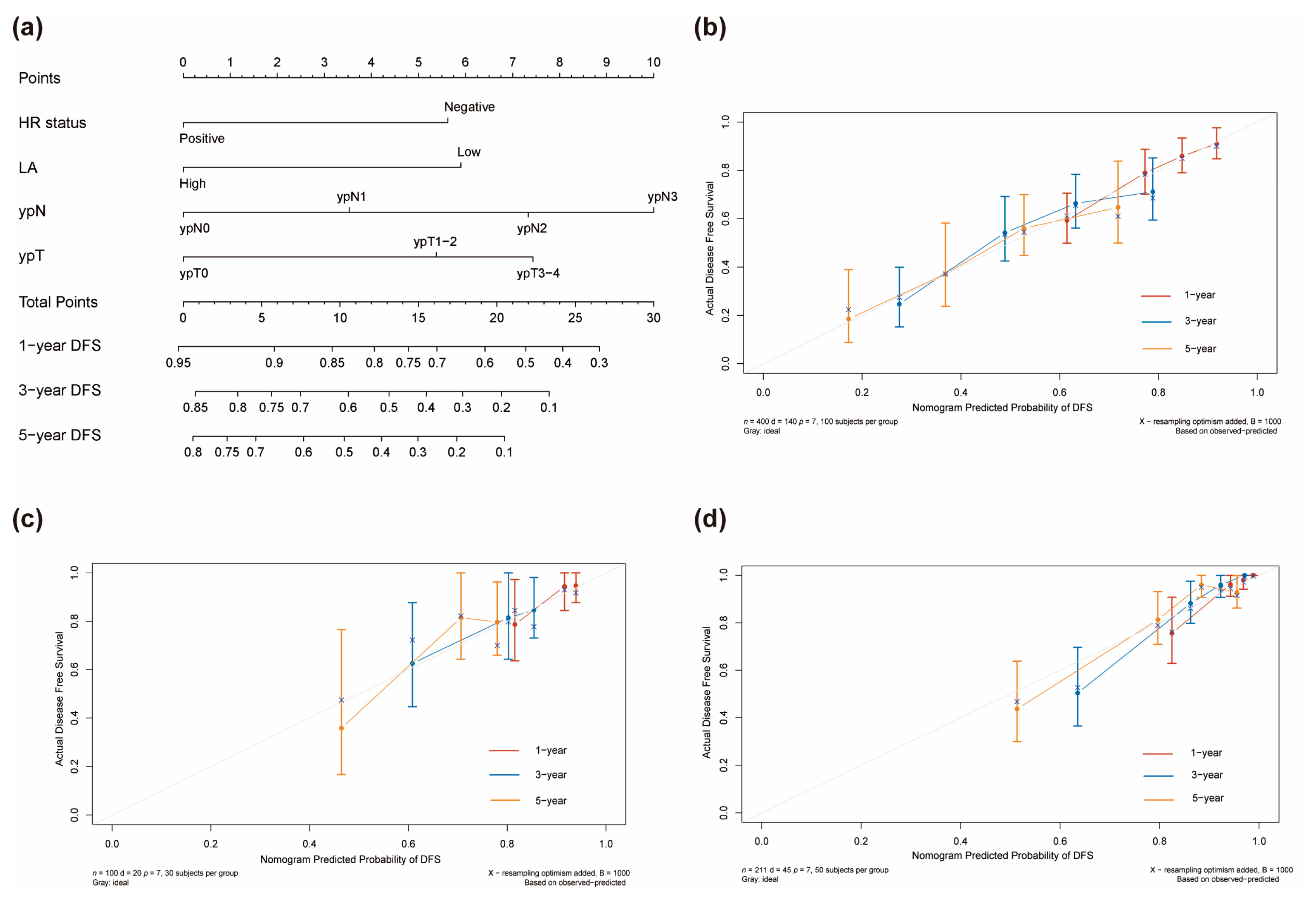
3.3. Development of the Prognostic Model
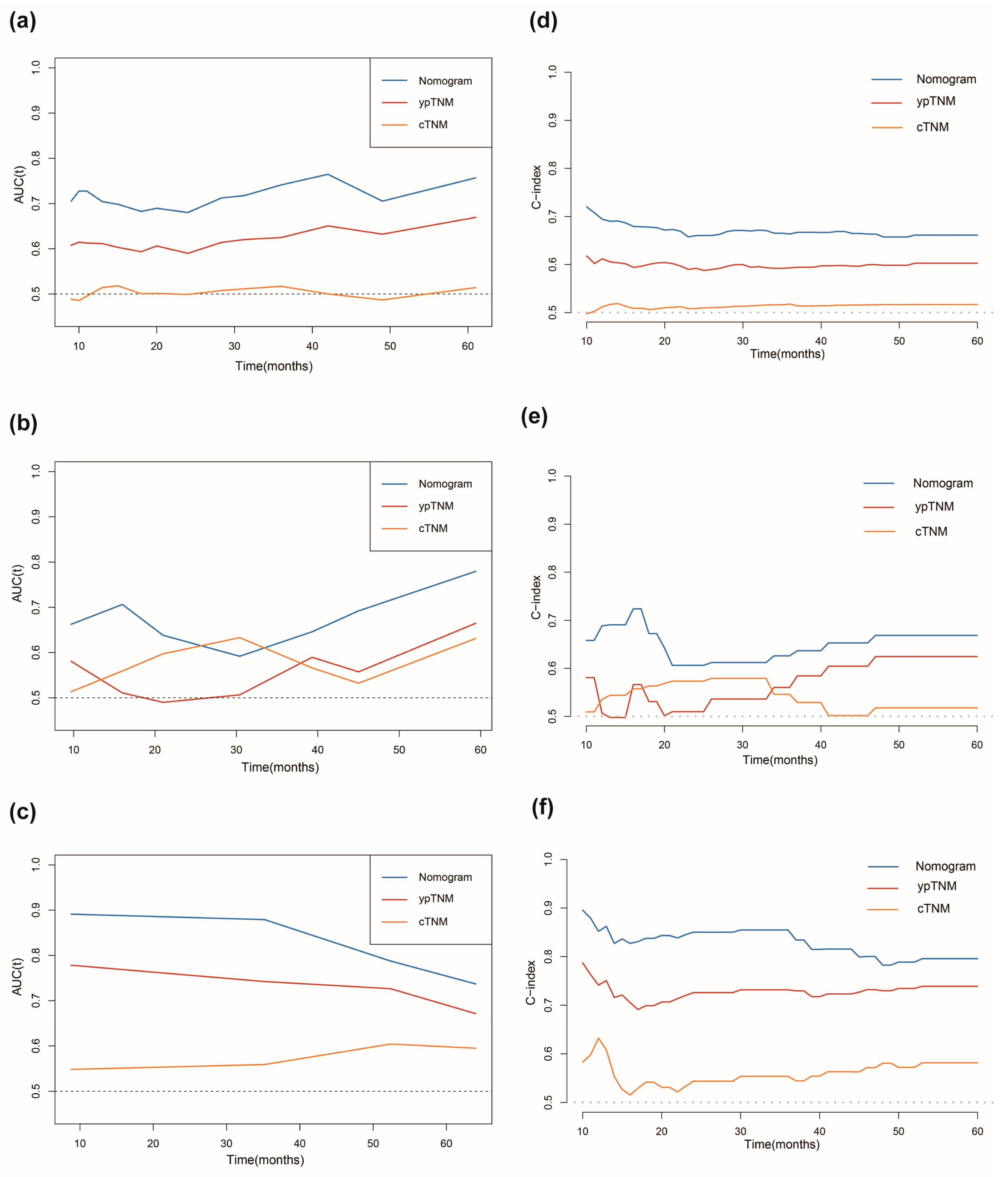
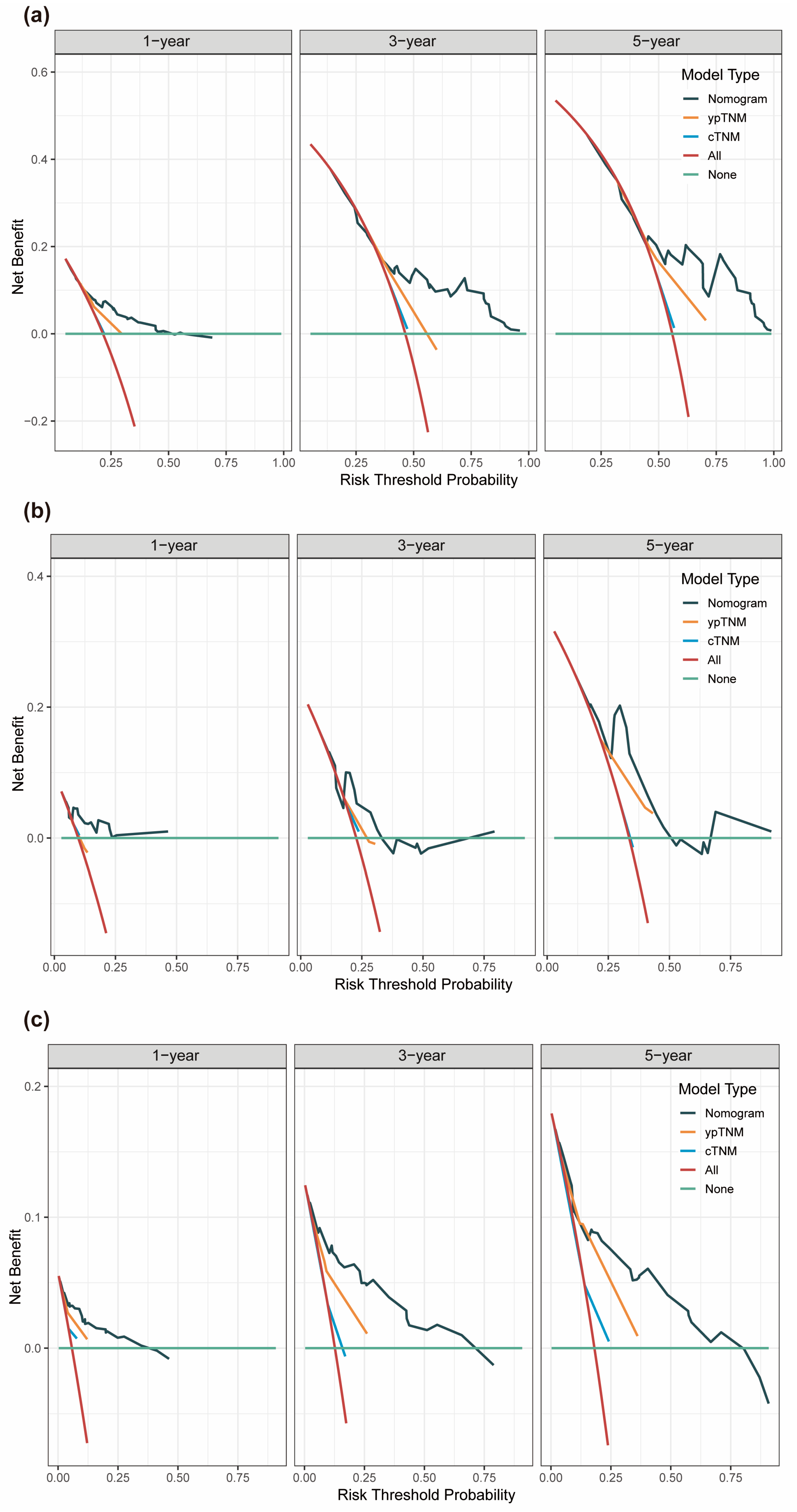
3.4. Assessment of Predictive Performance of the Prognostic Model
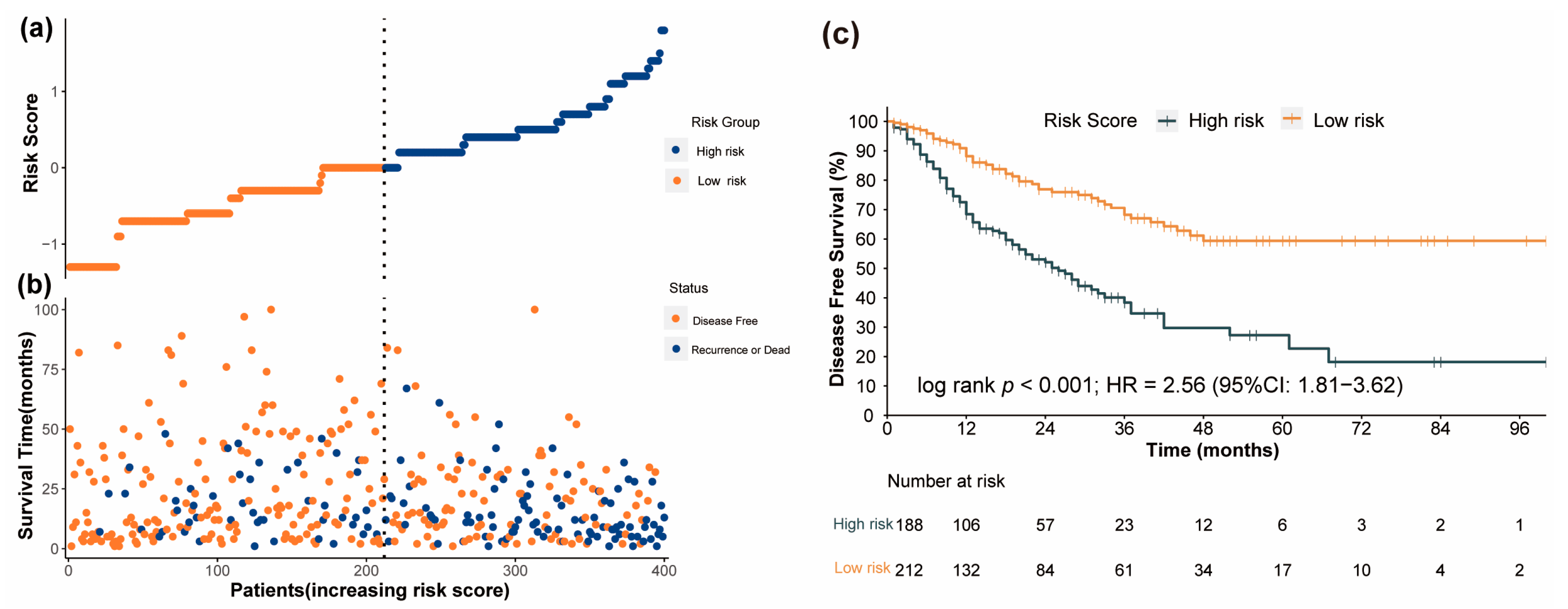
3.5. Performance of the Nomogram in Risk Stratification of Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hong, R.; Xu, B. Breast cancer: An up-to-date review and future perspectives. Cancer Commun. 2022, 42, 913–936. [Google Scholar] [CrossRef]
- Zhao, Y.; Schaafsma, E.; Cheng, C. Gene signature-based prediction of triple-negative breast cancer patient response to Neoadjuvant chemotherapy. Cancer Med. 2020, 9, 6281–6295. [Google Scholar] [CrossRef] [PubMed]
- Tabor, S.; Szostakowska-Rodzos, M.; Fabisiewicz, A.; Grzybowska, E.A. How to Predict Metastasis in Luminal Breast Cancer? Current Solutions and Future Prospects. Int. J. Mol. Sci. 2020, 21, 8415. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrari, P.; Duffy, M.J. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin. Cancer Biol. 2018, 52, 56–73. [Google Scholar]
- Tarighati, E.; Keivan, H.; Mahani, H. A review of prognostic and predictive biomarkers in breast cancer. Clin. Exp. Med. 2023, 23, 1–16. [Google Scholar]
- Garufi, G.; Carbognin, L.; Arcana, C.; Parola, S.; Ventriglia, A.; Doronzo, A.; Garutti, M.; Orlandi, A.; Palazzo, A.; Fabi, A.; et al. Tailoring neoadjuvant treatment of HR-positive/HER2-negative breast cancers: Which role for gene expression assays? Cancer Treat. Rev. 2022, 110, 102454. [Google Scholar] [PubMed]
