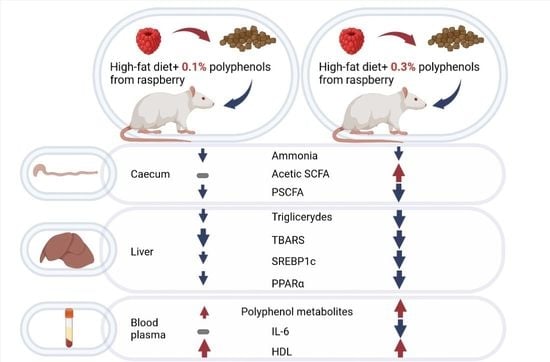
Dose-Related Regulatory Effect of Raspberry Polyphenolic Extract on Cecal Microbiota Activity, Lipid Metabolism and Inflammation in Rats Fed a Diet Rich in Saturated Fats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raspberry Extract Preparation
2.2. Chemical Analyses of the Raspberry Extract
2.3. Analysis of the Raspberry Extract Polyphenols
2.4. In Vivo Experiment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Zhou, B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci. Rep. 2018, 8, 1465. [Google Scholar] [CrossRef] [Green Version]
- Fotschki, B.; Laparra, J.M.; Sójka, M. Raspberry Polyphenolic Extract Regulates Obesogenic Signals in Hepatocytes. Molecules 2018, 23, 2103. [Google Scholar] [CrossRef] [Green Version]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—Results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, J.; Zhang, H.; Pang, J.; Li, Q.; Wang, X.; Xu, H.; Sun, X.; Zhao, H.; Yang, Y.; et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia—A randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 345–354. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Sójka, M.; Jurgoński, A.; Zduńczyk, Z. Ellagitannins and Flavan-3-ols from Raspberry Pomace Modulate Caecal Fermentation Processes and Plasma Lipid Parameters in Rats. Molecules 2015, 20, 22848–22862. [Google Scholar] [CrossRef] [Green Version]
- Antoine, T.; Georgé, S.; Leca, A.; Desmarchelier, C.; Halimi, C.; Gervais, S.; Aupy, F.; Marconot, G.; Reboul, E. Reduction of pulse “antinutritional” content by optimizing pulse canning process is insufficient to improve fat-soluble vitamin bioavailability. Food Chem. 2022, 370, 131021. [Google Scholar] [CrossRef]
- Nath, H.; Samtiya, M.; Dhewa, T. Beneficial attributes and adverse effects of major plant-based foods anti-nutrients on health: A review. Hum. Nutr. Metab. 2022, 28, 200147. [Google Scholar] [CrossRef]
- Savi, M.; Bocchi, L.; Mena, P.; Dall’Asta, M.; Crozier, A.; Brighenti, F.; Stilli, D.; Del Rio, D. In vivo administration of urolithin A and B prevents the occurrence of cardiac dysfunction in streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2017, 16, 80. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef] [Green Version]
- Milala, J.; Kosmala, M.; Karlińska, E.; Juśkiewicz, J.; Zduńczyk, Z.; Fotschki, B. Ellagitannins from Strawberries with Different Degrees of Polymerization Showed Different Metabolism through Gastrointestinal Tract of Rats. J. Agric. Food Chem. 2017, 65, 10738–10748. [Google Scholar] [CrossRef]
- Saha, P.; Yeoh, B.S.; Singh, R.; Chandrasekar, B.; Vemula, P.K.; Haribabu, B.; Vijay-Kumar, M.; Jala, V.R. Gut Microbiota Conversion of Dietary Ellagic Acid into Bioactive Phytoceutical Urolithin A Inhibits Heme Peroxidases. PLoS ONE 2016, 11, e0156811. [Google Scholar] [CrossRef] [Green Version]
