1. Introduction
Obesity is one of the most common nutritional disorders, and its medical, psychological, social, and economic effects have a significant burden on healthcare resources [
1], leading to high rates of morbidity and mortality worldwide [
2]. The development of obesity has multifactorial causes, so the interaction among multiple genes, as well as environmental factors and behaviors, has an impact on body weight and eating habits [
3]. The comorbidity rate between obesity and Eating Disorders (EDs) is high [
4,
5]. In particular, the lifetime prevalence of obesity in EDs is reported to be 28% on average, ranging from 5% in Anorexia Nervosa (AN) to 87% in Binge Eating Disorder (BED) [
6]. In this light, different phenotypes of obese patients in relation to eating dysfunctional behaviors were recently described [
7]. Many biomarkers were analyzed to explain the variability in eating behaviors and the biological mechanisms [
8] that could affect the brain response to food intake [
9]. The hypothalamus, the main regulating center for energy balance, is abundant with peptides that regulate satiety [
10]. Many of these peptides have also been described in peripheral sites (e.g., adipose tissue) to be important in regulating body weight homeostasis and providing the rise and progression of the metabolic abnormalities observed in obese patients [
11]. A newly discovered neuropeptide hormone, nesfatin-1, has been recently associated with the development of obesity [
12,
13,
14]. Studies on lung cell lines provided, for the first time, information on nesfatin-1 [
15], disclosing the expression of leptin receptors succeeding the activation by the PPAR-γ ligand troglitazone. This molecule was identified as an anorexigenic peptide derived from its precursor non-esterified fatty acid (NEFA)/nucleobindin-2 (
NUCB2) protein after post-translational cleavage by specific convertases, PC2 and PC3/1 [
15,
16].
The cleavage of
NUCB2 by PCs in other potential sites produces the nesfatin-2 (residues 85–163) and nesfatin-3 (residues 166–396) forms, but only nesfatin-1 or the full-length
NUCB2 has the potential role to induce satiety [
17]. Furthermore, when
NUCB2 is proteolytically transformed into nesfatin-1, it can stimulate anorexia [
17]. Polymorphisms in the
NUCB2 gene have been associated with susceptibility to obesity [
18].
Nesfatin-1 is abundantly secreted both in central and peripheral regions. It has been detected in several regions of the hypothalamus and demonstrated to play key roles in the control of food intake, while it seems to be modulated by starvation and refeeding [
19,
20]. As nesfatin-1 has been demonstrated to cross the blood–brain barrier bidirectionally [
21], it has been hypothesized that peripheral nesfatin-1 might enter into the brain and modulate appetite and feeding response [
22]. Recently, the gastrointestinal tract was also found to be an abundant source of peripheral nesfatin-1, suggesting its ability to improve mucosa regeneration, to restore the balance between pro-oxidants and antioxidants mechanisms, to interplay with the gut microbiota, and to modulate the structure of the intestinal barrier [
23]. Another potential source of peripheral nesfatin-1 is adipose tissue, which influences the integration of metabolic activity and energy balance by communicating, via adipokines, with peripheral organs and the brain [
24]. Nesfatin-1 is a good candidate to be part of the brain–adipose axis [
25]. Circulating nesfatin-1, which is supposed to be derived from peripheral tissues, might play a role in the transmission of anorexigenic signals from the periphery to the brain [
26]. Many studies aimed at understanding the role of nesfatin-1 in the development of obesity or diabetes point out inconsistent results. This may be due to the short life-time of circulating nesfatin-1 (* 10–20 min) [
27]. To date, the principal origin of circulating nesfatin-1, its receptor, the pharmaco-modulation of signaling, and the regulation of nesfatin-1 production in the periphery are still unknown [
19]. The possible relationship between nesfatin-1 and Body Mass Index (BMI) is yet uncertain and studies have shown conflicting results, with positive [
28,
29,
30,
31], negative [
32,
33], or no [
34] correlation. In addition, a growing body of recent evidence has suggested nesfatin-1 as a regulator of emotional stress responses [
35], affective [
36,
37], and EDs [
38], but results are still controversial. Despite an increasing number of neurochemical studies, there is not enough data on the effect of central nesfatinergic signaling on the course of affective and EDs [
39]. Furthermore, there are no fully appropriate yet available experimental animal models. In consideration of its pleiotropic functions, as well as with regard to its dual hypothalamic and peripheral sites of synthesis and localization, our hypothesis was that nesfatin-1 could play a role in food intake behaviors among obese patients in relation to the co-occurrence of ED and/or affective diseases. BMI, eating behaviors, anxiety, or depressive symptoms could, in fact, hypothetically, influence nesfatin-1 levels, both in Visceral Adipose Tissue (VAT) and in the bloodstream.