- Griguolo, G.; Bottosso, M.; Vernaci, G.; Miglietta, F.; Dieci, M.V.; Guarneri, V. Gene-expression signatures to inform neoadjuvant treatment decision in HR+/HER2- breast cancer: Available evidence and clinical implications. Cancer Treat. Rev. 2022, 102, 102323. [Google Scholar] [CrossRef]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, inflammation and cancer. Nat. Immunol. 2017, 18, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, Q.; Chen, Y.; Su, C.; Lin, W.; Wei, D.; Zhang, L.; Liu, H. Prognostic Evaluation of Metastasis-Related Lymphocyte/Monocyte Ratio in Stage I–III Breast Cancer Receiving Chemotherapy. Front. Oncol. 2021, 11, 782383. [Google Scholar]
- Yin, Y.; Zhang, Y.; Li, L.; Zhang, S.; Liu, N.; Yuan, S. Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio and Development of a Nomogram in Breast Cancer Patients. Front. Oncol. 2021, 11, 650980. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Long, Z.Q.; Huang, X.; Deng, J.P.; He, Z.Y.; Guo, L.; Zhang, W.W.; Lin, H.X. The Value of Prognostic Nutritional Index (PNI) in Predicting Survival and Guiding Radiotherapy of Patients With T1-2N1 Breast Cancer. Front. Oncol. 2019, 9, 1562. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Maeno, K.; Takekoshi, D.; Ono, M.; Ito, T.; Kanai, T.; Ito, K.I. Neoadjuvant chemotherapy-induced decrease of prognostic nutrition index predicts poor prognosis in patients with breast cancer. BMC Cancer 2020, 20, 160. [Google Scholar]
- Chen, L.; Qi, Y.; Kong, X.; Su, Z.; Wang, Z.; Wang, X.; Du, Y.; Fang, Y.; Li, X.; Wang, J. Nutritional Risk Index Predicts Survival in Patients With Breast Cancer Treated With Neoadjuvant Chemotherapy. Front. Nutr. 2021, 8, 786742. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, L.P.; Xie, S.Y.; Huang, H.Y.; Chen, X.Y.; Jiang, T.C.; Guo, L.; Lin, H.X. Pan-Immune-Inflammation Value: A New Prognostic Index in Operative Breast Cancer. Front. Oncol. 2022, 12, 830138. [Google Scholar] [PubMed]