- González-Barrio, R.; Truchado, P.; Ito, H.; Espín, J.C.; Tomás-Barberán, F.A. UV and MS identification of Urolithins and Nasutins, the bioavailable metabolites of ellagitannins and ellagic acid in different mammals. J. Agric. Food Chem. 2011, 59, 1152–1162. [Google Scholar] [CrossRef]
- Xu, Q.; Li, S.; Tang, W.; Yan, J.; Wei, X.; Zhou, M.; Diao, H. The Effect of Ellagic Acid on Hepatic Lipid Metabolism and Antioxidant Activity in Mice. Front. Physiol. 2021, 12, 751501. [Google Scholar] [CrossRef]
- Wu, T.H.; Wang, P.W.; Lin, T.Y.; Yang, P.M.; Li, W.T.; Yeh, C.T.; Pan, T.L. Antioxidant properties of red raspberry extract alleviate hepatic fibrosis via inducing apoptosis and transdifferentiation of activated hepatic stellate cells. Biomed. Pharmacother. 2021, 144, 112284. [Google Scholar] [CrossRef]
- Fotschki, B.; Wiczkowski, W.; Sawicki, T.; Sójka, M.; Myszczyński, K.; Ognik, K.; Juśkiewicz, J. Stimulation of the intestinal microbiota with prebiotics enhances hepatic levels of dietary polyphenolic compounds, lipid metabolism and antioxidant status in healthy rats. Food Res. Int. 2022, 160, 111754. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; de la Rosa, L.A. Modulation of PPAR Expression and Activity in Response to Polyphenolic Compounds in High Fat Diets. Int. J. Mol. Sci. 2016, 17, 1002. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Sójka, M. Fructo-Oligosaccharides and Pectins Enhance Beneficial Effects of Raspberry Polyphenols in Rats with Nonalcoholic Fatty Liver. Nutrients 2021, 13, 833. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Sójka, M.; Klimczak, E.; Macierzyński, J.; Kołodziejczyk, K. Nutrient and polyphenolic composition of industrial strawberry press cake. Eur. Food Res. Technol. 2013, 237, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotschki, B.; Milala, J.; Jurgoński, A.; Karlińska, E.; Zduńczyk, Z.; Juśkiewicz, J. Strawberry ellagitannins thwarted the positive effects of dietary fructooligosaccharides in rat cecum. J. Agric. Food Chem. 2014, 62, 5871–5880. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Płatosz, N.; Bączek, N.; Topolska, J.; Szawara-Nowak, D.; Wiczkowski, W. The Blood-Cerebrospinal Fluid Barrier Features Different Permeability to Cyanidin-3-galactoside and Cyanidin-3-diglucoside-5-glucoside and Their Metabolites Circulating in Blood. J. Agric. Food Chem. 2022, 70, 12852–12864. [Google Scholar] [CrossRef]
- Luo, T.; Miranda-Garcia, O.; Adamson, A.; Sasaki, G.; Shay, N. Development of obesity is reduced in high-fat fed mice fed whole raspberries, raspberry juice concentrate, and a combination of the raspberry phytochemicals ellagic acid and raspberry ketone. J. Berry Res. 2016, 6, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.P.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef]
- Visek, W.J. Diet and cell growth modulation by ammonia. Am. J. Clin. Nutr. 1978, 31, S216–S220. [Google Scholar] [CrossRef]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.; Sun, H.; Zhang, C. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef]