To our knowledge, this is the first study that compared nesfatin-1 levels according to eating behaviors and psychopathological features among patients at different BMI ranges, with or without an eating disorder. We aimed to investigate differences in NUCB2/nesfatin-1 levels in human VAT and in serum of obese patients compared to normal and underweight controls. Second, we tested the nesfatin-1 secretion profile in in vitro models of fully differentiated 3T3-L1 adipocytes. In order to estimate the link between obesity development and nesfatin-1 levels, polymorphisms of NUCB2/nesfatin-1 and the Fat mass and obesity gene (FTO) were also assessed. Finally, circulating nesfatin-1 plasma levels were evaluated in human subjects across a range of BMI and were compared to eating behaviors and affective symptoms.
4. Discussion
Nesfatin-1 has been identified as an anorexigenic peptide with multiple localizations, including adipose tissue. Whether this factor may play a role in obesity and eating behaviors is, however, poorly understood. We chose to address this issue by using a translational approach, and first analyzed 3T3-L1 cells, a murine adipose cell line commonly used to investigate the biology of adipose cells. It was previously demonstrated that nesfatin-1 is also secreted by cultured adipocytes, and its secretion levels vary according to the maturation state of adipocytes, although there exist mixed results. In these cells, nesfatin-1 has been previously shown to be synthesized and released into the culture medium in increasing concentrations during the differentiation from preadipocytes into adipocytes [
28], while Tagaya et al. [
54] reported the reduction in endogenous nesfatin-1 levels after the induction of 3T3-L1 cells differentiation. Consistent with the decrease in endogenous nesfatin-1 protein levels during 3T3-L1 adipogenesis, the stable knockdown of NUCB2 resulted in increased adipogenesis, while stable overexpression of NUCB2 decreased neutral lipid accumulation and reduced adipogenic gene expression [
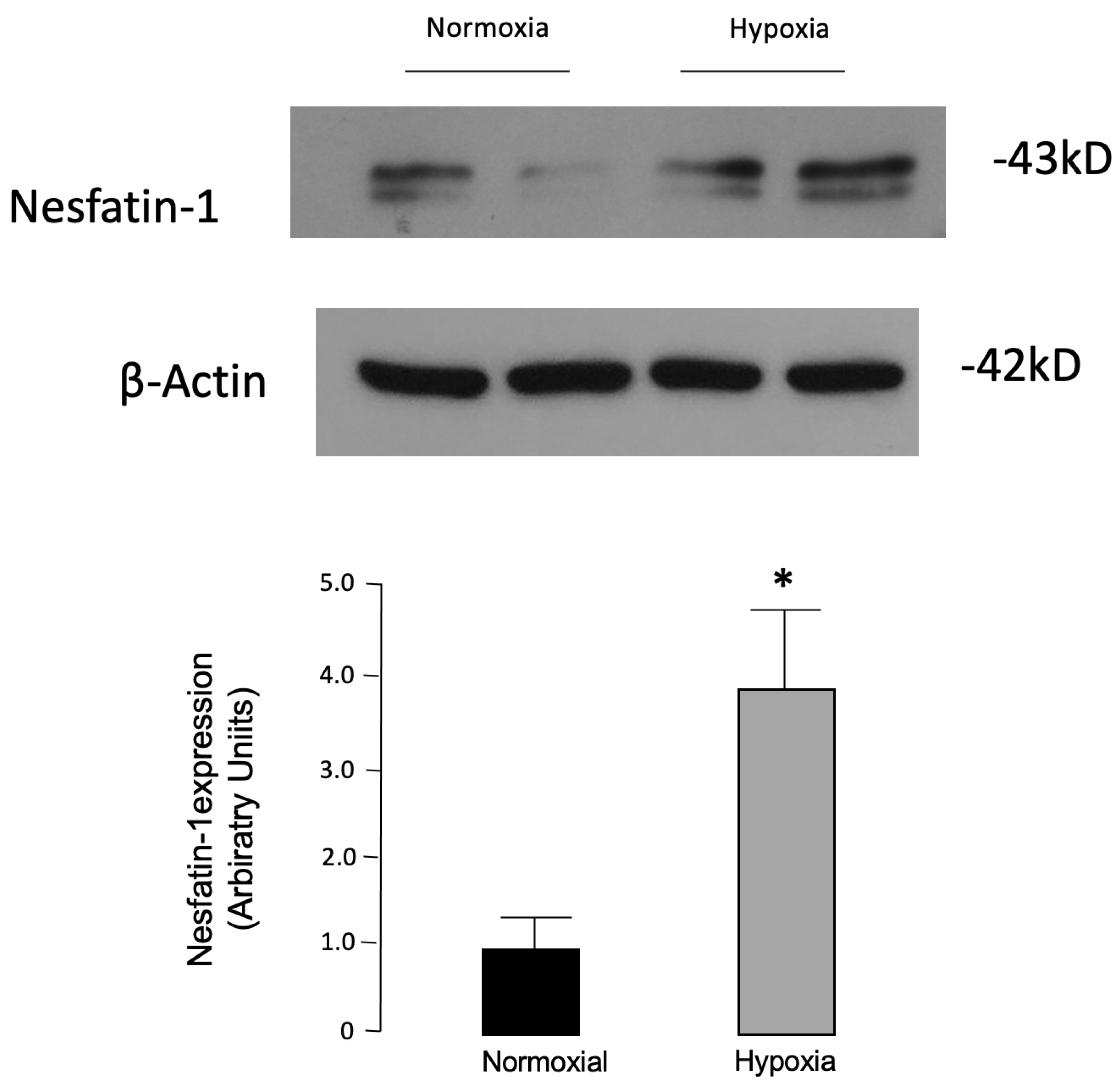
55]. Thus, despite the conflicting results, these findings hint that nesfatin-1 could contribute to the adipogenesis regulation. In consideration of this assessment, we determined nesfatin-1 protein levels in fully differentiated 3T3-L1 cultured adipocytes exposed to 24 h of hypoxia as a surrogate model of the molecular changes occurring in obesity [