- Şahin, A.B.; Cubukcu, E.; Ocak, B.; Deligonul, A.; Oyucu Orhan, S.; Tolunay, S.; Gokgoz, M.S.; Cetintas, S.; Yarbas, G.; Senol, K.; et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 2021, 11, 1–10. [Google Scholar]
- Yamamoto, T.; Kawada, K.; Hida, K.; Matsusue, R.; Itatani, Y.; Mizuno, R.; Yamaguchi, T.; Ikai, I.; Sakai, Y. Combination of lymphocyte count and albumin concentration as a new prognostic biomarker for rectal cancer. Sci. Rep. 2021, 11, 5027. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Molinaro, A.M.; Gauthier, M.L.; Berman, H.K.; Waldman, F.; Bennington, J.; Sanchez, H.; Jimenez, C.; Stewart, K.; Chew, K.; et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J. Natl. Cancer Inst. 2010, 102, 627–637. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [PubMed]
- Hothorn, T.; Zeileis, A. Generalized Maximally Selected Statistics. Biometrics 2008, 64, 1263–1269. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar]
- Van Calster, B.; Wynants, L.; Verbeek, J.F.M.; Verbakel, J.Y.; Christodoulou, E.; Vickers, A.J.; Roobol, M.J.; Steyerberg, E.W. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Eur. Urol. 2018, 74, 796–804. [Google Scholar] [PubMed]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [PubMed]
- Uno, H.; Tian, L.; Cai, T.; Kohane, I.S.; Wei, L.J. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat. Med. 2013, 32, 2430–2442. [Google Scholar] [CrossRef]
- Wang, H.K.; Wei, Q.; Yang, Y.L.; Lu, T.Y.; Yan, Y.; Wang, F. Clinical usefulness of the lymphocyte-to-monocyte ratio and aggregate index of systemic inflammation in patients with esophageal cancer: A retrospective cohort study. Cancer Cell Int. 2023, 23, 13. [Google Scholar]
- Wu, J.; Zhang, H.; Li, L.; Hu, M.; Chen, L.; Xu, B.; Song, Q. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. 2020, 40, 301–312. [Google Scholar]