- Corrêa, T.; Rogero, M.M.; Hassimotto, N.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H. Biological Function of Acetic Acid-Improvement in Obesity and Glucose Tolerance by Acetic Acid in Type 2 Diabetic Rats. Crit. Rev. Food Sci. Nutr. 2016, 56, 171–175. [Google Scholar] [CrossRef]
- Danielewski, M.; Matuszewska, A.; Szeląg, A.; Sozański, T. The Impact of Anthocyanins and Iridoids on Transcription Factors Crucial for Lipid and Cholesterol Homeostasis. Int. J. Mol. Sci. 2021, 22, 6074. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Henning, S.M.; Chan, B.; Long, J.; Zhong, J.; Acin-Perez, R.; Petcherski, A.; Shirihai, O.; Heber, D.; et al. Ellagic Acid and Its Microbial Metabolite Urolithin A Alleviate Diet-Induced Insulin Resistance in Mice. Mol. Nutr. Food Res. 2020, 64, e2000091. [Google Scholar] [CrossRef]
- Toney, A.M.; Fan, R.; Xian, Y.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Urolithin A, a Gut Metabolite, Improves Insulin Sensitivity Through Augmentation of Mitochondrial Function and Biogenesis. Obesity 2019, 27, 612–620. [Google Scholar] [CrossRef]
- Umesalma, S.; Sudhandiran, G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2010, 107, 650–655. [Google Scholar] [CrossRef]



| Compound | |
|---|---|
| Basic components (n = 3), g/100 g | |
| Dry matter (AOAC 940.26) | 94.79 ± 0.18 |
| Protein (AOAC 920.152) | 4.74 ± 0.45 |
| Fat (AOAC 930.09) | 0.51 ± 0.04 |
| Ash | 2.11 ± 0.06 |
| TDF (AOAC 985.29) | 0.00 ± 0.00 |
| Total polyphenols | 47.80 ± 1.06 |
| Saccharides | |
| Saccharose | 1.60 ± 0.00 |
| Glucose | 8.90 ± 0.60 |
| Fructose | 9.90 ± 0.60 |
| Polyphenols (n = 3), mg/100 g | |
| Total polyphenols (TPH) | 47804.8 ± 1060.5 |
| Ellagitannins (ETs) | |
| Sanguiin H-6 | 16975.8 ± 350.4 |
| Sanguiin H-6 minus a gallic acid moietya | 221.6 ± 9.7 |
| Sanguiin H-6 plus a gallic acid moietya | 356.3 ± 22.4 |
| Sanguiin H-10 isomer 1 a | 466.4 ± 14.0 |
| Sanguiin H-10 isomer 2 a | 533.0 ± 20.7 |
| Sanguiin H-10 isomer 3 a | 301.2 ± 10.7 |
| Lambertianin C | 18314.0 ± 1172.6 |
| Lambertianin C minus ellagic acid moiety 1 b | 764.1 ± 28.8 |
| Lambertianin C minus ellagic acid moiety 2 b | 196.4 ± 10.9 |
| Lambertianin C minus ellagic acid moiety 3 b | 426.6 ± 6.7 |
| Ellagic acid pentose conjugate c | 171.7 ± 11.4 |
| Ellagic acid (EA) | 196.7 ± 18.1 |
| Total ETs | 38088.9 ± 1503.6 |
| Total EACs | 368.3 ± 29.4 |
| Total ETs + EACs | 38923.6 ± 1547.0 |
| Flavanols | |
| Total flavanols | 8371.7 ± 486.5 |
| (+)-Catechin | 208.0 ± 5.4 |
| (-)-Epicatechin | 343.5 ± 5.5 |
| Proanthocyanidins | 7820.2 ± 475.7 |
| Anthocyanins (ACYs) | |
| Cyanidin-3-O-spohoroside d | 314.6 ± 11.5 |
| Cyanidin-3-O-glucosyl-rutinoside d | 27.0 ± 0.7 |
| Cyanidin-3-O-glucoside | 152.0 ± 0.9 |
| Cyanidin-3-O-rutinoside d | 11.5 ± 0.1 |
| Pelargonidin-3-O-glucoside d | 4.5 ± 0.3 |
| Total ACYs | 509.6 ± 10.9 |
| C | HF | HF + 0.1PP | HF + 0.3PP | |
|---|---|---|---|---|
| Casein 1 | 20.0 | 20.0 | 20.0 | 20.0 |