56]. In our study, significantly higher levels of nesfatin-1 were found in hypoxic vs. normoxic 3T3-L1 adipocytes. In previous reports, in the same in vitro model, it was shown that other adipokines, including visfatin, were induced by hypoxia [
52], and similar findings were later confirmed in human adipose tissue [
57]. Oh-I et al. [
15], trying to identify new appetite-regulating molecules, proved that the nesfatin-1 precursor, NUCB2, was secreted by 3T3-L1 adipocytes and that its levels increased after treatment with troglitazone in rat hypothalamic and 3T3-L1 adipocyte cells.
Thus, we next examined nesfatin-1 in human VAT in relation to BMI. Previous findings confirmed the expression of nesfatin-1 in VAT [
28,
58,
59], and even to a greater extent, in subcutaneous adipose tissue [
28]. However, we opted to search the nesfatin-1 protein in VAT because, as a major endocrine organ, it produces a variety of bioactive molecules, whose abnormal expression is strongly associated with systemic, low-grade inflammation, and insulin resistance, two conditions linked to obesity and to obesity-related disorders [
60,
61]. In adipose tissue, nesfatin-1 has been shown to increase with obesity and to be modulated by feeding and starvation [
59], while circulating and adipose tissue levels of nesfatin-1 were reported to be higher in diet-induced obese mice [
28]. Hypoxia is a characteristic feature of the adipose tissue in obesity, playing a determinant role in changes in the adipokine profile associated with obesity progression [
62,
63]. Adipose tissue hypoxia is considered an important trigger of adipose cell dysfunction in both animal models and humans with obesity [
44]. Although no previous study could confirm these results, we hypothesized that hypoxia could play a role in the overproduction of nesfatin-1 in VAT. Our data indeed demonstrated, for the first time, higher concentrations of nesfatin-1 in omental VAT of obese patients with respect to normal-weight controls, confirming a positive correlation between nesfatin-1 and BMI. Previous studies have demonstrated the presence of nesfatin-1 only in adipose tissue of normal-weight subjects [
28]. Thus, nesfatin-1 could be a good candidate to play a role in the brain–adipose axis. Indeed, it was tested to be secreted in the hypothalamus and in both white and brown adipose tissue, and its adipose, hypothalamic, and circulating levels are altered by states of feeding and starvation, being decreased by starvation and increased after re-feeding or a high-fat diet [
55].