- Chen, L.; Bai, P.; Kong, X.; Huang, S.; Wang, Z.; Wang, X.; Fang, Y.; Wang, J. Prognostic Nutritional Index (PNI) in Patients With Breast Cancer Treated With Neoadjuvant Chemotherapy as a Useful Prognostic Indicator. Front. Cell Dev. Biol. 2021, 9, 656741. [Google Scholar] [CrossRef]
- Chen, L.; Kong, X.; Wang, Z.; Wang, X.; Fang, Y.; Wang, J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J. Cell. Mol. Med. 2020, 24, 2993–3021. [Google Scholar]
- Zhu, M.; Chen, L.; Kong, X.; Wang, X.; Fang, Y.; Li, X.; Wang, J. The Systemic Inflammation Response Index as an Independent Predictor of Survival in Breast Cancer Patients: A Retrospective Study. Front. Mol. Biosci. 2022, 9, 856064. [Google Scholar]
- Chen, X.X.; Zhao, S.T.; Yang, X.M.; He, S.C.; Qian, F.H. Additional diagnostic value of the monocyte to red blood cell count ratio and the product of lymphocyte count and albumin concentration in lung cancer management. Oncol. Lett. 2023, 25, 135. [Google Scholar] [PubMed]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [PubMed]
- Quigley, D.A.; Kristensen, V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol. Oncol. 2015, 9, 2054–2062. [Google Scholar]
- Chen, L.; Kong, X.; Yan, C.; Fang, Y.; Wang, J. The Research Progress on the Prognostic Value of the Common Hematological Parameters in Peripheral Venous Blood in Breast Cancer. OncoTargets Ther. 2020, 13, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Tao, J.; Li, X.; Chen, H.; Lu, Q.; Yang, J.; Pan, H.; Wang, C.; Zhou, W.; Liu, X. Peripheral inflammation/immune indicators of chemosensitivity and prognosis in breast cancer patients treated with neoadjuvant chemotherapy. OncoTargets Ther. 2018, 11, 1423–1432. [Google Scholar] [CrossRef]