| DL-Methionine | 0.30 | 0.30 | 0.30 | 0.30 |
| Cellulose 2 | 5.0 | 3.0 | 3.0 | 3.0 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 |
| Cholesterol | 0.30 | 1.0 | 1.0 | 1.0 |
| Vitamin mix 3 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral mix 4 | 3.5 | 3.5 | 3.5 | 3.5 |
| Rapeseed oil | 2.0 | 2.0 | 2.0 | 2.0 |
| Lard | 6.0 | 23.0 | 23.0 | 23.0 |
| Raspberry polyphenolic extract | 0.00 | 0.00 | 0.21 | 0.64 |
| Saccharose | 10.0 | 10.0 | 10.0 | 10.0 |
| Maize starch 5 | 51.7 | 36.0 | 35.79 | 35.36 |
| kcal%, calculated | ||||
| Protein | 19.6 | 15.4 | 15.4 | 15.4 |
| Carbohydrate | 59.5 | 35.2 | 35.2 | 35.2 |
| Fat | 20.9 | 49.4 | 49.4 | 49.4 |
| C | HF | HF + 0.1PP | HF + 0.3PP | p Value | |
|---|---|---|---|---|---|
| Initial BW, g | 376 ± 19.4 | 385 ± 29.5 | 388 ± 32.3 | 380 ± 31.2 | 0.458 |
| BW gain, g/day | 1.70 ± 0.52 b | 2.97 ± 0.43 a | 3.42 ± 0.63 a | 3.14 ± 0.45 a | <0.001 |
| Daily diet intake, g | 17.4 ± 1.47 ab | 16.5 ± 1.27 b | 18.0 ± 1.32 a | 17.0 ± 1.19 ab | 0.042 |
| Intake per 1 g gain, g | 10.9 ± 2.68 a | 5.63 ± 0.72 b | 5.37 ± 0.74 b | 5.50 ± 0.61 b | <0.001 |
| Fat tissue 1, % | 19.8 ± 3.08 b | 29.8 ± 1.45 a | 30.4 ± 3.10 a | 28.7 ± 2.97 a | <0.001 |
| Lean tissue 1, % | 61.4 ± 2.68 a | 54.3 ± 1.08 b | 54.1 ± 2.97 b | 54.0 ± 3.06 b | <0.001 |
| eWAT 2, g/100 g BW | 3.66 ± 1.47 b | 5.09 ± 0.94 a | 4.75 ± 0.60 a | 4.53 ± 0.48 ab | 0.009 |
| Small intestine | |||||
| Tissue, g/100 g BW | 0.561 ± 0.084 | 0.557 ± 0.072 | 0.583 ± 0.065 | 0.625 ± 0.067 | 0.105 |
| pH of contents | 6.96 ± 0.24 | 7.00 ± 0.23 | 6.85 ± 0.33 | 6.83 ± 0.23 | 0.240 |
| C | HF | HF + 0.1PP | HF + 0.3PP | p Value | |
|---|---|---|---|---|---|
| Cecum | |||||
| Tissue, g/100 g BW | 0.164 ± 0.035 | 0.134 ± 0.018 | 0.132 ± 0.027 | 0.161 ± 0.031 | 0.052 |
| Ammonia, mg/g | 0.232 ± 0.028 b | 0.319 ± 0.067 a | 0.300 ± 0.069 ab | 0.286 ± 0.073 ab | 0.016 |
| pH of digesta | 7.11 ± 0.23 a | 7.09 ± 0.22 a | 6.97 ± 0.22 ab | 6.79 ± 0.25 b | 0.021 |
| SCFAs, µmol/g | |||||
| Acetic acid (C2) | 33.5 ± 3.38 a | 26.4 ± 5.24 b | 25.8 ± 4.61 b | 30.9 ± 2.77 a | 0.001 |
| Propionic acid (C3) | 10.2 ± 0.87 a | 7.84 ± 0.97 c | 9.02 ± 0.85 b | 8.53 ± 1.15 bc | <0.001 |
| Isobutyric acid(C4i) | 0.99 ± 0.31 a | 0.63 ± 0.13 b | 0.65 ± 0.09 b | 0.60 ± 0.21 b | 0.001 |
| Butyric acid (C4) | 4.13 ± 1.03 a | 1.86 ± 0.56 b | 1.78 ± 0.81 b | 1.80 ± 0.68 b | <0.001 |
| Isovaleric acid (C5i) | 1.37 ± 0.19 a | 0.78 ± 0.14 b | 0.97 ± 0.22 b | 0.79 ± 0.18 b | <0.001 |
| Valeric acid (C5) | 1.06 ± 0.08 a | 0.62 ± 0.09 c | 0.80 ± 0.15 b | 0.59 ± 0.14 c | <0.001 |
| PSCFAs | 3.42 ± 0.35 a | 2.03 ± 0.27 bc | 2.41 ± 0.41 b | 1.98 ± 0.44 c | <0.001 |
| Total SCFAs | 51.2 ± 4.16 a | 38.1 ± 6.33 b | 39.0 ± 6.15 b | 43.2 ± 3.65 b | <0.001 |
| Colon | |||||
| pH of the digesta | 7.04 ± 0.27 ab | 7.20 ± 0.20 a | 6.84 ± 0.28 b | 6.92 ± 0.31 ab | 0.021 |
| C | HF | HF + 0.1PP | HF + 0.3PP | p Value | |
|---|---|---|---|---|---|
| Liver | |||||