As a further step forward in the understanding of potential bonds between nesfatin-1 and BMI, we also evaluated plasma levels of nesfatin-1 and rs757081 polymorphism. In this regard, many studies have, in fact, tried to explain the link between the
NUCB2/nesfatin-1 gene and the development of obesity [
14]. Recently, Zegers et al. [
14] posited that mutations and polymorphisms in the nesfatin-1 encoding gene
NUCB2 might cause obesity in humans, and identified genetic variants in obese individuals, suggesting that nesfatin-1 might indeed be involved in the regulation of energy homeostasis and food intake [
14]. They analyzed a large sample of people with obesity and normal-weight controls, reporting an association between obesity and three Single-Nucleotide Polymorphisms (SNPs) (i.e., rs1330, rs214101, and rs757081). These SNPs were associated, only among males, with BMI, weight, and fat-free mass, leading to the concept that SNPs in the
NUCB2 gene could play an important role in the prediction of, and protection against, the development of obesity in male subjects. Chen et al. [
64] independently demonstrated that the association of the
NUCB2 variant c.1012C > G (Q338E or rs757081) is linked to childhood adiposity, while the less frequent GG genotype (Q338E) could be considered as protective against excessive adiposity gain, providing further evidence for the role of
NUCB2 in the determination of human adiposity. To note, even if our data did not reach significance, their trend is in line with this study. We also examined the possible correlation between nesfatin-1 serum levels and the
FTO gene variants. SNPs of the
FTO gene are intensely associated with obesity [
65]. Among these SNPs, the rs9939609 polymorphism exhibits a strong effect on BMI, body fat, and body composition [
66,
67]. The rs9939609 polymorphism A-allele was associated with higher caloric intake and eating a high-calorie diet [
68]. The
FTO gene plays an important role in fat metabolism, and the
FTO risk allele carriers display lower fat cell lipolysis compared with the others [
69]. The mechanism at the basis of the association between
FTO and obesity development remains unclear. Some studies reported that the
FTO gene may exert its effects by altering body composition and gaining adiposity [
69,
70]. Our results have shown that the AT genotype was the most frequent in our sample, and a significant effect of the interaction between the
FTO gene and BMI emerged on nesfatin-1 serum levels.
Despite this evidence, contradictory results have been reported regarding the association between BMI and circulating nesfatin-1 levels [
28,
29,
30,
31,
32,
33,
34]. Initial lines of evidence showed a negative correlation between nesfatin-1 levels and BMI [
32,
71]. However, none of these studies included obese subjects. Conversely, a significant association between fat percentage and circulating nesfatin-1 in obese and morbid obese subjects was found [
28,
72]. In our study, we did not find significant differences in plasma nesfatin-1 concentration in relation to BMI. Although this finding seems to be counterintuitive and needs to be explained, discrepancies between protein secretion in tissues and in circles should be evaluated within the complexity of the systemic responses in pathophysiology, and the limits of the analytical process. In addition, similar dynamics have been reported previously for other analytes with additional localizations, in addition to adipose fat [
73]. In this regard, as a “satiety molecule” secreted from adipose tissue, nesfatin-1 was described to cross the blood–brain barrier and to be secreted into the cerebrospinal fluid (CSF) [
30]. We speculated that the higher plasma nesfatin-1 levels observed in our obese subjects could be a consequence of reduced nesfatin-1 uptake into the CSF, possibly due to saturation of transporters [
29]. While confirming this hypothesis, Tan et al. [
30] reported significantly lower CSF/plasma ratios of nesfatin-1 that negatively correlated with BMI, body weight, and fat mass in obese adult subjects.
We also described that higher circulating nesfatin-1 could be linked to childhood obesity. Recent studies agreed with these results [
34]. In particular, Anwar et al. [
31] also demonstrated that plasma nesfatin-1 levels were significantly higher in obese children and adolescents than in control subjects. Controversial results may point toward other factors that influence circulating nesfatin-1 levels. For example, the postprandial level of nesfatin-1 and its relationship with various clinical and metabolic parameters were also discussed [
74]. It was demonstrated that fasting nesfatin-1 levels, after an oral glucose load, were not higher in obese than in healthy children, thus suggesting that oral glucose load may not be a proper test to induce the nesfatin-1 response and, conversely, that nesfatin-1 may not have an impact as a short-term regulator of food intake among obese children [
74]. Long-term changes in body weight could probably affect nesfatin-1 levels with reported higher levels in overweight and obese men and women [
28] and lower levels in female anorexic patients [
75], resulting in a positive correlation with BMI. Although further confirmation is still needed in humans, in rats, the regulation of circulating nesfatin-1 appears to be dependent on food intake with lower levels after fasting and the restoration after re-feeding [
28]. Concerning fasting plasma nesfatin-1 and its relation to dietary habits and daily intake, a study correlated nesfatin-1 positively with the percentage of calories derived from daily carbohydrates and saturated fat intake, and negatively with calories derived from daily protein intake [
31]. Thus, the evaluation of eating behaviors seemed to be an important issue, and nesfatin-1 could be a useful biomarker of eating habits among obese patients. Our aim was, therefore, to identify recurring eating behaviors among obese patients that could be linked to plasma nesfatin-1 levels. In terms of trends, an inverse correlation emerged with binge eating, grazing, emotional and sweet eating, and craving for carbohydrates, frequently described as dysfunctional eating behaviors in obese patients with BED [
7], and a positive correlation with hyperphagia and social eating, frequently described among obese patients without EDs [
7]. We determined that higher levels of nesfatin-1 were associated with lower scores of the BES. A previous study confirms our results [
13]. Although there are no studies to date that could explain this inverse relationship between binge eating and nesfatin-1 levels, we could consider important findings in other species to interpret our results. NUCB2/nesfatin-1 mRNA levels significantly decreased after a high-glucose and high-fat diet [
76], such as during binge eating. Macronutrients could exert a modulatory effect on preproghrelin and NUCB2/nesfatin-1 mRNA and protein secretion in the intestine and hepatopancreas of goldfish in a time- and concentration-dependent manner [
76]. This could be due to a distinct effect on nesfatin-1 transcription, translation, and post-translational processing. Thus, glucose induces changes not only at the mRNA level but also in the process that regulates the cleavage of nesfatin-1 from its precursor. Diet composition is considered an important factor for the regulation of metabolic hormones. In mammals, numerous studies have detailed a modulation of appetite-regulating hormones secretion, including ghrelin and nesfatin-1, by the macronutrient composition of diets [
77], independently to BMI.