- Jeng, L.B.; Chan, W.L.; Teng, C.F. Prognostic Significance of Serum Albumin Level and Albumin-Based Mono- and Combination Biomarkers in Patients with Hepatocellular Carcinoma. Cancers 2023, 15, 1005. [Google Scholar]
- Babson, A.L.; Winnick, T. Protein transfer in tumor-bearing rats. Cancer Res. 1954, 14, 606–611. [Google Scholar]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [PubMed]
- Gillon, P.; Touati, N.; Breton-Callu, C.; Slaets, L.; Cameron, D.; Bonnefoi, H. Factors predictive of locoregional recurrence following neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancer: An analysis of the EORTC 10994/BIG 1-00 study. Eur. J. Cancer 2017, 79, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Fayanju, O.M.; Ren, Y.; Thomas, S.M.; Greenup, R.A.; Plichta, J.K.; Rosenberger, L.H.; Tamirisa, N.; Force, J.; Boughey, J.C.; Hyslop, T.; et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Ann. Surg. 2018, 268, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shi, M.; Wang, W.H.; Shen, L.F.; Tang, Y.; Rong, Q.L.; Zhu, L.; Huang, X.B.; Tie, J.; Chen, J.Y.; et al. A novel nomogram for predicting locoregional recurrence risk in breast cancer patients treated with neoadjuvant chemotherapy and mastectomy. Radiother. Oncol. 2021, 161, 191–197. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 500) | Training Set (n = 400) | Internal Validation Set (n = 100) | p |
|---|---|---|---|---|
| Age (years) | ||||
| median (IQR) | 48 (40, 56) | 48 (40, 56) | 46 (39, 55) | 0.152 |
| ≤50 | 298 (59.60%) | 236 (59.00%) | 62 (62.00%) | 0.665 |
| >50 | 202 (40.04%) | 164 (41.00%) | 38 (38.00%) | |
| BMI (kg/m2) | ||||
| median (IQR) | 23.0 (21.3, 25.0) | 23.0 (21.4, 25.0) | 22.8 (20.7, 24.9) | 0.121 |
| ≤25 | 378 (75.6%) | 302 (75.5%) | 76 (76.0%) | 1.000 |
| >25 | 122 (24.4%) | 98 (24.5%) | 24 (24.0%) | |
| Menopausal status | ||||
| Pre-menopausal | 306 (61.20%) | 244 (61.00%) | 62 (62.00%) | 0.945 |