| Weight | 2.93 ± 0.14 b | 3.40 ± 0.23 a | 3.48 ± 0.26 a | 3.48 ± 0.43 a | <0.001 |
| Hepatic fat, % | 18.8 ± 3.06 b | 34.4 ± 4.16 a | 34.1 ± 5.70 a | 29.9 ± 4.98 a | <0.001 |
| TC, mg/g | 8.11 ± 1.51 b | 10.3 ± 1.00 a | 9.89 ± 1.18 a | 9.10 ± 0.76 a | 0.001 |
| TGs, mg/g | 12.5 ± 1.42 c | 18.8 ± 2.05 a | 17.9 ± 3.80 ab | 15.6 ± 3.01 b | <0.001 |
| TBARS, ng/g | 587 ± 47.7 b | 768 ± 96.9 a | 662 ± 80.4 b | 631 ± 70.7 b | <0.001 |
| Blood plasma | |||||
| TC, mmol/L | 2.52 ± 0.51 | 2.87 ± 0.58 | 2.59 ± 0.41 | 2.70 ± 0.58 | 0.232 |
| HDL, mmol/L | 0.495 ± 0.066 a | 0.404 ± 0.069b | 0.488 ± 0.054 a | 0.469 ± 0.028 a | 0.005 |
| LDL, mmol/L | 0.329 ± 0.069 | 0.498 ± 0.236 | 0.394 ± 0.151 | 0.508 ± 0.242 | 0.091 |
| TGs, mmol/L | 0.756 ± 0.132 | 0.824 ± 0.155 | 0.850 ± 0.193 | 0.841 ± 0.230 | 0.354 |
| TG/HDL ratio | 1.55 ± 0.30 | 2.17 ± 0.92 | 1.75 ± 0.42 | 1.79 ± 0.44 | 0.054 |
| LDL/HDL ratio | 0.66 ± 0.08 b | 1.31 ± 0.73 a | 0.81 ± 0.29 b | 1.08 ± 0.52 ab | 0.015 |
| TC/HDL ratio | 5.07 ± 0.54 b | 7.48 ± 2.83 a | 5.32 ± 0.68 b | 5.74 ± 1.12 b | 0.008 |
| AC | 4.07 ± 0.54 b | 6.48 ± 2.83 a | 4.32 ± 0.68 b | 4.74 ± 1.12 b | 0.008 |
| AIP | 0.182 ± 0.083 b | 0.310 ± 0.149 a | 0.234 ± 0.097 ab | 0.242 ± 0.097 ab | 0.039 |
| AST, U/L | 69.5 ± 6.90 b | 167 ± 26.9 a | 167 ± 52.5 a | 142 ± 22.5 a | <0.001 |
| ALP, U/L | 41.1 ± 35.3 b | 97.2 ± 21.9 a | 103 ± 47.0 a | 96.7 ± 35.3 a | 0.003 |
| ALP, U/L | 56.8 ± 8.95 b | 96.5 ± 22.8 a | 89.4 ± 13.2 a | 85.6 ± 29.8 a | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotschki, B.; Cholewińska, E.; Ognik, K.; Sójka, M.; Milala, J.; Fotschki, J.; Wiczkowski, W.; Juśkiewicz, J. Dose-Related Regulatory Effect of Raspberry Polyphenolic Extract on Cecal Microbiota Activity, Lipid Metabolism and Inflammation in Rats Fed a Diet Rich in Saturated Fats. Nutrients 2023, 15, 354. https://doi.org/10.3390/nu15020354
Fotschki B, Cholewińska E, Ognik K, Sójka M, Milala J, Fotschki J, Wiczkowski W, Juśkiewicz J. Dose-Related Regulatory Effect of Raspberry Polyphenolic Extract on Cecal Microbiota Activity, Lipid Metabolism and Inflammation in Rats Fed a Diet Rich in Saturated Fats. Nutrients. 2023; 15(2):354. https://doi.org/10.3390/nu15020354
Chicago/Turabian StyleFotschki, Bartosz, Ewelina Cholewińska, Katarzyna Ognik, Michał Sójka, Joanna Milala, Joanna Fotschki, Wiesław Wiczkowski, and Jerzy Juśkiewicz. 2023. "Dose-Related Regulatory Effect of Raspberry Polyphenolic Extract on Cecal Microbiota Activity, Lipid Metabolism and Inflammation in Rats Fed a Diet Rich in Saturated Fats" Nutrients 15, no. 2: 354. https://doi.org/10.3390/nu15020354
APA StyleFotschki, B., Cholewińska, E., Ognik, K., Sójka, M., Milala, J., Fotschki, J., Wiczkowski, W., & Juśkiewicz, J. (2023). Dose-Related Regulatory Effect of Raspberry Polyphenolic Extract on Cecal Microbiota Activity, Lipid Metabolism and Inflammation in Rats Fed a Diet Rich in Saturated Fats. Nutrients, 15(2), 354. https://doi.org/10.3390/nu15020354