Obese patients with higher scores of the BES, probably affected by BED, have shown lower levels of nesfatin-1, so nesfatin-1 could be related to the comorbidity of EDs in obese patients. In this regard, lower plasma nesfatin-1, by altering the satiety signaling, could be the trigger and the maintaining determinant of binge behaviors. Moreover, coherently, low circulating nesfatin-1 has been defined as a facilitating factor for the development of obesity [
78]. It is not yet clear if this variability in nesfatin-1 levels could account for the development and self-perpetuating of eating-related maladaptive behaviors. Chen et al. [
79] shed new and intriguing light on the hypothesis about the role of nesfatin-1 in the genesis of EDs, suggesting its direct action on dopaminergic reward circuitries. It was recently described that the nesfatin neurons in the lateral amygdala send their inhibitory efferent branches to the VTA neurons, which result in the anorexigenic effect [
39].
Few studies have been conducted to evaluate nesfatin-1 plasma levels in patients with EDs [
38,
39,
75]. We did not find a significant correlation between plasma nesfatin-1 levels and AN. Previous studies demonstrated decreased circulating levels of nesfatin-1 in patients diagnosed with restricting-type AN [
38,
75], but mixed findings have been reported in obese subjects, with increased [
80] or decreased [
81] levels. Moreover, a hormone with anorexigenic and anxiogenic effects might play a role in the etiology or maintenance of AN, which is often accompanied by depression and anxiety [
82]. Hoffman et al. [
37] investigated the relationship among nesfatin-1 plasma levels, anxiety, depressiveness, and perceived stress in obese men and women and their alterations during inpatient treatment. Nesfatin-1 levels in plasma positively correlated with perceived anxiety and may also change in the course of an eating disorder [
39]. However, we have not found significant differences in BDI-II and STAI scores among groups. Previous results have shown higher nesfatin-1 levels in women with high anxiety levels [
12]. A positive correlation of nesfatin-1 with depression was also reported [
36]. Our data have shown that eating behaviors could play a role in determining changes in plasma nesfatin levels rather than affective symptoms or BMI. Although previous results have shown that nesfatin-1 might be regulated in a sex-specific manner [
12,
83,
84], our data do not demonstrate significant differences in circulating nesfatin-1 levels according to gender. Similarly, Anwar et al. did not show a significant difference in nesfatin-1 levels in relation to sex, in the obese group [
31]. However, men with anxiety disorder displayed decreased plasma nesfatin-1 levels compared to healthy controls [
84]. These findings could suggest a different secretion of nesfatin-1 in males and females affected by affective symptoms that warrants further research to investigate this possible sex-specific effect.
Limits
Several limitations should be noted, prior to proceeding with the conclusions. First, the sample size limited the power in this study, and due to its exploratory nature, no corrections for multiple testing were made, thus increasing the likelihood of significant results. Second, the presence of nesfatin-1 in the adipose tissue of patients with or without BED was not verified, and it would be interesting to understand if nesfatin-1 levels also vary in relation to the reduction in binge behaviors. Overall, future investigations on nesfatin-1 on EDs need to ensure that such confounders are evaluated and described, and if possible, accounted for in statistical analyses. Limitations of cross-sectional studies obviate conclusions regarding causality.
Future studies with a longitudinal design could be useful to elucidate the variability in nesfatin-1 levels according to other variables, including the determination of nesfatin-1 in CSF in patients with varying degrees of BMIs.