| Post-menopausal | 194 (38.80%) | 156 (39.00%) | 38 (38.00%) | |
| Histological types | ||||
| IDC | 466 (93.20%) | 372 (93.00%) | 94 (94.00%) | 0.101 |
| ILC | 14 (2.80%) | 11 (2.80%) | 3 (3.00%) | |
| Others | 17 (3.40%) | 16 (4.00%) | 1 (1.00%) | |
| Missing data | 3 (0.60%) | 1 (0.20%) | 2 (2.00%) | |
| Histological grade | ||||
| I | 8 (1.60%) | 5 (1.20%) | 3 (3.00%) | 0.217 |
| II | 231 (46.20%) | 192 (48.00%) | 39 (39.00%) | |
| III | 156 (31.20%) | 124 (31.00%) | 32 (32.00%) | |
| Missing data | 105 (21.00%) | 79 (19.80%) | 26 (26.00%) | |
| cT Stage | ||||
| T1 | 21 (4.20%) | 19 (4.80%) | 2 (2.00%) | 0.176 |
| T2 | 288 (57.60%) | 225 (56.20%) | 63 (63.00%) | |
| T3 | 111 (22.20%) | 95 (23.80%) | 16 (16.00%) | |
| T4 | 80 (16.00%) | 61 (15.20%) | 19 (19.00%) | |
| cN Stage | ||||
| N0 | 20 (4.00%) | 14 (3.50%) | 6 (6.00%) | 0.186 |
| N1 | 75 (15.00%) | 56 (14.00%) | 19 (19.00%) | |
| N2 | 290 (58.00%) | 241 (60.20%) | 49 (49.00%) | |
| N3 | 115 (23.00%) | 89 (22.20%) | 26 (26.00%) | |
| ypT Stage | ||||
| Tis/T0 | 98 (19.60%) | 75 (18.80%) | 23 (23.00%) | 0.790 |
| T1 | 135 (27.00%) | 107 (26.80%) | 28 (28.00%) | |
| T2 | 193 (38.60%) | 156 (39.00%) | 37 (37.00%) | |
| T3 | 45 (9.00%) | 37 (9.20%) | 8 (8.00%) | |
| T4 | 29 (5.80%) | 25 (6.20%) | 4 (4.00%) | |
| ypN Stage | ||||
| N0 | 215 (43.00%) | 159 (39.80%) | 56 (56.00%) | 0.008 |
| N1 | 124 (24.80%) | 108 (27.00%) | 16 (16.00%) | |
| N2 | 86 (17.20%) | 75 (18.80%) | 11 (11.00%) | |
| N3 | 75 (15.00%) | 58 (14.50%) | 17 (17.00%) | |
| pCR | ||||
| No | 414 (82.80%) | 335 (83.80%) | 79 (79.00%) | 0.328 |
| Yes | 86 (17.20%) | 65 (16.20%) | 21 (21.00%) | |
| HR status | ||||
| Negative | 171 (34.20%) | 135 (33.80%) | 36 (36.00%) | 0.759 |
| Positive | 325 (65.80%) | 263 (66.30%) | 62 (64.00%) | |
| HER2 status | ||||
| Negative | 272 (54.40%) | 220 (55.00%) | 52 (52.00%) | 0.161 |
| Positive | 216 (43.20%) | 173 (43.20%) | 43 (43.00%) | |
| Missing data | 12 (2.40%) | 7 (1.80%) | 5 (5.00%) | |
| Ki-67 (%) | ||||
| median (IQR) | 30 (20, 50) | 30 (20, 53) | 30 (20, 40) | 0.274 |
| ≤14 | 69 (13.80%) | 52 (13.00%) | 17 (17.00%) | 0.466 |
| >14 | 420 (84.00%) | 340 (85.00%) | 80 (80.00%) | |
| Missing data | 11 (2.20%) | 8 (2.00%) | 3 (3.00%) | |
| Lymphovascular invasion | ||||
| No | 305 (61.00%) | 238 (59.50%) | 67 (67.00%) | 0.369 |
| Yes | 187 (37.40%) | 155 (38.80%) | 32 (32.00%) | |
| Missing data | 8 (1.60%) | 7 (1.80%) | 1 (1.00%) | |
| Type of primary surgery | ||||
| Mastectomy | 450 (90.00%) | 363 (90.80%) | 87 (87.00%) | 0.351 |
| BCS | 50 (10.00%) | 37 (9.20%) | 13 (13.00%) | |
| NAC regimens | ||||
| Anthracycline + taxane | 443 (88.60%) | 352 (88.00%) | 91 (91.00%) | 0.504 |
| Others | 57 (11.40%) | 48 (12.00%) | 9 (9.00%) | |
| LA | ||||
| Low | 66 (13.20%) | 51 (12.80%) | 13 (13.00%) | 1.000 |
| High | 434 (86.80%) | 349 (86.20%) | 87 (87.00%) | |
| Index | Training Cohort | Internal Validation Cohort | External Validation Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | p | Estimate | 95% CI | p | Estimate | 95% CI | p | |
| The nomogram vs. cTNM Staging | |||||||||
| NRI | |||||||||
| 1-year DFS | 0.65 | 0.21–0.96 | 0.71 | −0.43–1.55 | 1.33 | 0.62–1.57 | |||
| 3-year DFS | 0.55 | 0.22–0.81 | 0.45 | −0.23–1.22 | 1.26 | 0.75–1.50 | |||
| 5-year DFS | 0.64 | 0.25–0.93 | 0.80 | −0.20–1.40 | 0.83 | 0.33–1.24 | |||
| IDI | |||||||||
| 1-year DFS | 0.10 | 0.05–0.19 | <0.001 | 0.05 | −0.02–0.41 | 0.244 | 0.10 | 0.03–0.27 | <0.001 |
| 3-year DFS | 0.16 | 0.09–0.23 | <0.001 | 0.04 | −0.03–0.27 | 0.192 | 0.21 | 0.11–0.38 | <0.001 |
| 5-year DFS | 0.18 | 0.10–0.27 | <0.001 | 0.11 | −0.08–0.41 | 0.168 | 0.17 | 0.07–0.31 | <0.001 |
| The nomogram vs. ypTNM Staging | |||||||||
| NRI | |||||||||
| 1-year DFS | 0.60 | 0.28–0.90 | 0.51 | −0.37–1.54 | 0.99 | −0.05–1.37 | |||
| 3-year DFS | 0.60 | 0.18–0.84 | 0.17 | −0.23–1.11 | 0.63 | −0.16–1.26 | |||
| 5-year DFS | 0.68 | 0.07–0.88 | 0.14 | −0.28–1.34 | 0.25 | −0.19–0.91 | |||
| IDI | |||||||||
| 1-year DFS | 0.08 | 0.04–0.14 | <0.001 | 0.05 | −0.02–0.39 | 0.196 | 0.06 | 0.00–0.18 | 0.052 |
| 3-year DFS | 0.10 | 0.05–0.17 | <0.001 | 0.04 | 0.00–0.30 | 0.052 | 0.14 | 0.04–0.27 | <0.001 |
| 5-year DFS | 0.11 | 0.03–0.19 | 0.016 | 0.10 | −0.06–0.37 | 0.128 | 0.12 | 0.04–0.23 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-D.; Duan, F.-F.; Hua, X.; Cao, L.; Xia, W.; Chen, J.-Y. A Novel Albumin-Related Nutrition Biomarker Predicts Breast Cancer Prognosis in Neoadjuvant Chemotherapy: A Two-Center Cohort Study. Nutrients 2023, 15, 4292. https://doi.org/10.3390/nu15194292
Wang M-D, Duan F-F, Hua X, Cao L, Xia W, Chen J-Y. A Novel Albumin-Related Nutrition Biomarker Predicts Breast Cancer Prognosis in Neoadjuvant Chemotherapy: A Two-Center Cohort Study. Nutrients. 2023; 15(19):4292. https://doi.org/10.3390/nu15194292
Chicago/Turabian StyleWang, Meng-Di, Fang-Fang Duan, Xin Hua, Lu Cao, Wen Xia, and Jia-Yi Chen. 2023. "A Novel Albumin-Related Nutrition Biomarker Predicts Breast Cancer Prognosis in Neoadjuvant Chemotherapy: A Two-Center Cohort Study" Nutrients 15, no. 19: 4292. https://doi.org/10.3390/nu15194292
APA StyleWang, M.-D., Duan, F.-F., Hua, X., Cao, L., Xia, W., & Chen, J.-Y. (2023). A Novel Albumin-Related Nutrition Biomarker Predicts Breast Cancer Prognosis in Neoadjuvant Chemotherapy: A Two-Center Cohort Study. Nutrients, 15(19), 4292. https://doi.org/10.3390/nu15194292





